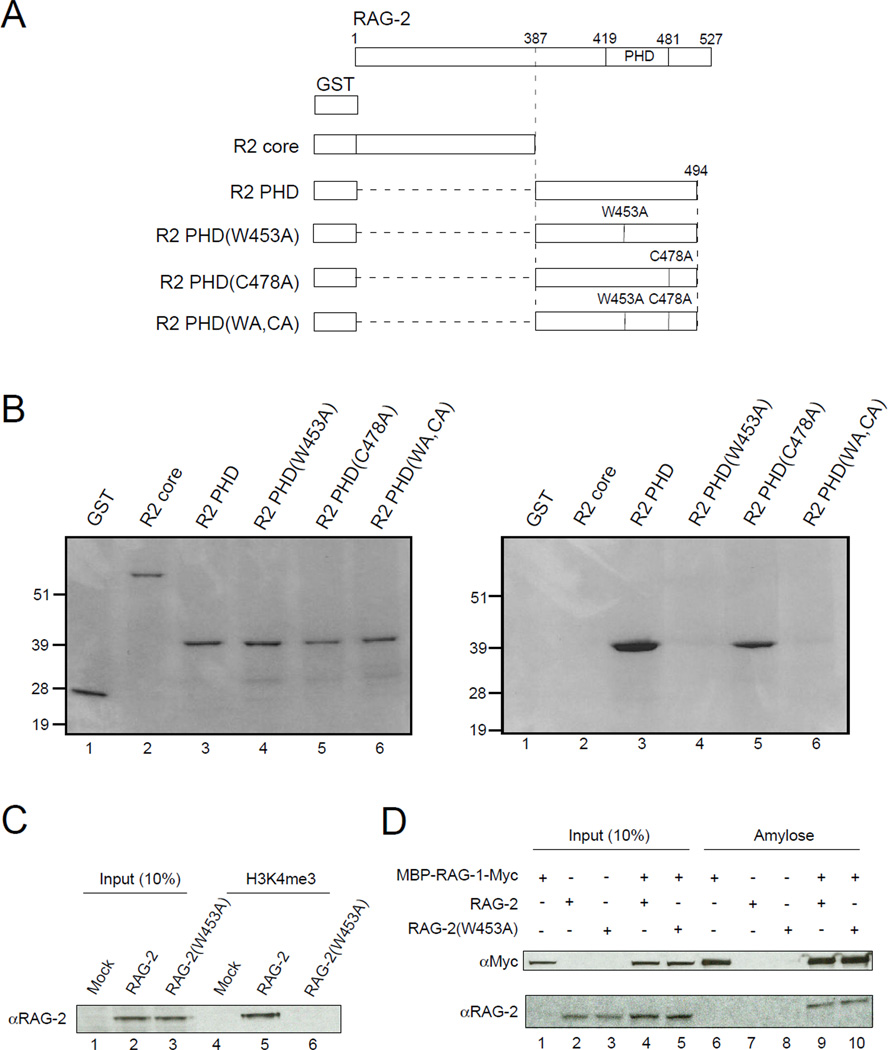
Figure 2.
Binding of RAG-2 to tri-Me H3K4 is mediated by the PHD finger. (A) Representation of proteins used in binding assays. All constructs carry an N-terminal GST tag. Full-length RAG-2 is shown at top; vertical lines indicate the C-terminal end of the core domain (387), the boundaries of the PHD domain (419 – 481) and the positions of amino acid substitutions (W453A, C478A). (B) The RAG-2 PHD finger mediates direct binding to tri-Me H3K4. Proteins described in (A) were expressed in E.coli, purified on glutathione-agarose and assayed for binding to biotinylated H3K4me3 peptide immobilized on streptavidin-linked beads. Bound protein (left) or 10% input protein (right) was fractionated by SDS-PAGE and detected by Coomassie blue. (C) Lysates of mock transfected 293T cells or cells expressing full-length wild-type RAG-2 or RAG-2(W453A) were assayed for binding to the biotinylated H3K4me3 peptide as above (lanes 4 – 6). Bound protein was fractionated by SDS-PAGE and detected by immunoblotting for RAG-2. Input protein (10%) was analyzed in parallel (lanes 1 – 3). (D) RAG-2(W453A) retains the ability to associate with RAG-1. Lysates of 293T cells coexpressing a c-myc-tagged MBP-RAG-1 fusion and RAG-2 or RAG-2(W453A) were adsorbed to amylose beads. Bound protein (lanes 6 – 10) was fractionated by SDS PAGE and detected by immunoblotting for c-myc (top) or RAG-2 (bottom). Input protein (10%) was assayed in parallel (lanes 1 – 5).