Abstract
The facultative intracellular pathogen Salmonella enterica resides within a membrane-bound compartment inside macrophages 1. This compartment must be acidified for Salmonella to survive within macrophages 2, possibly because acid pH promotes expression of Salmonella virulence proteins 3,4. We reasoned that Salmonella may sense its surroundings have turned acidic not only upon protonation of the extracytoplasmic domain of a protein sensor 5 but also by an increase in cytosolic ATP levels because conditions that enhance the proton gradient across the bacterial inner membrane stimulate ATP synthesis 6,7. We now report that an increase in cytosolic ATP promotes transcription of the coding region for the virulence gene mgtC, which is the most highly-induced horizontally-acquired gene when Salmonella is inside macrophages 8. This transcript was induced both upon media acidification as well as by physiological conditions that increase ATP levels independently of acidification. ATP is sensed via the coupling/uncoupling of transcription of the unusually long mgtC leader mRNA and translation of a short open reading frame located in this region. A mutation in the mgtC leader mRNA that eliminated the response to ATP hindered mgtC expression inside macrophages and attenuated Salmonella virulence in mice. Our results define a singular example of an ATP-sensing leader mRNA. Moreover, they indicate that pathogens can interpret extracellular cues by the impact they have on cellular metabolites.
The mgtC gene is required for survival inside macrophages and for growth in low Mg2+ media in several phylogenetically unrelated intracellular pathogens including S. enterica 9, Yersinia pestis 10, Brucella suis 11, Burkholderia cenocepacia 12 and Mycobacterium tuberculosis 13. In Salmonella, mgtC heads the mgtCBR operon, which specifies the inner membrane protein MgtC, the Mg2+ transporter MgtB and the MgtR peptide promoting MgtC degradation 14. Transcription from the mgtCBR operon is controlled by the virulence regulatory system PhoP/PhoQ 15. mgtC expression must be tightly regulated for a normal course of infection because inactivation of the mgtC gene attenuates Salmonella virulence in mice 9 whereas preventing transcription of AmgR, a PhoP-dependent anti-sense RNA that promotes the preferential degradation of the mgtC portion of the polycistronic mgtCBR message, renders Salmonella hypervirulent 16.
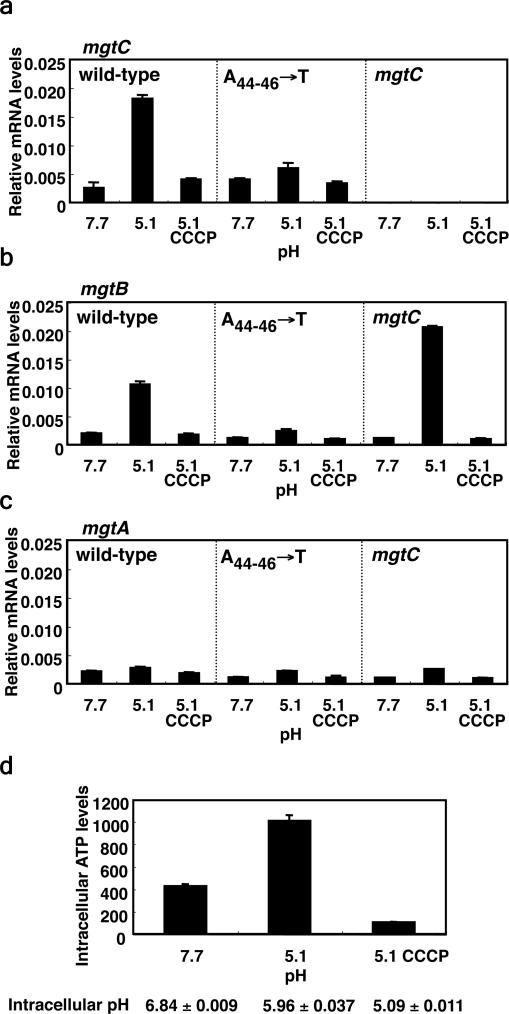
Mild acid pH induces expression of the mgtC 17 (Supplementary Fig. 1) and mgtB 18 genes in a phoP-dependent manner in wild-type Salmonella. This could be ascribed to acid pH sensing by PhoP's cognate sensor PhoQ 5. However, Salmonella appears to utilize a different mechanism to promote mgtC expression in response to mild acid pH because when bacteria were switched from media at pH 7.7 to media at pH 5.1, the mRNA levels of the mgtC (Fig. 1a) and mgtB (Fig. 1b) coding regions increased even in a phoP* phoQ- strain, which lacks the pH sensor PhoQ and harbors a PhoP allele that can function in its absence 19. This stimulation appears to be specific to mgtC because the mRNA levels corresponding to the mgtA coding region did not increase (Fig. 1c) despite the mgtA promoter also being under direct transcriptional control of PhoP 15.
Figure 1. Mild acid pH promotes transcription of the mgtC coding region in a Salmonella strain lacking the extracytoplasmic pH sensor PhoQ.
Relative mRNA levels of the coding regions of the mgtC (a), mgtB (b) and mgtA (c) genes produced by a Salmonella phoP* phoQ strain (EG10232) or derivatives with conserved A 44-46 nucleotides in the mgtCBR leader substituted by Ts (EL486). Bacteria were grown in N-minimal media with 500 μM Mg2+ at pH 7.7 for 1 h and then for an additional 1 h in the same media at pH 5.1 in the absence or presence of 0.5 μM of the protonophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP). mRNA levels of target genes were normalized to that of 16S ribosomal RNA rrs gene. Shown are the mean and SD from two independent experiments. d, Intracellular ATP levels and pH of Salmonella (EG10232) grown in media with pH 7.7 and one hour after being switched to media with pH 5.1 to an OD600: 0.453. Values are in pmol of ATP per ml of cells per OD600. Shown are the mean and SD from two independent measurements.
The stimulation of mgtC expression promoted by mild acid pH could be mediated by an increase in the proton gradient across the inner membrane, hence creating a change in cytosolic ATP. Indeed, the intracellular pH dropped by ~0.9 units and the ATP concentration rose ~2.5 fold within one hour of switching the phoP* phoQ- strain from media at pH 7.7 to media at pH 5.1 (Fig. 1d). Dissipation of the proton gradient with a protonophore decreased ATP levels (Fig. 1d) and prevented induction of mgtC and mgtB at pH 5.1 (Fig. 1a and 1b). (A similar drop in intracellular pH was displayed by wild-type Salmonella displayed [i.e., from pH 8.15±0.112 in media with pH 7.7 to pH 7.25±0.079 in media with pH 5.1 and to pH 5.04±0.031 in media with pH 5.1 in the presence of the protonophore].)
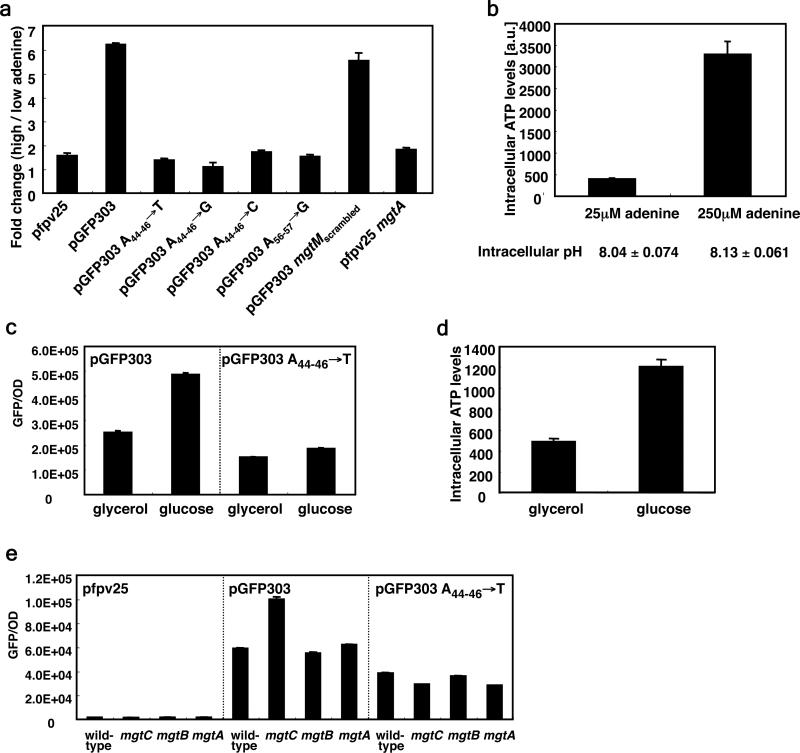
That cellular ATP is the signal upregulating mgtC transcription when Salmonella experiences mild acidification is supported by two additional experiments performed with strains carrying plasmid pGFP303, which harbors the nucleotide sequence corresponding to the natural mgtCBR promoter and leader region fused to a promoterless gfp gene at the mgtC start codon. Fluorescence was six fold higher when a purine auxotroph was grown in defined media with high adenine than with low adenine (Fig. 2a). The higher fluorescence is not due to differences in growth rates (data not shown) or a decrease in cellular pH (Fig. 2b), but reflects the higher ATP levels present in bacteria grown at the higher adenine concentration (Fig. 2b). Moreover, it is specific to ATP because a change in uridine levels in the growth media did not alter the fluorescence of a uridine auxotroph harboring pGFP303 (data not shown). Furthermore, it requires mgtCBR regulatory sequences because changes in the adenine concentration in the media did not affect the fluorescence produced by the adenine auxotroph harboring the plasmid vector (Fig. 2a). And when wild-type Salmonella harboring pGFP303 was grown in glucose, fluorescence was two times higher than when it was grown in glycerol (Fig. 2c), reflecting the larger ATP amounts generated when glucose is used as carbon source (Fig. 2d).
Figure 2. ATP promotes gene transcription in a manner dependent on conserved A nucleotides in the mgtCBR leader region.
a, Fold change in fluorescence produced by an adenine auxotroph (EG9652) harboring the plasmid vector pfpv25, pGFP303 or derivatives with substitutions of conserved A nucleotides at position 44-46 by Ts (pGFP303 A44-46→T), Gs (pGFP303 A44-46→G), or Cs (pGFP303 A44-46→C), with substitutions of conserved 55-56 A nucleotides by Gs (pGFP303 A56-57→G), or with substitution of most of the mgtM sequence except for A nucleotides at 44-46 and 55-56 (pGFP303 mgtMscrambled). Plasmid pfpv25mgtA harbors a transcriptional fusion between the PhoP-dependent mgtA promoter and mgtA leader sequence and a promoterless gfp gene. Bacteria were grown in N-minimal media with 10 μM Mg2+ in the presence of either 25 μM (low) or 250 μM (high) adenine. Fluorescence was monitored as described in Methods. Fold change was calculated by dividing the fluorescence of cells grown in the high adenine by that of cells grown in low adenine. Note that the ratio of fluorescence displayed at high and low adenine is the same for bacteria harboring pfpv25mgtA or the vector pfpv25. Data correspond to a representative of four independent experiments conducted in duplicate. b, Intracellular ATP levels and intracellular pH of purB Salmonella (EG9652) grown in the presence of the indicated concentrations of adenine were determined by labeling with 32P-phosphorus and then separating by TLC. c, Fluorescence produced by wild-type Salmonella (14028s) harboring pGFP303 or pGFP303 A44-46→T following growth for 4 h in N-minimal media with 10 μM Mg2+ in the presence of either 38 mM glycerol (~0.35%) or 0.2% glucose as a carbon source. d, Intracellular ATP levels of cells grown in the indicated carbon sources to an OD600: 0.419 were determined by luminescence as described in Methods. Shown are the mean and SD from two independent measurements. e, Fluorescence produced by wild-type (14028s), mgtC (EL4), mgtB (EL5) and mgtA (EG9521) Salmonella harboring the vector (pfpv25), pGFP303, or its derivative pGFP303 A44-46→T following growth for 4 h in N-minimal media with 10 μM Mg2+.
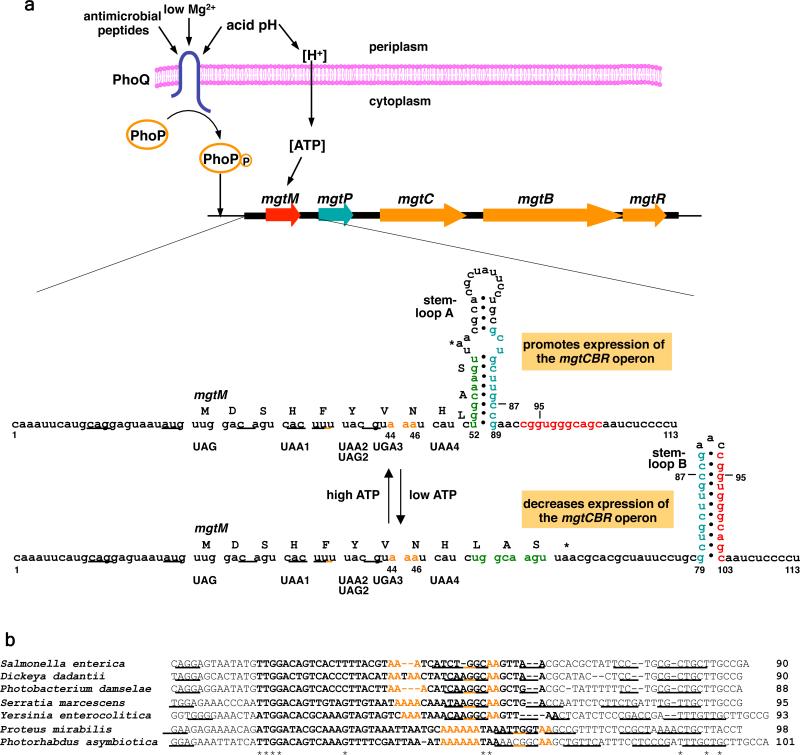
We analyzed the mgtCBR leader mRNA seeking sequence and structural features that may suggest how it might sense ATP. We identified two short ORFs – designated mgtM and mgtP – preceded by putative ribosome binding sites (RBS) (Fig. 4a). Here, we focus on mgtM because, as described below, it mediates the response to ATP. We determined that mgtM is translated in vivo (Supplementary Fig. 2) and that similarly sized ORFs preceded by potential RBSs are conserved in the mgtCBR leader regions from other enteric bacteria (Supplementary Fig. 3). The sequences adjacent to and including mgtM have the potential to adopt two alternative structures – stem-loops A and B – in Salmonella (Fig. 4a) and other examined species (Supplementary Fig. 3). Inline probing experiments verified the formation of stem-loop B in the wild-type mgtCBR leader RNA (Supplementary Fig. 4) and of stem-loop A in a mutant leader RNA with the G95→C substitution, which is predicted to hinder formation of stem-loop B (Supplementary Fig. 4).
Figure 4. Regulation of the Salmonella mgtCBR virulence operon by the PhoP/PhoQ system and mgtCBR leader region.
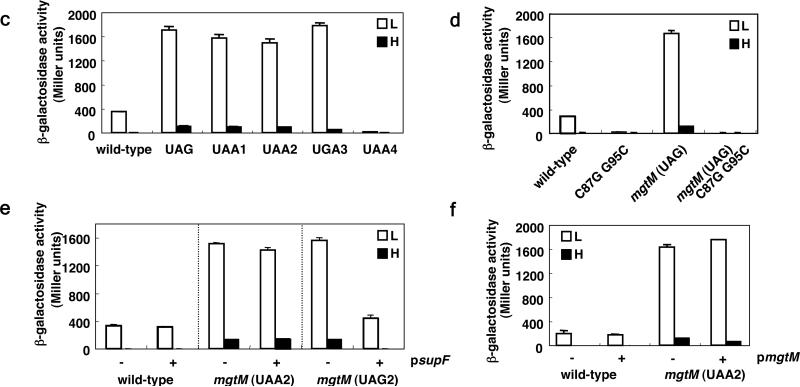
a, The sensor PhoQ responds to extracytoplasmic low Mg2+, acid pH and antimicrobial peptides by promoting phosphorylation of the PhoP protein, which binds to the mgtCBR promoter resulting in transcription initiation. Acidic pH also produces a proton gradient across the inner membrane resulting higher intracellular ATP levels. Intracellular ATP levels control transcription elongation into the mgtCBR coding regions by affecting the coupling/uncoupling of transcription of the mgtCBR leader and translation of the short ORF mgtM, which affects the formation of alternative stem-loops A versus B. The mgtM RBS is underlined. Positions and sequences of stop codon mutations or nucleotide substitutions in the strains used in the experiments presented in (c) and (d) are indicated and denoted below the mgtM sequence. b, Conservation of A nucleotides in mgtM relative to stem-loop structures in the mgtC leader. Alignment of the nucleotide sequences corresponding to mgtM from the indicated species. Sequences in bold correspond to mgtM. The mgtM RBSs are underlined. Asterisks correspond to nucleotides conserved in all listed species. Conserved A nucleotides are colored in orange and sequences with a potential to adopt stem-loop structures are underlined. c, β-galactosidase activity (Miller units) produced by Salmonella with a chromosomal mgtC-lac fusion (EG9527) and isogenic derivatives with mutation of the start codon (EG19269) or with stop codons at different positions (EG19285, EG19289, EG19293 and EG19298) in mgtM. Bacteria were grown in N-minimal media containing low (L; 10 μM) or high (H; 10 mM) Mg2+ for 4 h. d, β-galactosidase activity (Miller units) produced by Salmonella with a chromosomal mgtC-lac fusion (EL92) and isogenic derivatives with C87→G and G95→C substitution (EL96) or mutation of the start codon in mgtM (EL97) or both (EL98). Bacteria were grown as described in (c). Shown in (c) and (d) are the mean and SD from three independent experiments. e, β-galactosidase activity produced by Salmonella with a chromosomal mgtC-lac fusion harboring either plasmid psupF or the empty vector pUH21-2lacIq (EL86, EL87) and isogenic derivatives with an amber stop codon (EL90, EL91) or ochre stop codon (EL88, EL89) at position 39-41 of mgtM. Bacteria were grown as described above except in the presence of ampicillin and 0.2 mM IPTG. f, β-galactosidase activity produced by Salmonella with a chromosomal mgtC-lac fusion (EG9527) or a isogenic strain with a stop codon at position 39-41 in mgtM (EG19289) harboring either the vector or plasmid pmgtM. Bacteria were grown as described except in the presence of ampicillin and 0.1 mM IPTG. Shown in (e) and (f) are the mean and SD from two independent experiments.
The deduced amino acid sequence of mgtM is not conserved in other species (Supplementary Fig. 5), but its length and location relative to stem-loop A, as well as the presence of A nucleotides near the base and within stem-loop A are (i.e., positions 44-46 and 56-57; Fig. 4b). Because the left arm of stem-loop A includes the last four mgtM codons (Fig. 4a), translation of the complete mgtM is anticipated to hinder formation of stem-loop A and favor formation of stem-loop B. Therefore, changes in intracellular ATP levels may affect the coupling/uncoupling of transcription of the mgtCBR leader with translation of mgtM. This would determine whether stem-loop A versus B forms thereby dictating whether transcription continues into the mgtCBR coding regions. This is analogous to the mechanism by which cytoplasmic UTP levels control expression of the pyrimidine biosynthetic gene pyrBI in Escherichia coli, except that low UTP promotes pyrBI transcription when RNAP pauses at a U-rich segment of the pyrBI leader mRNA 20 whereas we propose that high ATP furthers expression of the mgtCBR coding regions.
In agreement with the notion that a transcription attenuation-like mechanism 20,21 regulates transcription elongation into the mgtCBR coding region, a strain with a chromosomal mutation in the mgtM start codon and harboring a lac fusion in the mgtC coding region produced over four times more β-galactosidase than the isogenic strain with the wild-type mgtC leader in response to low Mg2+ (Fig. 4c and 4d). Likewise, strains harboring stop codons at the 4th, 6th or 7th positions of mgtM (Fig. 4a) also derepressed mgtC-lac expression (Fig. 4c). This derepression is due to a defect in mgtM translation (as opposed to resulting from an effect on the structure of the mgtC leader mRNA) because a plasmid expressing the amber suppressor supF restored normal mgtC-lac transcription to an mgtM mutant with an amber stop codon at the 6th position but not to an isogenic mutant with an ochre stop codon at the same position (Fig. 4e). As expected, the supF-expressing plasmid had no effect on mgtC-lac transcription in a strain harboring the wild-type mgtC leader (Fig. 4e).
An mgtM derivative with a stop codon at the 9th position failed to express mgtC-lac (Fig. 4c), possibly because translation of mgtM beyond the 8th codon would result in formation of stem-loop B (Fig. 4a). mgtM translation exerts its regulatory effect on the associated mgtCBR coding regions in cis (as opposed to mgtM encoding a trans-acting peptide) because a plasmid expressing the mgtM ORF failed to restore normal mgC-lac expression to an mgtM stop codon mutant, behaving like the vector control (Fig. 4f); and it had no effect on a strain with a wild-type mgtC leader (Fig. 4f). Our data suggest that whenever translation stops before the ribosome reaches the 9th mgtM codon, stem-loop A forms, which promotes expression of the mgtCBR coding region; and when mgtM is translated beyond a certain position, stem-loop B is favored, which reduces transcription of the mgtCBR coding region. As predicted, the dramatic mgtC-lac derepression exhibited by the mgtM start codon mutant (Fig. 4c and 4d) was eliminated by the simultaneous introduction of C87→G and G95→C substitutions (Fig. 4d), which further formation of stem-loop B (Fig. 4a).
The phenotypes described above are not an artifact resulting from the absence of a fucntional mgtC gene in the mgtC-lac strains. This is because mutation of the mgtM start codon derepressed mgtC levels in an otherwise wild-type Salmonella that experienced mild acidification; and also because introduction of a stop codon at the 9th position of mgtM resulted silenced mgtC expression in an isogenic strain subjected to the same conditions (Supplementary Fig. 6)).
The conserved A nucleotides at positions 44-46 or 56-57 of the mgtCBR leader (Fig. 4a) are critical for the response to ATP because: on the one hand, the mild acid induction of the mgtC and mgtB genes disappeared in a strain with a chromosomal mutation at position 44-46 in the mgtCBR leader (Fig. 1a and 1b). Likewise, growth in glucose no longer promoted higher fluorescence than growth in glycerol in wild-type Salmonella carrying a pGFP303 derivative with the As at position 44-46 substituted by Ts (Fig. 2c). And the increased fluorescence displayed by the mgtC mutant also required the A nucleotides at position 44-46 (Fig. 2e). Yet, this mutant leader retained a wild-type structure (Supplementary Fig. 7) and ability to respond to Mg2+ (Supplementary Fig. 8) 22. Moreover, changes in the adenine concentration in the media failed to affect the fluorescence produced by the purine auxotroph with derivatives of pGFP303 where the A nucleotides at position 44-46 were replaced by either Ts, Gs or Cs, or with a plasmid with the A nucleotides at position 56-57 replaced by Gs (to avoid destabilizing stem-loop A, the latter two nucleotides were not replaced by Ts or Cs; Fig. 4a) (Fig. 2a). One the other hand, an engineered mgtCBR leader with a scrambled mgtM sequence that retained the As at positions 44-46 and 56-57 displayed a wild-type response to ATP (Fig. 2a).
The RNAs from the wild-type and ATP-blind mutant mgtC leader displayed indistinguishable in vitro profiles when investigated at two pHs (Supplementary Fig. 7b). This provides further support to the notion that the increase in mgtCBR expression resulting from mild acidification is mediated by cytosolic ATP levels as opposed to protons being sensed directly by the mgtC leader mRNA. Cumulatively, these data provide a singular example of a bacterial mRNA leader that senses ATP, utilizing a mechanism that is different from those previously described in eukaryotic organisms 23-25.
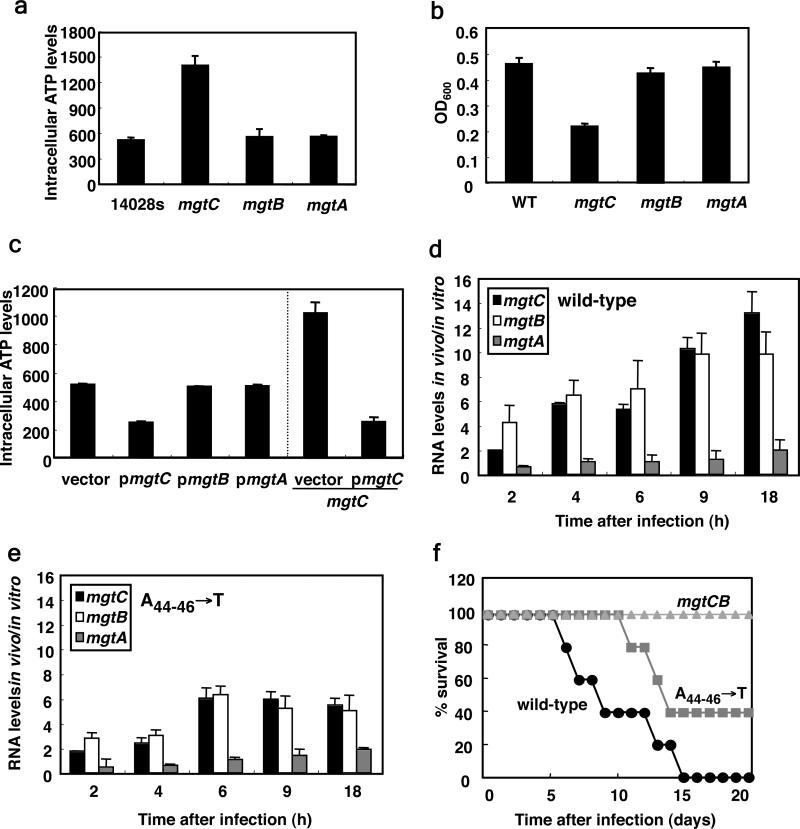
We wondered whether ATP sensing by the mgtCBR mRNA leader is required for Salmonella virulence given that mgtC is expressed in a variety of tissues during infection in several different animal hosts 8,26-28 and that inactivation of the mgtC gene hinders survival inside macrophages and virulence in mice 9. We established that when Salmonella was inside the macrophage-like cell line J774.A1, the mRNA levels of the mgtA and mgtC leaders region rose in parallel (Supplementary Fig. 9), which likely reflects activation of their respective promoters during infection 29. By contrast, the mRNA levels corresponding to the mgtC and mgtB coding regions increased dramatically (i.e., 10 fold by 9 h post internalization relative to Salmonella grown in tissue culture media; Fig. 3d), but no induction was observed for the coding region of the mgtA gene (Fig. 3d), which is not required for Salmonella virulence 9. This may reflect differences between the mgtCBR (Fig. 4a) and mgtA 30 leaders. Indeed, the mRNA levels of the mgtA coding region did not increase under conditions promoting higher ATP levels (Fig. 2a) or mild acidification (Fig. 1a). Induction of the mgtC and mgtB coding regions inside macrophages requires the ability of the mgtCBR leader mRNA to sense ATP because it was defective in a chromosomal mutant with the A nucleotides at position 44-46 replaced by Ts (Fig. 3e). This mutant was attenuated for virulence following intraperitoneal inoculation of mice (Fig. 3f), albeit not as much as a strain deleted for the mgtC and mgtB coding regions (Fig. 3f), implying that other signaling inputs remain functional in this mutant. Cumulatively, our findings demonstrate that the mgtCBR leader and the MgtC protein regulate ATP homeostasis by sensing and controlling cytosolic ATP levels, respectively. These properties are critical for Salmonella virulence, possibly because Salmonella resides within a phagosome that is mildly acidic 1, which is a condition that can generate high ATP levels in the bacterium 6,7. Finally, our data highlight how pathogens can interpret host-derived signals, such as acid pH, by the changes they experience in their cellular constituents.
Figure 3. Expression of the mgtC coding region inside macrophages is dependent on its leader region's ability to sense ATP, a property required for Salmonella virulence.
d, Relative mRNA levels of the mgtC, mgtB and mgtA coding regions of wild-type Salmonella harboring the wild-type mgtCBR leader (14028s) inside J774 A.1 macrophages at the indicated times after infection. e, Same as in (d) except using a mutant Salmonella with the chromosomal A nucleotides at position 44-46 of the mgtCBR leader substituted by Ts (EL341). f, Survival of C3H/HeN mice inoculated intraperitoneally with ~104 colony forming units of wild-type Salmonella (14028s) or ATP-sensing defective mutant (EL341) or deleted for both the mgtC and mgtB coding regions (EL6).
Methods
Bacterial strains, plasmids, oligodeoxynucleotides and growth conditions
Bacterial strains and plasmids used in this study are listed in Supplementary Table 1. All S. enterica serovar Typhimurium strains are derived from the wild-type strain 14028s 31 and were constructed by phage P22-mediated transductions as described 32. All DNA oligonucletides are listed in Supplementary Table 2. Bacteria were grown at 37°C in Luria-Bertani broth (LB), N-minimal media (pH 7.7) 33 supplemented with 0.1% casamino acids, 38 mM glycerol and the indicated concentrations of MgCl2. To examine the effect of pH or carbon source on gene expression, we used a modified N-minimal medium containing 0.2% glucose instead of 38 mM glycerol. Escherichia coli DH5α was used as the host for preparation of plasmid DNA. Ampicillin and was used at 50 μg ml-1, chloramphenicol was used at 20 μg ml-1, kanamycin at 20 μg ml-1, tetracycline at 10 μg ml-1 and fusaric acid at 12 μg ml-1.
Effect of pH on gene expression
Cells were grown overnight in N-minimal medium containing 10 mM Mg2+. 1/100 dilution of the overnight culture was used to inoculate 20 ml of the same medium and grown for 3 h. Cells were then washed and transferred to 20 ml of N-minimal medium containing 500 μM Mg2+ and grown for 1 h. The cells were harvested and washed with N-minimal medium containing 500 μM Mg2+ and an aliquot corresponding to 1/10 volume of cells for pH 7.7 before switching to pH 5.1. Then, cells were resuspended in 20 ml of N-minimal medium containing 500 μM Mg2+ at pH 5.1 with or without 0.5 μM of CCCP and growth continued for 1 h. Bacteria were stabilized using RNAprotect Bacteria Reagent (Qiagen) and RNA was isolated for further analysis.
Effect of exogenous adenine on gene expression
The adenine auxotrophic strain EG9652 harboring plasmid pGFP303, its derivatives, pfpv25mgtA or the plasmid vector were grown overnight in N-minimal medium containing 10 mM Mg2+, 500 μM adenine and ampicillin. 1 ml of the overnight culture was washed in the N-minimal medium without Mg2+ and adenine and resuspended in 1 ml of the same media. The suspended bacteria were inoculated 1/50 volume in N-minimal medium with 10 μM Mg2+ and ampicillin in the presence of either 25 μM or 250 μM adenine. Fluorescence and the OD600 of the cultures were monitored for 6.5 h with shaking 37°C in a Victor3 plate reader (Perkin Elmer). To prevent evaporation, the 96-well plate was covered with mineral oil. GFP expression of a given strain was determined by plotting fluorescence over OD600. Note that the purB strains were freshly transduced before each experiment to prevent the isolation of suppressor mutations.
Measurement of intracellular ATP in Salmonella
We measured intracellular ATP levels using a luminometer (BioTek Synergy H1) as described with modification 34. Briefly, bacteria were grown overnight in N-minimal media containing 10 mM Mg2+ and 0.2% glucose as a carbon source. 1 ml of the overnight culture was washed in the N-minimal medium without Mg2+ and resuspended in 1 ml of the same media. 1/100 dilution of bacteria were inoculated in 2 ml of N-minimal media containing 10 μM Mg2+ (with proper antibiotics if necessary) and grown for 4 h. Cells were normalized by OD600 and resuspended in 500 μl of phosphate-buffered saline (PBS). Nucleic acids were extracted by adding 100 μl of ice cold 3 M perchloric acid. After incubating for 5 min, extracts were neutralized with 225 μl of neutralization buffer (1 M KOH, 0.5 M Tris, 0.5 M KCl) and centrifuged for 10 min. 50 μl of the supernatant was diluted with 50 μl of L buffer (25 mM KCl, 50 mM MgSO4, and 100 mM HEPES pH 7.4) and intracellular ATP was measured using an ATP determination kit (Invitrogen) according to manufacturer's instruction. Intracellular ATP levels [pmoles/ml cells at given OD600] were converted using reference to standards of known concentration.
To measure intracellular ATP levels in response to exogenous adenine levels (Fig. 2b), purB Salmonella were grown overnight in N-minimal medium containing 10 mM Mg2+ and antibiotic. 1 ml of the overnight culture was washed in the N-minimal medium without Mg2+ and adenine and resuspended in 1 ml of the same media. 1/50 dilution of suspended bacteria were inoculated in modified N-minimal media containing 10 μM Mg2+ and 0.3 mM inorganic phosphate and grown for 6.5 h. Then, 120 μCi of P32-phosphorus was added and labeled for 15 min. The labeled NTPs were extracted by formic acid extraction as described previously and analyzed by PEI-cellulose TLC plate 35. The levels of intracellular ATP were quantified by Typhoon FLA-9000 phosphorimager (GE healthcare).
Examining gene expression inside macrophages
Macrophage infection was performed as described 8 with the following modifications: J774 A.1 macrophages were seeded in 6-well plates in RPMI medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine and 10 mM HEPES at a density of 106 per well one day prior to infection with Salmonella. Bacteria were grown overnight in LB media at 37°C, washed with PBS and used to infect macrophages at a multiplicity of infection (MOI) of 100:1. Plates were centrifugated at 1,000 g for 5 min (defined as time 0) and incubated for 1 h for phagocytosis. Extracellular bacteria were killed with RPMI media containing 50 μg/ml gentamicin. The media was replaced after 1 h with same media containing 10 μg/ml gentamicin and continued incubation as indicated in the legend to Figures 3d and 3e. At each time point, infected macrophages were lysed and stabilized with Tri reagent (Applied Biosystems) and RNA was extracted according to manufacturer's instruction. Control RNA from Salmonella cultured in tissue culture media was obtained as described previously 8. Salmonella mRNA levels at each time point were normalized by the mRNA levels following growth in tissue culture media. To estimate the intracellular bacteria at each time point, cells were lysed with PBS containing 0.1% triton X-100 and plated on LB plate with proper dilutions or DNA was extracted from the same RNA sample according to manufacturer's instruction (Applied Biosystems) and measured by quantitative RT-PCR.
Mouse virulence assays
Six to eight week old female C3H/HeN mice were inoculated intraperitoneally with ~104 colony forming units. Mouse survival was followed for 21 days. Virulence assays were conducted three times with similar outcomes and data correspond to groups of five mice.
Supplementary Material
Acknowledgements
We thank Charles Turnbough for discussions, Martina Wade for help with the mouse virulence assays, and Ronald Breaker and Adam Roth for help with in-line probing experiments. This work was supported, in part, by grant AI49561 from the National Institutes of Health to EAG, who is an investigator of the Howard Hughes Medical Institute.
Footnotes
Author Contribution
E.L. conducted the experiments. E.L. and E.A.G. designed the study and wrote the paper. All authors read the paper and contributed to its final form.
References
- 1.Garcia-del Portillo F. Salmonella intracellular proliferation: where, when and how? Microbes Infect. 2001;3:1305–1311. doi: 10.1016/s1286-4579(01)01491-5. [DOI] [PubMed] [Google Scholar]
- 2.Rathman M, Sjaastad MD, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpuche Aranda CM, Swanson JA, Loomis WP, Miller SI. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci U S A. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu XJ, McGourty K, Liu M, Unsworth KE, Holden DW. pH sensing by intracellular Salmonella induces effector translocation. Science. 2010;328:1040–1043. doi: 10.1126/science.1189000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prost LR, et al. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 2007;26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Harold FM, Maloney PC. Energy transduction by ion currents. In: Neidhart FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, et al., editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. American Society for Microbiology; 1996. pp. 283–306. [Google Scholar]
- 7.Senior AE. The proton-translocating ATPase of Escherichia coli. Annu Rev Biophys Biophys Chem. 1990;19:7–41. doi: 10.1146/annurev.bb.19.060190.000255. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- 9.Blanc-Potard AB, Groisman EA. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. Embo J. 1997;16:5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun. 2006;74:3727–3741. doi: 10.1128/IAI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavigne JP, O'Callaghan D, Blanc-Potard AB. Requirement of MgtC for Brucella suis intramacrophage growth: a potential mechanism shared by Salmonella enterica and Mycobacterium tuberculosis for adaptation to a low-Mg2+ environment. Infect Immun. 2005;73:3160–3163. doi: 10.1128/IAI.73.5.3160-3163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maloney KE, Valvano MA. The mgtC gene of Burkholderia cenocepacia is required for growth under magnesium limitation conditions and intracellular survival in macrophages. Infect Immun. 2006;74:5477–5486. doi: 10.1128/IAI.00798-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchmeier N, et al. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol Microbiol. 2000;35:1375–1382. doi: 10.1046/j.1365-2958.2000.01797.x. [DOI] [PubMed] [Google Scholar]
- 14.Alix E, Blanc-Potard AB. MgtC: a key player in intramacrophage survival. Trends Microbiol. 2007;15:252–256. doi: 10.1016/j.tim.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Soncini FC, Garcia Vescovi E, Solomon F, Groisman EA. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J Bacteriol. 1996;178:5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EJ, Groisman EA. An antisense RNA that governs the expression kinetics of a multifunctional virulence gene. Mol Microbiol. 2010;76:1020–1033. doi: 10.1111/j.1365-2958.2010.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Retamal P, Castillo-Ruiz M, Mora GC. Characterization of MgtC, a virulence factor of Salmonella enterica Serovar Typhi. PLoS One. 2009;4:e5551. doi: 10.1371/journal.pone.0005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bearson BL, Wilson L, Foster JW. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J Bacteriol. 1998;180:2409–2417. doi: 10.1128/jb.180.9.2409-2417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamnongpol S, Groisman EA. Acetyl phosphate-dependent activation of a mutant PhoP response regulator that functions independently of its cognate sensor kinase. J Mol Biol. 2000;300:291–305. doi: 10.1006/jmbi.2000.3848. [DOI] [PubMed] [Google Scholar]
- 20.Turnbough CL, Jr., Switzer RL. Regulation of pyrimidine biosynthetic gene expression in bacteria: repression without repressors. Microbiol Mol Biol Rev. 2008;72:266–300. doi: 10.1128/MMBR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merino E, Yanofsky C. Transcription attenuation: a highly conserved regulatory strategy used by bacteria. Trends Genet. 2005;21:260–264. doi: 10.1016/j.tig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Spinelli SV, Pontel LB, Garcia Vescovi E, Soncini FC. Regulation of magnesium homeostasis in Salmonella: Mg(2+) targets the mgtA transcript for degradation by RNase E. FEMS Microbiol Lett. 2008;280:226–234. doi: 10.1111/j.1574-6968.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 23.Amiott EA, Jaehning JA. Mitochondrial transcription is regulated via an ATP “sensing” mechanism that couples RNA abundance to respiration. Mol Cell. 2006;22:329–338. doi: 10.1016/j.molcel.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Dennis PB, et al. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 25.Shu D, Guo P. A viral RNA that binds ATP and contains a motif similar to an ATP-binding aptamer from SELEX. J Biol Chem. 2003;278:7119–7125. doi: 10.1074/jbc.M209895200. [DOI] [PubMed] [Google Scholar]
- 26.Harvey PC, et al. Salmonella enterica serovar Typhimurium colonizing the lumen of the chicken intestine are growing slowly and up-regulate a unique set of virulence and metabolism genes. Infect Immun. 2011;79:4105–4121. doi: 10.1128/IAI.01390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawley TD, et al. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2006;2:e11. doi: 10.1371/journal.ppat.0020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehfuss MY, Parker CT, Brandl MT. Salmonella transcriptional signature in Tetrahymena phagosomes and role of acid tolerance in passage through the protist. ISME J. 2011;5:262–273. doi: 10.1038/ismej.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heithoff DM, et al. Bacterial infection as assessed by in vivo gene expression. Proc Natl Acad Sci U S A. 1997;94:934–939. doi: 10.1073/pnas.94.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SY, Cromie MJ, Lee EJ, Groisman EA. A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell. 2010;142:737–748. doi: 10.1016/j.cell.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis RW, Bolstein D, Roth JR. Advanced Bacterial Genetics. Cold Spring Harbor Lab; 1980. [Google Scholar]
- 33.Snavely MD, Miller CG, Maguire ME. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991;266:815–823. [PubMed] [Google Scholar]
- 34.Rust MJ, Golden SS, O'Shea EK. Light-driven changes in energy metabolism directly entrain the cyanobacterial circadian oscillator. Science. 2011;331:220–223. doi: 10.1126/science.1197243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen KF, Houlberg U, Nygaard P. Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal Biochem. 1979;98:254–263. doi: 10.1016/0003-2697(79)90138-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.