Abstract
This review examines the signals encoded in the discharge of cerebellar neurons during voluntary arm and hand movements, assessing the state of our knowledge and the implications for hypotheses of cerebellar function. The evidence for the representation of forces, joint torques, or muscle activity in the discharge of cerebellar neurons is limited, questioning the validity of theories that the cerebellum directly encodes the motor command. In contrast, kinematic parameters such as position, direction, and velocity are widely and robustly encoded in the activity of cerebellar neurons. These findings favor hypotheses that the cerebellum plans or controls movements in a kinematic framework, such as the proposal that the cerebellum provides a forward internal model. Error signals are needed for on-line correction and motor learning, and several hypotheses postulate the need for their representations in the cerebellum. Error signals have been described mostly in the complex spike discharge of Purkinje cells, but no consensus has emerged on the exact information signaled by complex spikes during limb movements. Newer studies suggest that simple spike firing may also encode error signals. Finally, Purkinje cells located more posterior and laterally in the cerebellar cortex and dentate neurons encode nonmotor, task-related signals such as visual cues. These results suggest that cerebellar neurons provide a complement of information about motor behaviors. We assert that additional single unit studies are needed using rich movement paradigms, given the power of this approach to directly test specific hypotheses about cerebellar function.
Keywords: Cerebellum, Purkinje cell, Simple spike, Cerebellar nuclei, Kinematics, Force
Introduction
The cerebellum is essential for the production of smooth, continuous movements. However, the cerebellum’s specific contributions to motor control remain controversial. Numerous hypotheses have been formulated regarding cerebellar function, including motor learning [1–3], providing internal models [4–6], movement timing [7–10], and error detection and correction [11]. These hypotheses are not necessarily exclusive of one another. For example, motor learning may represent the updating or formation of accurate internal models and likely requires information about movement timing and errors. Testing the validity of these or future hypotheses requires identification of the movement and nonmovement information signaled by cerebellar neurons and understanding how this information is processed. Ideally, we need to understand the signals encoded in the inputs of the mossy and climbing fibers and the transformations occurring in the cerebellar cortex. Critical are the parameters carried by the Purkinje cells (PCs), the sole output of the cerebellar cortex to the cerebellar nuclei (CN). Finally, we need to understand the representations in the CN, the final output of the cerebellum.
This review strives to summarize the present knowledge of the signals encoded by cerebellar neurons during voluntary movements and to identify deficits in our understanding. While a rich literature exists regarding the role of the cerebellum in many types of behaviors including locomotion, licking, reflexes, and eye movements [6, 8, 12, 13], we concentrate on arm and hand movements. This review also focuses on signal processing in the context of motor control as opposed to nonmotor functions of the cerebellum [14–17].
Do Cerebellar Neurons Specify the Motor Command?
Many hypotheses surrounding cerebellar function suggest that the cerebellum directly contributes to the motor command that specifies the forces and torques required to produce a movement. Patients with cerebellar lesions often display symptoms related to abnormal force production [18]. Early electrophysiology studies found that PC simple spikes exhibited reciprocal activity during single-joint flexion/extension movements [19–21], consistent with the viewpoint that the cerebellum directly regulates force production and/or individual muscle activity. Others postulated that the cerebellum encodes information in motor coordinates and is responsible for the motor command [22]. Patients with cerebellar pathology have difficulty compensating for interaction torques during multi-joint movements [23, 24]. Patients also fail to adapt their arm movements in a novel external force field [25, 26] or compensate for predictable changes in load forces during precision grasp tasks [27–29], supporting the hypothesis that cerebellar processing is a major component in an inverse dynamics internal model that transforms the desired movement trajectory into a motor command [4, 5, 30]. All of these hypotheses require that cerebellar neuronal discharge encodes force-related parameters such as forces, joint torques, or muscle activity.
However, electrophysiological evidence that PC simple spike discharge encodes force-related information is not compelling. In visuomotor tracking, the simple spike firing has stronger correlations with arm kinematics than with electromyographic (EMG) activity [31, 32] and the spatial tuning functions of individual arm muscles differs from those of the PCs [32]. Likewise, during grasp and lift of objects with different weights and textures, only a small percentage of PCs exhibit simple spike firing that significantly correlates with the grasp force or rate of force production [21, 33, 34]. The simple spike discharge often lags forearm muscle contraction and does not persist for the duration of the grasp [33]. When grasping differently shaped objects at predefined force levels, simple spike activity is extensively modulated with hand shape but the average encoding strength for grasp force is minimal (<2 spikes/s/N) [35].
Two recent studies further addressed PC encoding of force and muscle activity. In one, monkeys produced invariant movement kinematics while performing elbow rotation movements under assistive or resistive force fields [36]. Although brief changes in simple spike discharge occurred when switching between resistive and assistive forces, the magnitude of the loads was not systematically varied nor was a null field used. Consequently, the encoding strength for the loads and/or EMG activity was not evaluated, and it was not determined if the simple spike firing encodes the specific joint torques and/or muscle forces expected from the output of an inverse dynamics model of the arm. Another study trained monkeys to push a series of buttons in response to a light cue. Cross-correlation analyses were used to evaluate the relationship between PC simple spike firing and arm muscle activity [37]. On average, only 6–7% of the firing variability was explained by the muscle activity. Moreover, movement kinematics were not explicitly controlled or included in the analyses, and therefore not disentangled from the muscle activity. Strong evidence for PC encoding of force-related or motor command-like signals is still lacking.
Multi-joint movement studies that unambiguously dissociate force production from the movement kinematics while varying the forces required by the task have not been done until recently. To fill this gap, monkeys were trained to track a target moving in a circular path under varying force fields and loads [38]. Hand forces and arm EMG activity changed appropriately with the applied force fields and loads. In contrast, hand position and speed remained constant, allowing dissociation between hand forces/arm EMG activity and kinematic parameters. For the vast majority of PCs, simple spike firing was neither modulated by the type (e.g., viscous or elastic) nor amplitude of the force field or by changes in EMG activity. These results are inconsistent with hypotheses postulating that PC firing encodes muscle activity, interaction torques, or the motor command [4, 5, 20, 22, 24, 30].
Nonetheless, these results based on PC recordings do not exclude the possibility that force-related parameters or motor commands are encoded in the CN. Stimulation in the interposed nucleus facilitates bilateral muscle activity, suggesting the nuclei have the capacity to influence muscle activation [39]. Also, damage or inactivation of the interposed nuclei leads to reaching movement errors consistent with an inability to compensate for interaction torques [40].
Electrophysiology studies provide an inconsistent picture on the involvement of the CN in controlling force production, joint torques, or EMG activity. Fastigial neuronal discharge is only weakly correlated to muscle activity during reaching [41] and encodes a delayed representation of load forces during wrist movements [42], possibly reflecting processing of sensory feedback as opposed to contributing to the motor command. Firing in dentate and interposed nuclei is correlated to the EMG activity during wrist postural holds [43] and reach-to-pinch [44]. However, only weak correlations are found for isometric pinching and wrist manipulations [45]. During rapid wrist movements, firing modulates with the joint torques but not with the load forces [46]. Downstream from the CN, the neural activity in the red nucleus shows correlations with the EMG activity of distal segments [22], but neurons in the cerebellar thalamus were reported to only encode movement duration [47]. Therefore, no clear answer emerges on the signaling of muscle forces or joint torques in the CN. However, if the simple spike discharge of PCs in the intermediate zones is dominated by kinematics, it seems unlikely that the target CN of these PCs would then primarily signal limb forces or muscle activity.
Some of the conflicting results on the encoding of force-related parameters may be due to the confounding factors and behaviors studied. For example, PC sensitivity to object properties (e.g., shape and texture) could covary with force production at grasp onset [21, 35, 48], and anticipation of perturbations may change grasp kinematics or the rate of force application [48]. Even the feedback modality, for example, visually displaying a quantitative level of grasp force [35], might alter firing activity and be misinterpreted as encoding force production rather than a feedback response. Another source of inconsistencies could arise from the covariance between force-related and kinematic factors. For reaching movements, larger distances are achieved using greater arm velocities and accelerations and, consequently, increased muscle force [49]. The importance of task complexity is revealed by patient studies suggesting that the cerebellum is more engaged in multi-joint movements (contrast Refs. [25] vs. [50]). This raises the question of whether single-joint movement studies are optimal for investigating cerebellar function. Future elec-trophysiology experiments must decouple force-related parameters from the kinematics and systematically examine how task characteristics such as complexity affect representations in the cerebellar output.
Are Kinematic Signals Robustly Encoded at all Levels of Cerebellum Processing?
Although the motor command must eventually be expressed in terms of forces or joint torques, a vast psychophysical literature shows that the central nervous system explicitly plans movements in kinematic reference frames. Point-to-point reaching movements are executed with little variability in the trajectory or velocity profile, despite changes to load forces or movement speeds [51–54]. Errors made during reaching movements depend on the direction and distance to the target, as well as the initial position of the hand [55–57]. Consequently, visually guided movements, in which the cerebellum is thought to play a crucial role [58], require the representation and processing of kinematic signals. One predominant hypothesis is that the cerebellum acts as a forward internal model, using information about the motor command and the current state of the limb to provide an estimate of the consequences arising from the motor command. The model may predict sensory feedback or the resultant state of the limb, both of which could be used to estimate errors without long delays in sensory feedback, permitting rapid, yet, finely tuned movements [5, 59].
Lesion and imaging studies implicate the cerebellum in the control of motor kinematics. Cerebellar damage produces deficits in movement velocity and acceleration [60–63], disrupts the normal bell-shaped velocity profile [64], and degrades intersegmental coordination of kinematic parameters [65]. Functional imaging studies show that the cerebellum is one of a few central structures modulated by tracking speed [66]. During a precision grip force task, imaging reveals activity in the contralateral cerebellum that is dissociated from ipsilateral muscle control, and this activity may reflect a forward internal model that predicts arm kinematics [67]. Other evidence for a forward model is that patients with cerebellar lesions can react, but cannot adapt, to predictable task perturbations [25–27, 68, 69]. During adaptation to movement perturbations, patients with cerebellar ataxia make corrections at compensatory angles that would have been appropriate at earlier time points in the movement, but incorrect for the current arm kinematics [70]. One explanation is that these patients do not have an intact forward model of arm kinematics to facilitate fast error detection, and instead rely upon delayed sensory feedback.
Electrophysiological evidence for encoding movement kinematics is found at all levels of the cerebellum. Global kinematic parameters such as position, direction, and velocity of limb movements are encoded as early as the dorsal spinocerebellar tract and the cuneate nucleus during passive limb manipulations in anesthetized cats [71, 72] and rats [73, 74]. Cerebellar inputs conveyed by the mossy fibers encode position, direction, and velocity of single joints in the behaving monkeys [75]. Mossy fiber signals appear conserved in the granule cell activity, the first level of integration in the cerebellar cortex [76, 77].
Numerous studies have documented that PC simple spike firing signals kinematic variables in the cerebellar cortex. Early single-joint movement studies showed that the simple spike discharge of PCs exhibits some degree of directional sensitivity (for review, see [78]). In more complex, multi-joint movements, the simple spike discharge from intermediate zone PCs in lobules IV–VI of awake monkeys correlates with arm/hand position, direction, speed, and movement distance [31, 32, 38, 49, 79–81]. Similar kinematic representations are found in the simple spike discharge of floccular PCs during smooth pursuit and ocular following [82–85], as well as the encoding of saccade timing by vermal PCs [86]. Position and velocity signals have also been observed in anesthetized or decerebrate cats and rats during passive limb movements [76, 87, 88], demonstrating that simple spike encoding of kinematics is robustly represented in PC discharge across preparations and effectors. These conclusions have to be tempered by the same caution expressed above over confounding variables in which the forces and torques may covary with kinematics. Few studies have explicitly dissociated kinematic and force variables [38]. However, the failure to find simple spike firing modulated in relation to hand forces or arm EMG activity while keeping kinematics constant argues that the reported signaling of kinematics by PCs is not primarily due to confounds with force-related parameters [38].
An example of kinematic encoding in simple spike firing recorded in monkeys performing a circular tracking task is shown in Fig. 1 [32, 38]. The animals tracked both in the clockwise (CW) and counterclockwise (CCW) directions at a fixed speed (±60 or ±80°/s) for a full circle. Modulation in the simple spike firing relative to the mean firing is shown as a function of hand position in the working space. This cell’s firing pattern shows that the maximal and minimal firings are approximately 180° out of phase in both tracking directions (245° and 45° for CCW; 25° and 205° for CW). The position of the maximal firing is shifted approximately 140° between CCWand CW directions. This firing pattern demonstrates that this PC signals a combination of position and movement direction [32] as found in smooth pursuit eye movements [89, 90]. Tracking at different speeds shows that the simple spike firing also encodes movement speed [32].
Fig. 1.
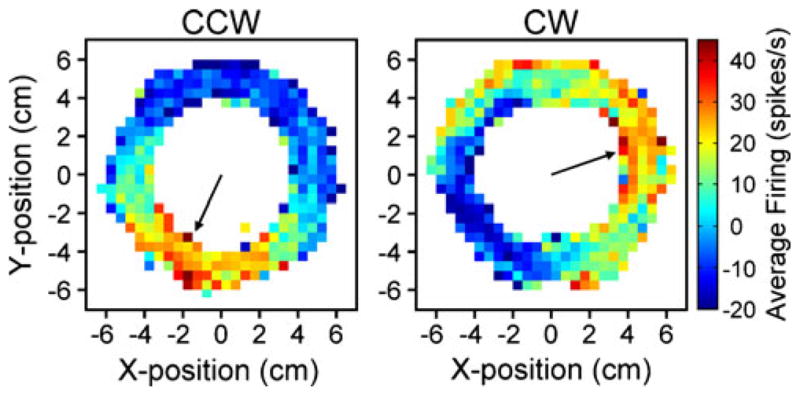
PC simple spike activity during circular tracking. Using a two-joint manipulandum to control a cursor (“+”, 0.5×0.5 cm), monkeys tracked a target (2.5 cm diameter) moving in a circular trajectory (5 cm radius) for 360° in the counterclockwise (CCW; left panel) or clockwise (CW; right panel) directions. The 12×12-cm position workspace (−6 to 6 cm) was partitioned into X–Y bins at a resolution of 0.5×0.5 cm. Simple spike activity was averaged relative to hand position within each bin. Color coding depicts the average change in simple spike firing rate relative to the mean firing rate at each position bin. Arrows point to the position of the maximal simple spike activity (245° for CCW and 25° for CW). Paradigm and experimental details have been described in previous publications [32, 38]
Like simple spike firing, the complex spike discharge in the monkey correlates with kinematic parameters of limb movement, including amplitude and direction [91], tracking direction and speed [92], and endpoint position [93]. However, others failed to find any evidence for inferior olivary discharge coupled to reaching in cats [94, 95]. Inferior olive neurons and complex spikes respond robustly to passive limb movements or to cutaneous stimulation at rest but are inhibited during active, intentional movements [95, 96], consistent with observations that inferior olive activity is inhibited by activation of the red nucleus [97]. However, inferior olive cells are active during exploratory movements or when the receptive field unexpectedly makes contact with an object during active movement [95]. These findings suggest that the complex spike discharge contains kinematic information, but encoding is highly context dependent.
Representations of kinematic parameters are also found in the cerebellar nuclei. Movement direction is encoded in the dentate and interposed nuclei during arm reaching [79] or wrist manipulation [98]. Speed and velocity modulate interposed and dentate neuronal discharge [99, 100], while fastigial neurons encode velocity during ongoing, dynamic movements [42]. The firing of dentate neurons is also modulated with static wrist position [43]. However, there is no universal agreement among studies. Correlations with kinematic parameters were not found in the interposed nuclei during elbow or shoulder movements [43] or in the interposed and dentate nuclei during wrist flexion/extension [46, 101]. As discussed above, an important factor may be task complexity, specifically whether the task requires single- or multi-joint movements. The few studies evaluating PC simple spike activity across multi-joint versus single-joint movements found that many cells exhibited different patterns of discharge for the two different tasks [21, 102].
Whether these kinematic signals in the cerebellum are leading or lagging the movements is fundamental to interpreting how they are used for motor control. If the firing discharge lags kinematics, this is consistent with a role in error detection or monitoring feedback. Conversely, firing leading the actual kinematics would support feed-forward control, the output of a forward model, or encoding of task-related signals (see section below). Simple spike firing often leads hand kinematics by approximately 100 ms during arm tracking movements [31, 32] or center-out reaching tasks [49, 80]. Interestingly, the latencies of PC simple spike responses to passive limb movements are greater in cells recorded medially that project to the fastigial or lateral vestibular nuclei compared with those recorded more laterally that likely project to the interpositus nucleus [103]. Likewise, during intentional arm movements, PCs with the greatest “leads” tend to be recorded more laterally [80], suggesting that timing relationships between firing discharge and movement kinematics may segregate in the different corticonuclear zones.
Only limited information is available on the timing relationships for other cerebellar neurons. Mossy fiber activation varies from leading movement onset by 80 ms to lagging by 100 ms [75]. Activity from the dentate and interposed nuclei tend to precede movements [43], while neuronal firing in the fastigial nucleus lags movement onset [42], consistent with the mediolateral arrangement of timing observed in PCs. Additionally, blocking the dentate nucleus results in slower speeds and longer reaction times in ballistic arm movements [104].
The evidence shows that kinematics are encoded in the cerebellar cortex and its inputs, with PC simple spike firing often leading the movement parameters. Kinematics are also signaled in the cerebellar nuclei but the representations are more variable. Part of the uncertainty may be due to the methods employed in prior electrophysiology studies. Studies in awake monkeys have, by necessity, relied on overtraining in repetitive, predictable paradigms that covered limited areas of the kinematic workspace. This may have resulted in oversimplified models of firing that likely would not translate well to more complex natural movements. Predictable tasks also prevent conclusively determining whether the neural activity encodes future limb kinematics or reflects that the highly trained animals predict task stimuli (e.g., target trajectory). A final question is whether kinematic encoding generalizes across different movement tasks. One study found that regression coefficients calculated from kinematic and PC firing data during circular manual tracking could reconstruct the cell firing during the nontracking, intercept epoch of the task [32]. However, invariant encoding must be tested more rigorously using movement paradigms with different control strategies and properties.
Is Movement Error Signaled by Cerebellar Neurons?
A long-standing hypothesis is that the cerebellum detects and corrects for movement errors [11]. Psychophysical and imaging studies provide support for this concept [25, 26, 105]. The error signals have been generally assumed to be transmitted by the climbing fiber input to PCs [11, 106]. Complex spike discharge driven by retinal slip in oculo-motor behaviors provides the most compelling evidence for an explicit error representation [107–109]. Complex spike discharge increases during learning of a smooth pursuit task where the target predictably changes direction [110]. Similarly in the monkey, complex spike discharge increases during transient limb perturbations, including redirection of reaching [111] and unexpected loads [1], and during adaptation to visuomotor transformations [112]. Complex spike firing also modulates with end point reaching errors [93]. These “motor” error signals, that is errors related to poor motor performance, have been postulated to be used for either on-line control of movement [113, 114] or long-term adaptive modification of the cerebellar circuitry [2, 115–117]. However, several arm movement, as well as eye movement studies, reported that complex spike firing does not encode error signals. During center-out reach tasks performed by monkeys, complex spike modulation did not signal direction or speed errors [92]. Also, inferior olive neurons did not respond to perturbations or errors during reaching movements in the cat [94]. Recent studies of saccade [118] and smooth pursuit [119] adaptation found that the complex spike activity of PCs in the posterior vermis appears unrelated to error signaling. Also undetermined is the reliability and robustness of the climbing fiber error signal. For example, in a study that examined reaching errors with pointing movements [93] complex spikes occurred in only a small fraction of the trials and extensive averaging was required to extract the error-related information. Therefore, there is no uniform agreement if complex spikes strongly signal errors or the precise error information encoded.
Direct evidence that PC simple spike discharge is correlated with specific errors during limb movements is limited. Error signal encoding was found in a reaching task as the simple spike activity modulated with trial success or failure [120]. In a circular tracking task, simple spike activity was correlated with both direction and speed errors, in a manner consistent with error feedback [121]. Although the study acknowledged that the kinematic and error measures evaluated were not strictly independent, the findings suggest that PC simple spike activity could encode performance errors in addition to arm kinematics.
Does the Cerebellum Encode Task-Related Cues?
A final question is whether simple spike firing carries information about task cues. Separating motor activity from visual and/or cutaneous stimuli can be difficult, because many movement paradigms rely upon task-related cues to ensure precise, reproducible movements. One study separated hand and cursor kinematics by changing the cursor gain during a reaching task. Some cursor related modulation was found in PCs recorded from intermediate regions and the modulation tended to lag the cursor actions [91]. However, during visually guided tracking, few intermediate zone PCs modulated in response to the target cue, suggesting that encoding of task parameters is limited or inconsistent [31].
To further examine this issue, we designed a visuomotor dissociation task in which five angular offsets were randomly introduced between the position of the hand and its representation on the screen during the circular tracking task (Fig. 2a). Preliminary results from 16 PCs recorded in the intermediate cerebellar cortex show that the simple spike firing profiles are invariant when aligned relative to hand position, but exhibit phase shifts consistent with the angular offsets when aligned to target position (Fig. 2b, c). The mean phase shift is almost zero and significantly reduced when the firing is aligned to the hand position as opposed to the target position (Fig. 2d). These results suggest that in the intermediate zone, PC simple spike activity reflects limb movement more closely than target movement.
Fig. 2.
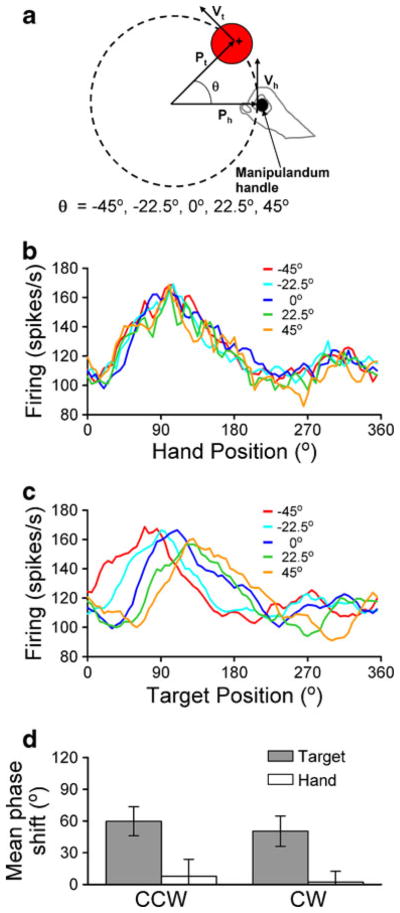
Simple spike activity is phase locked to hand movements during circular tracking with a visuomotor offset. a For each trial, a constant offset (θ) randomly selected from five values was introduced between the angular position of the manipulandum and the hand representation on the screen. b, c The simple spike activity was binned in 5° bins relative to the hand (b) or target (c) angular position, and then averaged across similar trials (same tracking direction and offset value). Firing modulation and phase is unaffected by the offsets when binned relative to hand position (b). However, the phase for each offset value is markedly shifted when binned relative to target position (c). d The magnitude of the offset effect was quantified as the phase shift between −45° and 45° offsets for each tracking direction. The population summary (n=16; mean±SEM) shows the average offset effect is small when neural activity is binned relative to hand (white bars, 7.8± 15.9°CCW and 2.2±10.1°CW) versus target position (gray bars, 59.7± 14.4°CCW and 50.3±13.7°CW) for both directions of tracking (p=0.028 CCW; p=0.023 CW, paired t test)
In contrast, the simple spike firing of PCs in posterolat-eral regions of the cerebellar cortex is highly influenced by visual inputs and visuomotor dissociations [122–125]. During a reaching task in which the direction of cursor movement was opposite from the hand movement, the majority of laterally located PCs modulated with the direction of the cursor movement and not the arm movement [124]. In the cat, posterolateral PCs signal visual events and are modulated in relation to a moving target [126, 127]. The authors suggest this activity may reflect an internal model of the moving visual target. Similarly, dentate neurons discharge in relation to task cues, including selectivity to the cue modality [98] and future movement direction [128]. Therefore, lateral and intermediate zones of the cerebellum exhibit different properties, with PCs in the lateral cortex and dentate neurons exhibiting greater modulation in relation to task cues.
Discussion and Conclusions
Behavioral studies in patients and in animals demonstrate cerebellar involvement in motor control. However, these studies need to be complemented by additional approaches to test specific hypotheses of cerebellar function. Electro-physiological investigations examining the signals encoded in the discharge of cerebellar neurons during limb movements have yielded important insights. As reviewed, the evidence strongly argues that PCs in the intermediate and neighboring lateral zones do not provide the motor command, neither specifying the forces/torques or muscle activity needed to generate a movement. Therefore, these results are inconsistent with hypotheses postulating that Purkinje cells specify forces and/or muscle activity [20, 22], compute interaction torques [23, 24], or act as an inverse dynamics model [4, 5, 30]. This illustrates how single cell recording studies can be used to test specific hypotheses of cerebellar function. The exact nature and extent of the forces or muscle activity signaled in the CN is less clear. Some of the confusion regarding the CN may be that the signals encoded depend on task-related factors like complexity or predictability, underscoring the need for CN electrophysiological studies using rich movement tasks that control and dissociate kinematics from force-related parameters.
During limb movements, cerebellar neurons at every level of the circuitry encode a variety of kinematic parameters. The most robust representations have been identified in the simple spike firing of PCs; however, the majority of investigations have focused on these neurons. Numerous studies also show that simple spike firing tends to lead limb kinematics. These findings are consistent with hypotheses that the cerebellum is involved with feed-forward or predictive control [68, 129], for example, the forward internal model hypothesis [4, 5]. The predictive function of the cerebellum has been hypothesized to transcend the motor control context. Predicting the consequences of current motor commands provides an additional stream of information that when combined with direct sensory input may enhance the perception of the world [130].
Cerebellar neurons also encode other signals. Error signals are a primary interest as these are needed for online correction and motor learning. Most of the efforts have focused on the complex spike discharge or the firing of inferior olivary neurons. Consensus on the nature of the signals in the complex spike discharge during limb movements has not emerged. While there is some evidence for error signals in PC simple spike discharge, electrophysiol-ogy studies in the CN have not addressed this question. Nonmotor, task-related signals are encoded in the discharge of PCs and nuclear neurons, particularly neurons located more laterally. Understanding the relationships among the motor, error, and task-related signals is needed. For example, are kinematic parameters and error signals represented at the population level only or are both integrated at the single cell level? Are these signals encoded in a common coordinate frame?
Major questions remain about the signals encoded in the discharge of cerebellar neurons during limb movements. The degree to which a single cell signals the same information during different movements has not been extensively investigated. Addressing this question will provide insights into the strength and robustness of the encoding and whether the encoding is task dependent. Also, this question is central to the hypothesis that the cerebellar cortex is the site of multiple internal models [131, 132]. Another question is the role of feed-forward versus feedback signals. Most previous studies used tasks that were highly predictable and the animals made very accurate movements with few errors. Investigating the discharge of cerebellar neurons during feedback-dependent tasks will help elucidate error processing in the cerebellum. The nature of the transformation from input to output must be addressed. It would be extremely valuable to compare neuronal activity at each stage of the cerebellar circuitry during a single complex task to better understand how information is encoded and transformed by the cerebellum.
Finally, a critical question is the localization of limb movement related signals in the cerebellar cortex or nuclei but this was only briefly touched upon in this review. This reflects a lack of systematic studies. The importance of localization can be appreciated from the prominent longitudinal architecture of the cerebellum [133, 134]. Both the climbing fiber projection from the inferior olive and the PC corticonuclear projections are organized in parasagittal zones [135–138]. This parasagittal compartmentalization is present at the molecular level on PCs as exemplified by the parasagittal bands of zebrin II/aldolase C as well as a host of other molecular markers [139–141]. While activation of spinocerebellar and olivocerebellar afferents results in parasagittally oriented responses in the cerebellar cortex [8, 11, 142–144], the relation between the representation of specific parameters of limb movements and this parasagittal architecture is essentially unknown [133].
Understanding cerebellar function continues to present numerous challenges as the cerebellum appears to be involved not only in motor control but also sensory and cognitive processes [14, 15, 17, 145]. Studying cerebellar function in the context of motor control remains a very powerful direction of investigation as it allows well-controlled experiments involving quantifiable behaviors. Future studies of cerebellar neuronal activity must be carefully designed to systematically manipulate variables, eliminate confounding factors, and evaluate the influence of contextual task factors like complexity and predictability.
Acknowledgments
We wish to thank Michael McPhee for graphics and Kris Bettin for preparation of the manuscript. This study was supported in part by NIH grants NS18338 and NS071686-01.
Footnotes
Conflicts of Interest: The authors declare no current or potential conflicts of interest.
References
- 1.Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–28. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- 2.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–70. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito M. Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning. Ann NY Acad Sci. 2002;978:273–88. doi: 10.1111/j.1749-6632.2002.tb07574.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–47. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 5.Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–27. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 6.Lisberger SG. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience. 2009;162:763–76. doi: 10.1016/j.neuroscience.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braitenberg V, Atwood RP. Morphological observations on the cerebellar cortex. J Comp Neurol. 1958;109:1–33. doi: 10.1002/cne.901090102. [DOI] [PubMed] [Google Scholar]
- 8.Welsh JP, Lang EJ, Suglhara I, Llinas R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–7. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 9.Keele SW, Ivry R. Does the cerebellum provide a common computation for diverse tasks? A timing hypothesis. Ann NY Acad Sci. 1990;608:179–207. doi: 10.1111/j.1749-6632.1990.tb48897.x. [DOI] [PubMed] [Google Scholar]
- 10.O’Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci. 2008;28:2252–60. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oscarsson O. Functional organization of olivary projection to the cerebellar anterior lobe. In: Courville J, editor. The inferior olivary nucleus: anatomy and physiology. New York: Raven; 1980. pp. 279–90. [Google Scholar]
- 12.Morton SM, Bastian AJ. Mechanisms of cerebellar gait ataxia. Cerebellum. 2007;6:79–86. doi: 10.1080/14734220601187741. [DOI] [PubMed] [Google Scholar]
- 13.Bloedel JR, Bracha V. On the cerebellum, cutaneomuscular reflexes, movement control and the elusive engrams of memory. Behav Brain Res. 1995;68:1–44. doi: 10.1016/0166-4328(94)00171-b. [DOI] [PubMed] [Google Scholar]
- 14.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 15.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–13. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 16.Glickstein M, Doron K. Cerebellum: connections and functions. Cerebellum. 2008;7:589–94. doi: 10.1007/s12311-008-0074-4. [DOI] [PubMed] [Google Scholar]
- 17.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 18.Holmes G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain. 1917;40:461–535. [Google Scholar]
- 19.Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968;31:785–97. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- 20.Smith AM. The coactivation of antagonist muscles. Can J Physiol Pharmacol. 1981;59:733–47. doi: 10.1139/y81-110. [DOI] [PubMed] [Google Scholar]
- 21.Frysinger RC, Bourbonnais D, Kalaska JF, Smith AM. Cerebellar cortical activity during antagonist cocontraction and reciprocal inhibition of forearm muscles. J Neurophysiol. 1984;51:32–49. doi: 10.1152/jn.1984.51.1.32. [DOI] [PubMed] [Google Scholar]
- 22.Miller LE, Houk JC. Motor co-ordinates in primate red nucleus: preferential relation to muscle activation versus kinematic variables. J Physiol. 1995;488(Pt 2):533–48. doi: 10.1113/jphysiol.1995.sp020988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastian AJ, Zackowski KM, Thach WT. Cerebellar ataxia: torque deficiency or torque mismatch between joints? J Neuro-physiol. 2000;83:3019–30. doi: 10.1152/jn.2000.83.5.3019. [DOI] [PubMed] [Google Scholar]
- 24.Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- 25.Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91:230–8. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- 26.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–21. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 27.Muller F, Dichgans J. Dyscoordination of pinch and lift forces during grasp in patients with cerebellar lesions. Exp Brain Res. 1994;101:485–92. doi: 10.1007/BF00227341. [DOI] [PubMed] [Google Scholar]
- 28.Nowak DA, Hermsdorfer J, Marquardt C, Fuchs HH. Grip and load force coupling during discrete vertical arm movements with a grasped object in cerebellar atrophy. Exp Brain Res. 2002;145:28–39. doi: 10.1007/s00221-002-1079-8. [DOI] [PubMed] [Google Scholar]
- 29.Rost K, Nowak DA, Timmann D, Hermsdorfer J. Preserved and impaired aspects of predictive grip force control in cerebellar patients. Clin Neurophysiol. 2005;116:1405–14. doi: 10.1016/j.clinph.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Schweighofer N, Arbib MA, Kawato M. Role of the cerebellum in reaching movements in humans. I. Distributed inverse dynamics control. Eur J Neurosci. 1998;10:86–94. doi: 10.1046/j.1460-9568.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- 31.Coltz JD, Johnson MT, Ebner TJ. Cerebellar Purkinje cell simple spike discharge encodes movement velocity in primates during visuomotor arm tracking. J Neurosci. 1999;19:1782–803. doi: 10.1523/JNEUROSCI.19-05-01782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roitman AV, Pasalar S, Johnson MT, Ebner TJ. Position, direction of movement, and speed tuning of cerebellar Purkinje cells during circular manual tracking in monkey. J Neurosci. 2005;25:9244–57. doi: 10.1523/JNEUROSCI.1886-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AM, Bourbonnais D. Neuronal activity in cerebellar cortex related to control of prehensile force. J Neurophysiol. 1981;45:286–303. doi: 10.1152/jn.1981.45.2.286. [DOI] [PubMed] [Google Scholar]
- 34.Espinoza E, Smith AM. Purkinje cell simple spike activity during grasping and lifting objects of different textures and weights. J Neurophysiol. 1990;64:698–714. doi: 10.1152/jn.1990.64.3.698. [DOI] [PubMed] [Google Scholar]
- 35.Mason CR, Hendrix CM, Ebner TJ. Purkinje cells signal hand shape and grasp force during reach-to-grasp in the monkey. J Neurophysiol. 2006;95:144–58. doi: 10.1152/jn.00492.2005. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto K, Kawato M, Kotosaka S, Kitazawa S. Encoding of movement dynamics by Purkinje cell simple spike activity during fast arm movements under resistive and assistive force fields. J Neurophysiol. 2007;97:1588–99. doi: 10.1152/jn.00206.2006. [DOI] [PubMed] [Google Scholar]
- 37.Holdefer RN, Miller LE. Dynamic correspondence between Purkinje cell discharge and forelimb muscle activity during reaching. Brain Res. 2009;1295:67–75. doi: 10.1016/j.brainres.2009.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;9:1404–11. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- 39.Soteropoulos DS, Baker SN. Bilateral representation in the deep cerebellar nuclei. J Physiol. 2008;586:1117–36. doi: 10.1113/jphysiol.2007.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper SE, Martin JH, Ghez C. Effects of inactivation of the anterior interpositus nucleus on the kinematic and dynamic control of multijoint movement. J Neurophysiol. 2000;84:1988–2000. doi: 10.1152/jn.2000.84.4.1988. [DOI] [PubMed] [Google Scholar]
- 41.MacKay WA. Cerebellar nuclear activity in relation to simple movements. Exp Brain Res. 1988;71:47–58. doi: 10.1007/BF00247521. [DOI] [PubMed] [Google Scholar]
- 42.Bava A, Grimm RJ, Rushmer DS. Fastigial unit activity during voluntary movement in primates. Brain Res. 1983;288:371–4. doi: 10.1016/0006-8993(83)90121-x. [DOI] [PubMed] [Google Scholar]
- 43.Thach WT. Correlation of neural discharge with pattern and force of muscular activity, joint position, and direction of intended next movement in motor cortex and cerebellum. J Neurophysiol. 1978;41:654–76. doi: 10.1152/jn.1978.41.3.654. [DOI] [PubMed] [Google Scholar]
- 44.Goodkin HP, Thach WT. Cerebellar control of constrained and unconstrained movements. II. EMG and nuclear activity. J Neurophysiol. 2003;89:896–908. doi: 10.1152/jn.00115.2002. [DOI] [PubMed] [Google Scholar]
- 45.Wetts R, Kalaska JF, Smith AM. Cerebellar nuclear cell activity during antagonist cocontraction and reciprocal inhibition of forearm muscles. J Neurophysiol. 1985;54:231–44. doi: 10.1152/jn.1985.54.2.231. [DOI] [PubMed] [Google Scholar]
- 46.Schieber MH, Thach WT. Trained slow tracking. II. Bidirectional discharge patterns of cerebellar nuclear, motor cortex, and spindle afferent neurons. J Neurophysiol. 1985;54:1228–70. doi: 10.1152/jn.1985.54.5.1228. [DOI] [PubMed] [Google Scholar]
- 47.Ivanusic JJ, Bourke DW, Xu ZM, Butler EG, Horne MK. Cerebellar thalamic activity in the macaque monkey encodes the duration but not the force or velocity of wrist movement. Brain Res. 2005;1041:181–97. doi: 10.1016/j.brainres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Dugas C, Smith AM. Responses of cerebellar Purkinje cells to slip of a hand-held object. J Neurophysiol. 1992;67:483–95. doi: 10.1152/jn.1992.67.3.483. [DOI] [PubMed] [Google Scholar]
- 49.Fu QG, Flament D, Coltz JD, Ebner TJ. Relationship of cerebellar Purkinje cell simple spike discharge to movement kinematics in the monkey. J Neurophysiol. 1997;78:478–91. doi: 10.1152/jn.1997.78.1.478. [DOI] [PubMed] [Google Scholar]
- 50.Richter S, Maschke M, Timmann D, Konczak J, Kalenscher T, Illenberger AR, et al. Adaptive motor behavior of cerebellar patients during exposure to unfamiliar external forces. J Mot Behav. 2004;36:28–38. doi: 10.3200/JMBR.36.1.28-38. [DOI] [PubMed] [Google Scholar]
- 51.Atkeson CG, Hollerbach JM. Kinematic features of unrestrained vertical arm movements. J Neurosci. 1985;5:2318–30. doi: 10.1523/JNEUROSCI.05-09-02318.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacquaniti F, Soechting JF, Terzuolo SA. Path constraints on point-to-point arm movements in three-dimensional space. Neuroscience. 1986;17:313–24. doi: 10.1016/0306-4522(86)90249-6. [DOI] [PubMed] [Google Scholar]
- 53.Abend W, Bizzi E, Morasso P. Human arm trajectory formation. Brain. 1982;105:331–48. doi: 10.1093/brain/105.2.331. [DOI] [PubMed] [Google Scholar]
- 54.Georgopoulos AP, Kalaska JF, Massey JT. Spatial trajectories and reaction times of aimed movements: effects of practice, uncertainty, and change in target location. J Neurophysiol. 1981;46:725–43. doi: 10.1152/jn.1981.46.4.725. [DOI] [PubMed] [Google Scholar]
- 55.Soechting JF, Flanders M. Extrapolation of visual motion for manual interception. J Neurophysiol. 2008;99:2956–67. doi: 10.1152/jn.90308.2008. [DOI] [PubMed] [Google Scholar]
- 56.Soechting JF, Flanders M. Errors in pointing are due to approximations in sensorimotor transformations. J Neurophysiol. 1989;62:595–608. doi: 10.1152/jn.1989.62.2.595. [DOI] [PubMed] [Google Scholar]
- 57.Vindras P, Viviani P. Frames of reference and control parameters in visuomanual pointing. J Exp Psychol Hum Percept Perform. 1998;24:569–91. doi: 10.1037//0096-1523.24.2.569. [DOI] [PubMed] [Google Scholar]
- 58.Stein JF, Glickstein M. Role of the cerebellum in visual guidance of movement. Physiol Rev. 1992;72:967–1017. doi: 10.1152/physrev.1992.72.4.967. [DOI] [PubMed] [Google Scholar]
- 59.Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–2. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- 60.Holmes G. The cerebellum of man. Brain. 1939;62:1–30. [Google Scholar]
- 61.Hore J, Wild B, Diener HC. Cerebellar dysmetria at the elbow, wrist, and fingers. J Neurophysiol. 1991;65:563–71. doi: 10.1152/jn.1991.65.3.563. [DOI] [PubMed] [Google Scholar]
- 62.Beppu H, Suda M, Tanaka R. Analysis of cerebellar motor disorders by visually guided elbow tracking movement. Brain. 1984;107(Pt 3):787–809. doi: 10.1093/brain/107.3.787. [DOI] [PubMed] [Google Scholar]
- 63.Miall RC, Weir DJ, Stein JF. Visuo-motor tracking during reversible inactivation of the cerebellum. Exp Brain Res. 1987;65:455–64. doi: 10.1007/BF00236319. [DOI] [PubMed] [Google Scholar]
- 64.Diener HC, Dichgans J. Pathophysiology of cerebellar ataxia. Mov Disord. 1992;7:95–109. doi: 10.1002/mds.870070202. [DOI] [PubMed] [Google Scholar]
- 65.Milak MS, Shimansky Y, Bracha V, Bloedel JR. Effects of inactivating individual cerebellar nuclei on the performance and retention of an operantly conditioned forelimb movement. J Neurophysiol. 1997;78:939–59. doi: 10.1152/jn.1997.78.2.939. [DOI] [PubMed] [Google Scholar]
- 66.Turner RS, Grafton ST, Votaw JR, DeLong MR, Hoffman JM. Motor subcircuits mediating the control of movement velocity: a PET study. J Neurophysiol. 1998;80:2162–76. doi: 10.1152/jn.1998.80.4.2162. [DOI] [PubMed] [Google Scholar]
- 67.Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res. 2003;142:171–88. doi: 10.1016/S0079-6123(03)42013-X. [DOI] [PubMed] [Google Scholar]
- 68.Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16:645–9. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 69.Nowak DA, Topka H, Timmann D, Boecker H, Hermsdorfer J. The role of the cerebellum for predictive control of grasping. Cerebellum. 2007;6:7–17. doi: 10.1080/14734220600776379. [DOI] [PubMed] [Google Scholar]
- 70.Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- 71.Bosco G, Rankin A, Poppele R. Representation of passive hindlimb postures in cat spinocerebellar activity. J Neurophysiol. 1996;76:715–26. doi: 10.1152/jn.1996.76.2.715. [DOI] [PubMed] [Google Scholar]
- 72.Bosco G, Poppele RE. Representation of multiple kinematic parameters of the cat hindlimb in spinocerebellar activity. J Neurophysiol. 1997;78:1421–32. doi: 10.1152/jn.1997.78.3.1421. [DOI] [PubMed] [Google Scholar]
- 73.Garifoli A, Caserta C, Bosco G, Lombardo SA, Casabona A, Perciavalle V. Kinematic features of passive forelimb movements and rat cuneate neuron discharges. NeuroReport. 2002;13:267–71. doi: 10.1097/00001756-200203040-00004. [DOI] [PubMed] [Google Scholar]
- 74.Giaquinta G, Casabona A, Valle MS, Bosco G, Perciavalle V. On the relation of rat’s external cuneate activity to global parameters of forelimb posture. NeuroReport. 1999;10:3075–80. doi: 10.1097/00001756-199909290-00037. [DOI] [PubMed] [Google Scholar]
- 75.van Kan PL, Gibson AR, Houk JC. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol. 1993;69:74–94. doi: 10.1152/jn.1993.69.1.74. [DOI] [PubMed] [Google Scholar]
- 76.Kolb FP, Rubia FJ, Bauswein E. Cerebellar unit responses of the mossy fibre system to passive movements in the decerebrate cat. I. Responses to static parameters. Exp Brain Res. 1987;68:234–48. doi: 10.1007/BF00248790. [DOI] [PubMed] [Google Scholar]
- 77.Bengtsson F, Jorntell H. Sensory transmission in cerebellar granule cells relies on similarly coded mossy fiber inputs. Proc Natl Acad Sci USA. 2009;106:2389–94. doi: 10.1073/pnas.0808428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ebner TJ, Fu Q. What features of visually guided arm movements are encoded in the simple spike discharge of cerebellar Purkinje cells? Prog Brain Res. 1997;114:431–47. doi: 10.1016/s0079-6123(08)63379-8. [DOI] [PubMed] [Google Scholar]
- 79.Fortier PA, Kalaska JF, Smith AM. Cerebellar neuronal activity related to whole-arm reaching movements in the monkey. J Neurophysiol. 1989;62:198–211. doi: 10.1152/jn.1989.62.1.198. [DOI] [PubMed] [Google Scholar]
- 80.Marple-Horvat DE, Stein JF. Cerebellar neuronal activity related to arm movements in trained rhesus monkeys. J Physiol. 1987;394:351–66. doi: 10.1113/jphysiol.1987.sp016874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mano N, Yamamoto K. Simple-spike activity of cerebellar Purkinje cells related to visually guided wrist tracking movement in the monkey. J Neurophysiol. 1980;43:713–28. doi: 10.1152/jn.1980.43.3.713. [DOI] [PubMed] [Google Scholar]
- 82.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol. 1990;63:1241–61. doi: 10.1152/jn.1990.63.5.1241. [DOI] [PubMed] [Google Scholar]
- 83.Shidara M, Kawano K, Gomi H, Kawato M. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature. 1993;365:50–2. doi: 10.1038/365050a0. [DOI] [PubMed] [Google Scholar]
- 84.Medina JF, Lisberger SG. Encoding and decoding of learned smooth pursuit eye movements in the floccular complex of the monkey cerebellum. J Neurophysiol. 2009;102:2039–54. doi: 10.1152/jn.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gomi H, Shidara M, Takemura A, Inoue Y, Kawano K, Kawato M. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys I. Simple spikes. J Neurophysiol. 1998;80:818–31. doi: 10.1152/jn.1998.80.2.818. [DOI] [PubMed] [Google Scholar]
- 86.Thier P, Dicke PW, Haas R, Barash S. Encoding of movement time by populations of cerebellar Purkinje cells. Nature. 2000;405:72–6. doi: 10.1038/35011062. [DOI] [PubMed] [Google Scholar]
- 87.Valle MS, Bosco G, Poppele R. Information processing in the spinocerebellar system. NeuroReport. 2000;11:4075–9. doi: 10.1097/00001756-200012180-00033. [DOI] [PubMed] [Google Scholar]
- 88.Giaquinta G, Valle MS, Caserta C, Casabona A, Bosco G, Perciavalle V. Sensory representation of passive movement kinematics by rat’s spinocerebellar Purkinje cells. Neurosci Lett. 2000;285:41–4. doi: 10.1016/s0304-3940(00)01020-x. [DOI] [PubMed] [Google Scholar]
- 89.Leung HC, Suh M, Kettner RE. Cerebellar flocculus and paraflocculus Purkinje cell activity during circular pursuit in monkey. J Neurophysiol. 2000;83:13–30. doi: 10.1152/jn.2000.83.1.13. [DOI] [PubMed] [Google Scholar]
- 90.Suh M, Leung HC, Kettner RE. Cerebellar flocculus and ventral paraflocculus Purkinje cell activity during predictive and visually driven pursuit in monkey. J Neurophysiol. 2000;84:1835–50. doi: 10.1152/jn.2000.84.4.1835. [DOI] [PubMed] [Google Scholar]
- 91.Fu QG, Mason CR, Flament D, Coltz JD, Ebner TJ. Movement kinematics encoded in complex spike discharge of primate cerebellar Purkinje cells. NeuroReport. 1997;8:523–9. doi: 10.1097/00001756-199701200-00029. [DOI] [PubMed] [Google Scholar]
- 92.Ebner TJ, Johnson MT, Roitman A, Fu Q. What do complex spikes signal about limb movements? Ann NY Acad Sci. 2002;978:205–18. doi: 10.1111/j.1749-6632.2002.tb07568.x. [DOI] [PubMed] [Google Scholar]
- 93.Kitazawa S, Kimura T, Yin PB. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature. 1998;392:494–7. doi: 10.1038/33141. [DOI] [PubMed] [Google Scholar]
- 94.Horn KM, van Kan PL, Gibson AR. Reduction of rostral dorsal accessory olive responses during reaching. J Neurophysiol. 1996;76:4140–51. doi: 10.1152/jn.1996.76.6.4140. [DOI] [PubMed] [Google Scholar]
- 95.Gellman R, Gibson AR, Houk JC. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol. 1985;54:40–60. doi: 10.1152/jn.1985.54.1.40. [DOI] [PubMed] [Google Scholar]
- 96.Rushmer DS, Roberts WJ, Augter GK. Climbing fiber responses of cerebellar Purkinje cells to passive movement of the cat forepaw. Brain Res. 1976;106:1–20. doi: 10.1016/0006-8993(76)90069-x. [DOI] [PubMed] [Google Scholar]
- 97.Weiss C, Houk JC, Gibson AR. Inhibition of sensory responses of cat inferior olive neurons produced by stimulation of red nucleus. J Neurophysiol. 1990;64:1170–85. doi: 10.1152/jn.1990.64.4.1170. [DOI] [PubMed] [Google Scholar]
- 98.Chapman CE, Spidalieri G, Lamarre Y. Activity of dentate neurons during arm movements triggered by visual, auditory, and somesthetic stimuli in the monkey. J Neurophysiol. 1986;55:203–26. doi: 10.1152/jn.1986.55.2.203. [DOI] [PubMed] [Google Scholar]
- 99.Soechting JF, Burton JE, Onoda N. Relationships between sensory input, motor output and unit activity in interpositus and red nuclei during intentional movement. Brain Res. 1978;152:65–79. doi: 10.1016/0006-8993(78)90134-8. [DOI] [PubMed] [Google Scholar]
- 100.van Kan PL, Houk JC, Gibson AR. Output organization of intermediate cerebellum of the monkey. J Neurophysiol. 1993;69:57–73. doi: 10.1152/jn.1993.69.1.57. [DOI] [PubMed] [Google Scholar]
- 101.Aumann TD, Rawson JA, Horne MK. The relationship between monkey dentate cerebellar nucleus activity and kinematic parameters of wrist movement. Exp Brain Res. 1998;119:179–90. doi: 10.1007/s002210050332. [DOI] [PubMed] [Google Scholar]
- 102.Thach WT. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970;33:537–47. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- 103.Gray C, Perciavalle V, Poppele R. Sensory responses to passive hindlimb joint rotation in the cerebellar cortex of the cat. Brain Res. 1993;622:280–4. doi: 10.1016/0006-8993(93)90829-c. [DOI] [PubMed] [Google Scholar]
- 104.Lu X, Hikosaka O, Miyachi S. Role of monkey cerebellar nuclei in skill for sequential movement. J Neurophysiol. 1998;79:2245–54. doi: 10.1152/jn.1998.79.5.2245. [DOI] [PubMed] [Google Scholar]
- 105.Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25:9919–31. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ito M. Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886:237–45. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- 107.Graf W, Simpson JI, Leonard CS. Spatial organization of visual messages of the rabbit’s cerebellar flocculus. II. Complex and simple spike responses of Purkinje cells. J Neurophysiol. 1988;60:2091–121. doi: 10.1152/jn.1988.60.6.2091. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi Y, Kawano K, Takemura A, Inoue Y, Kitama T, Gomi H, et al. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys II. Complex spikes. J Neurophysiol. 1998;80:832–48. doi: 10.1152/jn.1998.80.2.832. [DOI] [PubMed] [Google Scholar]
- 109.Barmack NH, Shojaku H. Vestibular and visual climbing fiber signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol. 1995;74:2573–89. doi: 10.1152/jn.1995.74.6.2573. [DOI] [PubMed] [Google Scholar]
- 110.Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–92. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim JH, Wang JJ, Ebner TJ. Climbing fiber afferent modulation during treadmill locomotion in the cat. J Neurophysiol. 1987;57:787–802. doi: 10.1152/jn.1987.57.3.787. [DOI] [PubMed] [Google Scholar]
- 112.Ojakangas CL, Ebner TJ. Purkinje cell complex spike activity during voluntary motor learning: relationship to kinematics. J Neurophysiol. 1994;72:2617–30. doi: 10.1152/jn.1994.72.6.2617. [DOI] [PubMed] [Google Scholar]
- 113.Ebner TJ, Bloedel JR. Climbing fiber afferent system: intrinsic properties and role in cerebellar information processing. In: King JS, editor. New concepts in cerebellar neurobiology. New York: Alan R. Liss, Inc; 1987. pp. 371–86. [Google Scholar]
- 114.Bloedel JR. Functional heterogeneity with structural homogeneity: how does the cerebellum operate? Behav Brain Sci. 1992;15:666–78. [Google Scholar]
- 115.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–95. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 116.Kawato M. Learning internal models of the motor apparatus. In: Bloedel JR, Ebner TJ, Wise SP, editors. The acquisition of motor behavior in vertebrates. Cambridge: MIT Press; 1996. pp. 409–30. [Google Scholar]
- 117.Thach WT. A role for the cerebellum in learning movement coordination. Neurobiol Learn Mem. 1998;70:177–88. doi: 10.1006/nlme.1998.3846. [DOI] [PubMed] [Google Scholar]
- 118.Catz N, Dicke PW, Thier P. Cerebellar complex spike firing is suitable to induce as well as to stabilize motor learning. Curr Biol. 2005;15:2179–89. doi: 10.1016/j.cub.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 119.Dash S, Catz N, Dicke PW, Thier P. Specific vermal complex spike responses build up during the course of smooth-pursuit adaptation, paralleling the decrease of performance error. Exp Brain Res. 2010;205:41–55. doi: 10.1007/s00221-010-2331-2. [DOI] [PubMed] [Google Scholar]
- 120.Greger B, Norris S. Simple spike firing in the posterior lateral cerebellar cortex of Macaque Mulatta was correlated with success-failure during a visually guided reaching task. Exp Brain Res. 2005;167:660–5. doi: 10.1007/s00221-005-0155-2. [DOI] [PubMed] [Google Scholar]
- 121.Roitman AV, Pasalar S, Ebner TJ. Single trial coupling of Purkinje cell activity to speed and error signals during circular manual tracking. Exp Brain Res. 2009;192:241–51. doi: 10.1007/s00221-008-1580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Norris SA, Greger B, Hathaway EN, Thach WT. Purkinje cell spike firing in the posterolateral cerebellum: correlation with visual stimulus, oculomotor response, and error feedback. J Neurophysiol. 2004;92:1867–79. doi: 10.1152/jn.01251.2003. [DOI] [PubMed] [Google Scholar]
- 123.Marple-Horvat DE, Stein JF. Neuronal activity in the lateral cerebellum of trained monkeys, related to visual stimuli or to eye movements. J Physiol. 1990;428:595–614. doi: 10.1113/jphysiol.1990.sp018230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, Robertson E, Miall RC. Neuronal activity related to the visual representation of arm movements in the lateral cerebellar cortex. J Neurophysiol. 2003;89:1223–37. doi: 10.1152/jn.00817.2002. [DOI] [PubMed] [Google Scholar]
- 125.Marple-Horvat DE, Criado JM, Armstrong DM. Neuronal activity in the lateral cerebellum of the cat related to visual stimuli at rest, visually guided step modification, and saccadic eye movements. J Physiol. 1998;506:489–514. doi: 10.1111/j.1469-7793.1998.489bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miles OB, Cerminara NL, Marple-Horvat DE. Purkinje cells in the lateral cerebellum of the cat encode visual events and target motion during visually guided reaching. J Physiol. 2006;571:619–37. doi: 10.1113/jphysiol.2005.099382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cerminara NL, Apps R, Marple-Horvat DE. An internal model of a moving visual target in the lateral cerebellum. J Physiol. 2009;587:429–42. doi: 10.1113/jphysiol.2008.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mushiake H, Strick PL. Preferential activity of dentate neurons during limb movements guided by vision. J Neurophysiol. 1993;70:2660–4. doi: 10.1152/jn.1993.70.6.2660. [DOI] [PubMed] [Google Scholar]
- 129.Ebner TJ, Pasalar S. Cerebellum predicts the future motor state. Cerebellum. 2008;7:583–8. doi: 10.1007/s12311-008-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- 131.Kawato M, Wolpert D. Internal models for motor control. Novartis Found Symp. 1998;218:291–304. doi: 10.1002/9780470515563.ch16. [DOI] [PubMed] [Google Scholar]
- 132.Imamizu H, Kuroda T, Miyauchi S, Yoshioka T, Kawato M. Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci. 2003;100:5461–6. doi: 10.1073/pnas.0835746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–81. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- 134.Hawkes R, Herrup K. Aldolase C/zebrin II and the regionalization of the cerebellum. J Mol Neurosci. 1995;6:147–58. doi: 10.1007/BF02736761. [DOI] [PubMed] [Google Scholar]
- 135.Voogd J. Comparative aspects of the structure and fibre connexions of the mammalian cerebellum. Prog Brain Res. 1967;25:94–134. doi: 10.1016/S0079-6123(08)60963-2. [DOI] [PubMed] [Google Scholar]
- 136.Voogd J, Bigare F. Topographical distribution of olivary and corticonuclear fibers in the cerebellum. A review. In: Courville J, DeMontigny C, Lamarre Y, editors. The inferior olivary nucleus. New York: Raven; 1980. pp. 207–34. [Google Scholar]
- 137.Brodal A, Kawamura K. Olivocerebellar projection: a review. Adv Anat Embryol Cell Biol. 1980;64:1–140. [PubMed] [Google Scholar]
- 138.Sugihara I, Quy PN. Identification of aldolase C compartments in the mouse cerebellar cortex by olivocerebellar labeling. J Comp Neurol. 2007;500:1076–92. doi: 10.1002/cne.21219. [DOI] [PubMed] [Google Scholar]
- 139.Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990;291:538–52. doi: 10.1002/cne.902910405. [DOI] [PubMed] [Google Scholar]
- 140.Sillitoe RV, Hawkes R. Whole-mount immunohistochemistry: a high-throughput screen for patterning defects in the mouse cerebellum. J Histochem Cytochem. 2002;50:235–44. doi: 10.1177/002215540205000211. [DOI] [PubMed] [Google Scholar]
- 141.Ahn AH, Dziennis S, Hawkes R, Herrup K. The cloning of zebrin II reveals its identity with aldolase C. Development. 1994;120:2081–90. doi: 10.1242/dev.120.8.2081. [DOI] [PubMed] [Google Scholar]
- 142.Ekerot CF, Larson B. Correlation between sagittal projection zones of climbing and mossy fibre paths in cat cerebellar anterior lobe. Brain Res. 1973;64:446–50. doi: 10.1016/0006-8993(73)90203-5. [DOI] [PubMed] [Google Scholar]
- 143.Hanson C, Chen G, Ebner TJ. Climbing fiber afferents contributed significantly to optically recorded parasagittal banding evoked by peripheral stimulation. Soc Neurosci. 1997;23:750. [Google Scholar]
- 144.Llinas R, Sasaki K. The functional organization of the olivo-cerebellar system as examined by multiple Purkinje cell recordings. Eur J Neurosci. 1989;1:587–602. doi: 10.1111/j.1460-9568.1989.tb00365.x. [DOI] [PubMed] [Google Scholar]
- 145.Bower JM. Control of sensory data acquisition. Int Rev Neurobiol. 1997;41:489–513. doi: 10.1016/s0074-7742(08)60367-0. [DOI] [PubMed] [Google Scholar]


