Abstract
Purpose.
In response to ischemia, retinal neuronal cells express nerve growth factor (NGF), which can be proangiogenic. Endothelial progenitor cells (EPCs) can participate with the resident vasculature to promote angiogenesis. We postulated that NGF may stimulate CD34+ EPCs to convert to an angiogenic phenotype.
Methods.
Human CD34+ cells and human retinal endothelial cells (HRECs) were used to examine the effect of NGF on key steps associated with neovascularization. CD34+ cells and HRECs were stimulated with NGF (1 to 4 pM) for 24, 48, and 72 hours. Cell migration was measured using a modified Boyden chamber assay. Expression of the receptor for the cytokine stromal derived growth factor 1 (SDF-1), CXCR-4, was assessed by flow cytometry. In vitro angiogenesis was tested using a three-dimensional (3D) extracellular matrix with HRECs/CD34+ cell cocultures. NGF receptor activation was assessed by western analysis.
Results.
NGF promoted proliferation of CD34+ cells but not HRECs. Pretreatment of CD34+ cells with NGF increased CXCR-4 expression in CD34+ cells, resulting in enhanced migration to SDF-1 (P < 0.0001). The enhanced tubule-forming effect of NGF in HRECs was further potentiated by coculture with NGF-pretreated CD34+ cells (P < 0.01). The beneficial effect of NGF was blocked (P < 0.0001) by the ERK inhibitor PD98059. In both CD34+ and HRECs, NGF increased phosphorylation of neurotrophic tyrosine kinase receptor type 1 (TrkA) receptor by ERK1 activation (P < 0.01).
Conclusions.
Our in vitro results suggest that NGF released from ischemic nerves in vivo may contribute to the “angiogenic switch” by stimulating the angiogenic behavior of CD34+ cells while minimally affecting resident retinal endothelial cells.
NGF in the physiologic range significantly increases proliferation of CD34+ cells, but not human retinal endothelial cells. Pretreatment of CD34+ cells with NGF increases CXCR-4 expression and angiogenesis. NGF increases phosphorylation of TrkA receptors in both CD34+ and HRECs by ERK1 activation.
Introduction
Diabetic retinopathy (DR) is the leading cause of blindness among working aged adults.1 DR affects about 700,000 Americans, with 63,000 new cases of DR developing each year.2 Over 40% of Americans diagnosed with diabetes have DR and it affects 80% of individuals with a 10-year history of diabetes. Over the course of diabetes, vasodegeneration (capillary dropout) leads to widespread ischemia3 and subsequent release of the hypoxia-regulated factors, vascular endothelial growth factor (VEGF), stromal derived factor 1 (SDF-1), and erythropoietin.
Central nervous system neurons and retinal neurons are part of the “neurovascular unit.”4 This term specifically emphasizes the significant interactions between neurons and endothelial cells. Nerve growth factor (NGF) is secreted by neurons in response to mechanical or ischemic stress and induces reparative angiogenesis.5 NGF action is mediated by the activation of tyrosine kinase receptor (TrkA) and has been shown to prevent apoptosis of endothelial cells in ischemic wounds of diabetic mice.6,7 NGF also binds to the low-affinity receptor p75NTR8 and mediates apoptosis by proteolytic cleavage.9 However, the effect of NGF on retinal endothelial cells is largely unknown. Only a single study by Steinle and Granger10 reported that NGF stimulates human choroidal, but not retinal, endothelial cell migration and proliferation. Interestingly, NGF levels in serum and tears are higher in patients with proliferative DR and higher levels are associated with higher HbA1c and longer diabetes duration. The correlation between NGF and retinopathy is so strong that a tear fluid assay for NGF has been suggested as an effective, noninvasive diagnostic tool for retinopathy assessment.11
Although there is considerable evidence to support that NGF can stimulate in vitro and in vivo angiogenesis, the effects are certainly vascular-bed specific.12 We asked whether NGF could regulate a critical circulating endothelial progenitor cell population (CD34+), which has been implicated in both physiologic vascular repair and pathologic neovascularization. Specifically, we tested whether exposure to physiologically relevant levels of NGF could alter the behavior of human CD34+ cells and promote their proliferation, migration, and ability to modulate the angiogenic potential of human retinal endothelial cells (HRECs).
Materials and Methods
Isolation and Culture of HRECs and CD34+ Cells
Donor human eyes were obtained from the National Disease Resource Interchange (Philadelphia, PA) within 36 hours of death. HRECs were isolated and maintained as previously described.13 The identity of HRECs was validated by demonstrating endothelial cell incorporation of fluorescence-labeled acetylated LDL.13 Briefly, HRECs were cultured in T-75 flasks pretreated with attachment factor in media [Ham's F-12: Dulbecco's modified Eagle's medium (DMEM) (1:1)], insulin–transferrin–selenium (ITS), penicillin–streptomycin–glutamine (PSG) (2%) (Mediatech, Inc., Manassas, VA), endothelial cell growth supplement (ECGS; Sigma- Aldrich, St. Louis, MO), and 10% fetal bovine serum (Invitrogen Corp., Carlsbad, CA). Passage was performed when cells reached 85–90% confluency using trypsin/EDTA (Lonza, Walkersville, MD). Passages 3–5 were used for experiments. Basal media consisted of Ham's:DMEM (1:1).
The study protocol was approved by the Institutional Review Board (IRB 2010-163) at the University of Florida, which allowed removal of peripheral blood from healthy patients. Written informed consent was obtained from each patient. Additional peripheral blood was obtained from Life South Blood Center, Gainesville, FL. CD34+ cells were isolated by magnetic bead separation using CD34+ isolation kit (StemCell Technologies, Vancouver, CA) and commercial media (StemSpan; StemCell Technologies) was used for culture.
CD34+ Cell Proliferation
CD34+ cells were isolated as described earlier, and plated into 96-well plates. NGF has previously been used at 10 μM to study cell migration and proliferation in human choroidal and retinal endothelial cells.10 Yet, other reports indicated maximal proliferation of human airway smooth muscle cells at 3 pM concentration14 and human Müller glial cells at 7.5 pM concentration.15 Thus, for the purpose of this study, low concentrations were used. Cells were treated with 1, 2, and 4 pM concentration of NGF (ProSpec, Rehovot, Israel). Untreated cells were used as controls. Culture medium was replenished every 48 hours with NGF. After 24, 48, and 72 hours of NGF treatment, cells were collected and nonadherent cells were counted manually using the trypan-blue exclusion method.16
HREC Proliferation
Because HRECs are adherent, proliferation was assessed using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cell proliferation assay kit (Vybrant; Invitrogen) in accordance with the manufacturer's specifications. HRECs were plated into 96-well plates. Once they were confluent to 85–90%, they were treated with 1, 2, and 4 pM concentrations of NGF.
Protein Extraction
CD34+ cells or HRECs were obtained as described earlier. About 0.5 million cells were suspended in 100 μL media. CD34+ cells proliferated maximally at 24 hours with 2 and 4 pM of NGF in the previous experiment; therefore, we treated cells with NGF at 3 pM concentration for 0-, 0.5-, 1-, 5-, 15-, and 30-minute intervals. Following centrifugation, lysis buffer (Cell Signaling, Boston, MA) (1×) with 1% protease inhibitor cocktail, phosphatase cocktail 1 inhibitor, and phosphatase cocktail 2 inhibitor (Sigma) was added and the samples were centrifuged again at 290g for 10 minutes. The supernatant was taken for protein quantification using a commercial assay kit (BCA; Thermo Scientific Pierce, Rockford, IL) and absorbance was read at 562 nm using a plate reader (Synergy 2; BioTek Instruments Inc., Winooski, VT).
Western Blotting
For CD34+, the extracted proteins (100 μg) were loaded on 4–15% SDS-PAGE gels (Bio-Rad, Hercules, CA) and electrophoresis was performed under 80 V until bands were clearly separated. Proteins were transferred from the gel onto the nitrocellulose membrane for 30 minutes at 20 V. The blot was blocked with a commercial blocking solution (Odyssey; LI-COR Biosciences, Lincoln, NE) and PBS (1:1) for 45 minutes at room temperature. Primary antibodies (1:500 dilution; 5 μg/mL) to phospho-TrkA (Cell Signaling Technology, Danvers/Boston, MA), TrkA (R&D Systems, Minneapolis, MN), ERK, pERK, and β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in blocking solution (Odyssey) with Tween-20 (0.1%) was added overnight at 4°C, then washed with PBS and Tween-20 (0.1%) to remove excess primary antibodies. Blots were probed with secondary antibody (species-specific) (Rockland, Gilbertsville, PA) at a 1:2000 dilution for 1 hour at room temperature. Excess secondary antibody was removed by washing with PBS with 0.1% Tween-20 and excess Tween-20 was removed by washing the blot with PBS. The bands were detected using the LI-COR instrument and band intensity was determined using software functions (Odyssey V 1.2; LI-COR Biosciences).
For pTrkA and TrkA expression in HRECs, western blotting was performed.17 Secondary antibodies conjugated to horseradish peroxidase (species-specific, 1:5000; Promega, Madison, WI) were used. Antibody–antigen interactions were visualized using enhanced chemiluminescence (ECL Reagent; Amersham Biosciences, Little Chalfort, UK). Densitometric analysis was carried out using a commercial device (Kodak Image Station 4000MM). Data are expressed as a ratio of phosphorylated protein levels to total protein levels in arbitrary units.
Migration of CD34+ Cells
CD34+ cells (20,000) were treated with NGF at 1, 5, or 10 pM for 8 hours. The positive control group was treated with a 1% cytokine commercial cocktail (StemSpan CC100; StemCell Technologies), whereas the negative control group was treated without cytokines. Cells were spun at the end of an 8 hour NGF treatment or StemSpan with 1% cytokine cocktail or StemSpan without cytokines. Each pellet was suspended in PBS. CD34+ cells in PBS (100 μL) were loaded into the upper chamber of a migration assay kit (Chemicon International/Millipore, Temecula, CA). SDF-1 (R&D Systems, Minneapolis, MN) was used as the stimulatory chemokine for the second part of this study and was added to the lower chamber. Cells were allowed to migrate for 16 hours at 37°C at 5% CO2. A diluted fluorescent dye (CyQuant GR Dye; Millipore) with lysis buffer was added to lower wells, lysing the cells and binding to the cellular nucleic acids. This fluorescent dye was read by using a multidetection microplate reader (Synergy HT, Synergy 2; BioTek Instruments) with an excitation of 485 ± 20 and an emission of 528 ± 20.
CXCR-4 Expression on CD34+ Cells Using Flow Cytometry
Chemokine receptor type 4 (CXCR-4) on CD34+ cells was assessed following treatment with 1, 5, and 10 pM NGF. CD34+ cells were incubated with 0 (control), 1, 5, and 10 pM NGF overnight at 37°C. Cells were blocked with PBS containing 1% bovine serum albumin (BSA) for 1 hour. Phycoerythrin (PE, 20 μg) anti-human CXCR-4 (eBiosciences, San Diego, CA) diluted in PBS containing 1% BSA was added to cells and then the cells were incubated on ice for 30 minutes. Cells were washed twice with PBS. After a final wash, cells were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer. Samples were analyzed for CXCR-4 expression by flow cytometry performed at the University of Florida ICBR Core facility (FACScan; BD Biosciences, Lincoln Park, NJ). Results were quantified by plotting the relative fluorescence units as histograms over control group readings.
In Vitro 3D Angiogenesis Study
Endothelial tube formation was studied using synthetic basement membrane as the matrix. This angiogenesis assay was conducted as described by Stitt et al.18 Briefly, HRECs (1000 cells for each treatment) were mixed with an equal volume of angiogenesis assay (Matrigel; BD Biosciences, San Jose, CA) (1:1 ratio) at 4°C. Of this suspension, a 10 μL aliquot was plated in a 35-mm petri dish and allowed to polymerize at 37°C for 30 minutes. A second layer of 15 μL assay (Matrigel) containing NGF (3 pM), NGF (3 pM) + ERK inhibitor (PD98059, 10 μM; Cell Signaling Technology)14,19 or Matrigel alone (as control) was placed on the top of the first layer and incubated at 37°C for 30 minutes.
In a second group, untreated HRECs were placed into a petri dish as described earlier. After 24 hours CD34+ cells (200 cells for each treatment) (untreated, treated with 3 pM NGF, or 3 pM NGF + 10 μM PD98059) were mixed with assay Matrigel (1:1 ratio) at 4°C; a 15 μL aliquot of this suspension was added above the first layer (containing HRECs) and maintained as described earlier.
By day 2, HRECs formed sprouts and crossed the primary and secondary borders of the Matrigel and sprouts crossing the second border were enumerated using microscopy. In each assay, five fields of view in each group were randomly selected and captured by the digital camera under the microscope (magnification ×10; Carl Zeiss Inc., Thornwood, NY). The analysis of tube formation was then carried out by counting the number of sprouts.
Statistical Analysis
Data were summarized as mean ± SE. Post hoc Bonferroni corrections for multiple comparisons were made following ANOVA. Statistical differences between means were assessed with two-way ANOVA for data pertaining to cell proliferation and sprout formation (angiogenesis study), one-way ANOVA for ERK expression, CD34+ cells migration, and CXCR-4 expression data and paired t-test for TRK expression data. All the statistical analyses were performed with a commercial software package (Prism Software Version 5.0; GraphPad, San Diego, CA).
Results
NGF Increases Proliferation of CD34+ Cells but Not HRECs
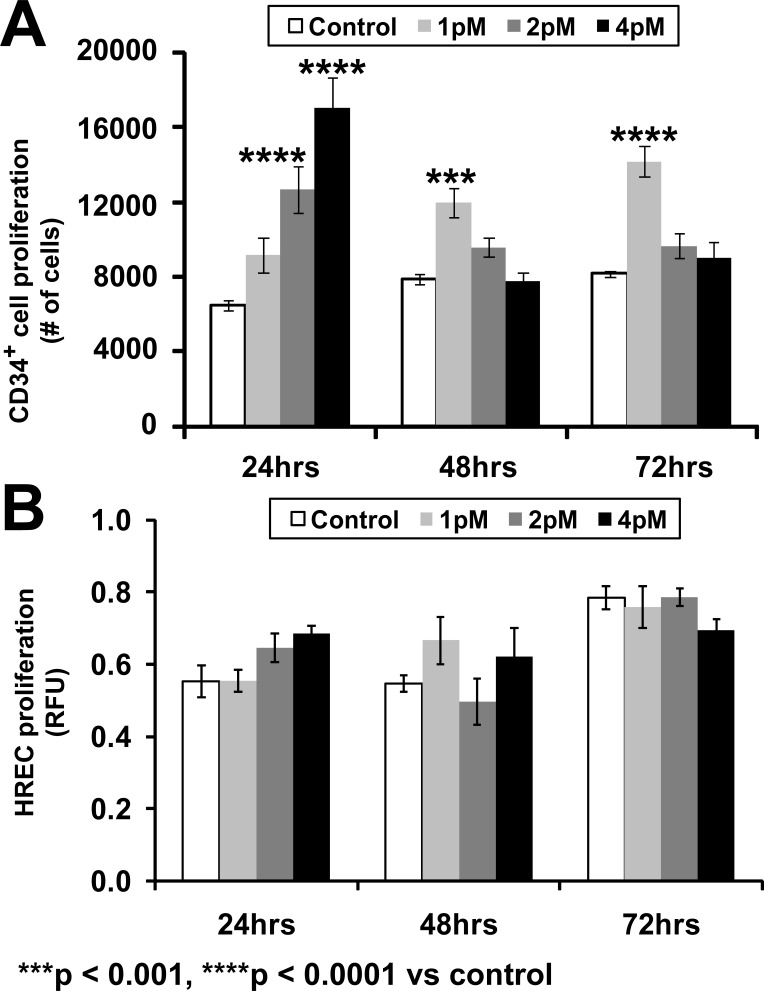
NGF increased proliferation of CD34+ cells in a concentration- and time-dependent manner (Fig. 1A). NGF at a concentration of 1 pM increased proliferation at 48 hours (t = 4.373, P < 0.001) and 72 hours (t = 6.425; P < 0.0001), whereas 2 and 4 pM increased proliferation at 24 hours (t = 6.604; P < 0.001 and t = 11.24; P < 0.0001). A proliferation assay of HRECs was performed using the identical concentrations of NGF and the identical time course; however, no proliferation was observed (Fig. 1B).
>Figure 1.
Effect of varying concentrations of NGF on proliferation of (A) CD34+ cells and (B) HRECs during a 72-hour period. Proliferation of CD34 cells was examined using the trypan blue–exclusion method and HREC proliferation was examined using the MTT assay. MTT activity was expressed in arbitrary fluorescence units. Data presented as mean ± SE (n = 6). Data analysis: two-way ANOVA revealed significant effects of concentration of NGF (F[3,60] = 24.97; P < 0.0001), duration of incubation with NGF (F[2,60] = 9.38; P = 0.0003), and interaction of concentration and incubation period (F[6,60] = 23.91; P < 0.0001) on proliferation of CD34+ cells; each of these factors accounted for 25.21%, 6.32%, and 48.28% of the total variance, respectively. Asterisks indicate significant difference compared with corresponding control (post hoc Bonferroni multiple comparisons).
Phosphorylation of TrkA after NGF Treatment
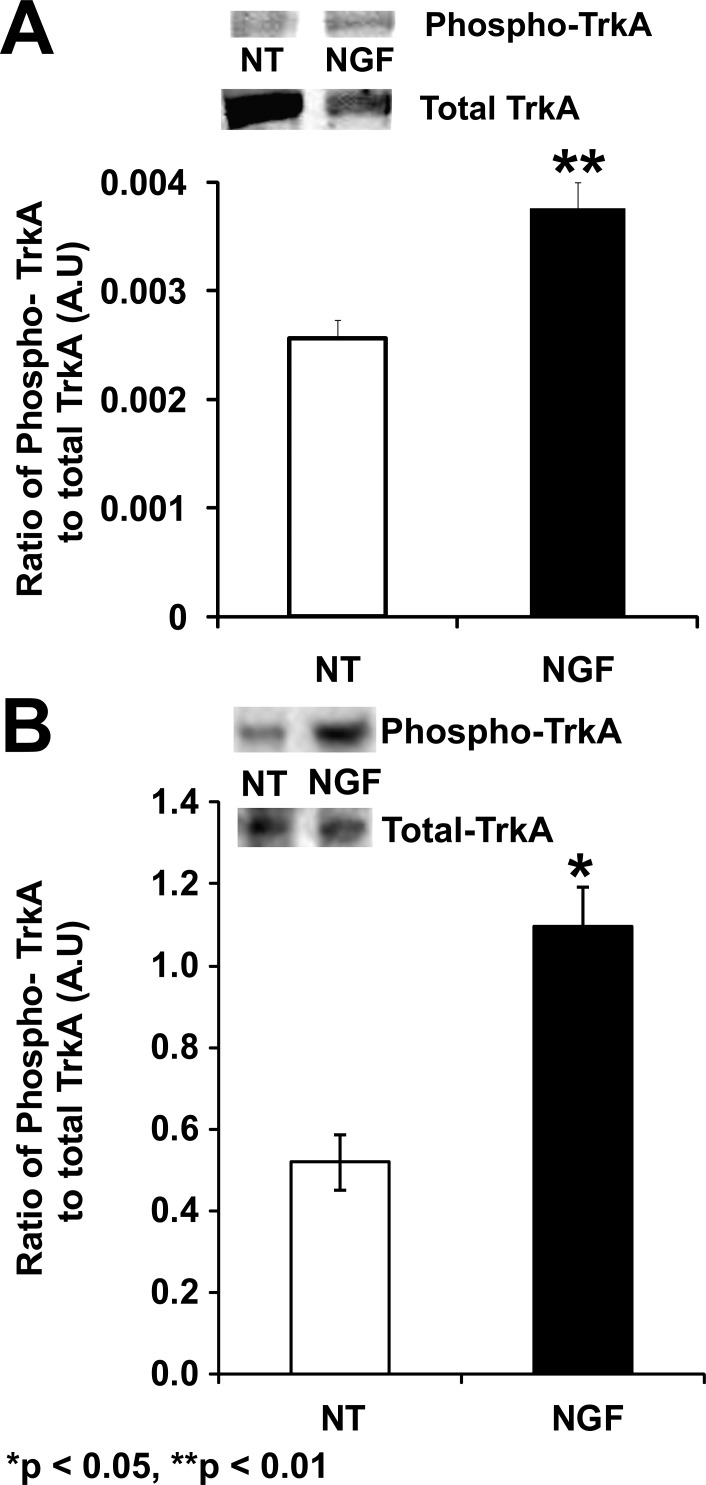
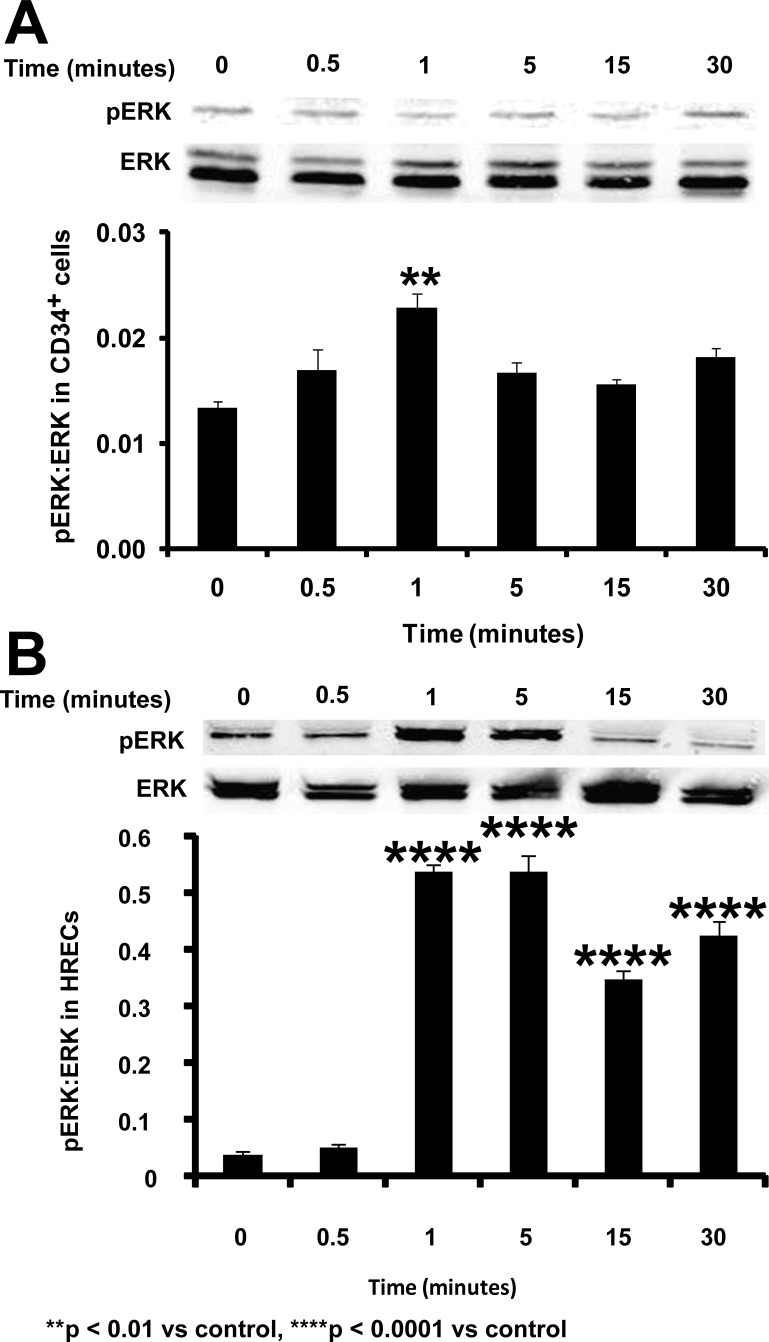
Typically, activation of NGF occurs through TrkA, which induces autophosphorylation via activation of the mitogen-activated protein kinase (MAPK) pathway. Phosphorylation of TrkA in response to the NGF treatment was observed in both CD34+ cells (P = 0.0095, Fig. 2A) and HRECs (P = 0.037; Fig. 2B). In CD34+ cells, NGF treatment resulted in ERK phosphorylation in a time-dependent manner (F[5,12] = 7.396, P = 0.0022) with maximal phosphorylation occurring at 1 minute (Bonferroni post hoc test, P < 0.01; Fig. 3A). Similar to the CD34+ cells, HRECs treated with NGF demonstrated increased ERK phosphorylation over time (F[5,12] = 159.7, P < 0.0001) compared with untreated controls. Maximal phosphorylation was noted at 1 and 5 minutes post-NGF treatment in these cells (Bonferroni post hoc test, P < 0.0001 for both; Fig. 3B).
Figure 2.
Phosphorylation of TrkA in CD34+ cells and HREC following NGF treatment. CD34+ cells (A) and HRECs (B) were either nontreated (NT) or treated with 3 pM NGF for 1 minute and protein expression was evaluated. Bars represent ratio of integrated optical density of phosphorylated TrkA to total TrkA. Data were analyzed by Student's t-test and represented as mean ± SE (n = 3). Asterisks indicate significant difference compared with control (NT).
Figure 3.
Phosphorylation of ERK in CD34+ cells and HRECs following NGF treatment overtime. Bars represent ratio of integrated optical density of phosphorylated ERK to total ERK. Data are presented as mean ± SE (n = 3), Data analysis: one-way ANOVA and post hoc Bonferroni multiple comparisons. Asterisks indicate significant difference compared with corresponding control.
NGF Promotes Migration of CD34+ Cells
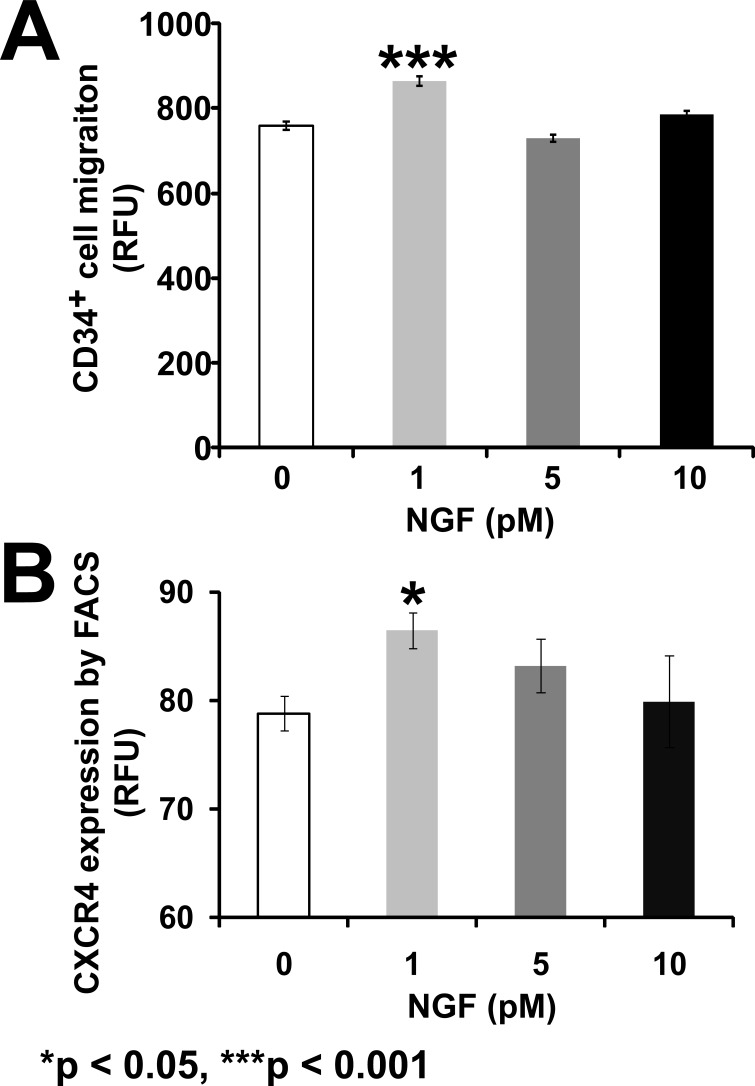
Ischemic tissue expresses SDF-1 to recruit progenitor cells from the circulation to areas of tissue injury. We reasoned that within the retina of diabetics, local NGF may serve to prime the CD34+ cells for SDF-1–induced migration. We hypothesized that this may occur by increasing the expression of CXCR-4, the SDF-1 receptor, on CD34+, thus preparing these cells to optimally respond to local SDF-1 in the ischemic retinal environment.20 CD34+ cells showed a significant migratory response to SDF-1 when they were pretreated with varying concentrations of NGF (F[3,20] = 21.60, P < 0.0001). Maximal migration was observed at the lowest concentration of NGF pretreatment (1 pM) (P < 0.001; Bonferroni post hoc test), but not at higher concentrations (Fig. 4A).
Figure 4.
NGF pretreatment promotes CD34+ cell migration by increasing CXCR-4 expression. (A) CD34+ cells were pretreated with NGF and then stimulated with SDF-1 in a modified Boyden chamber assay. (B) Flow cytometric analysis of CXCR-4 expression in CD34+ cells treated with NGF. Data are presented as mean ± SE (n = 4). Data analysis: one-way ANOVA and post hoc Bonferroni multiple comparisons. Asterisks indicate significant difference compared with corresponding control.
To examine the mechanism by which NGF enhanced SDF-1–induced migration in CD34+ cells, we asked whether NGF increased the expression of CXCR-4 on these cells. Only in the CD34+ cells treated with 1 pM concentration of NGF did we observe a significant increase of CXCR-4 expression compared with untreated cells (F[3,12]) = 4.260, P = 0.0289, Bonferroni post hoc test, P < 0.05, Fig. 4B).
NGF Promotes CD34+ Cell-Mediated HREC Tube Formation
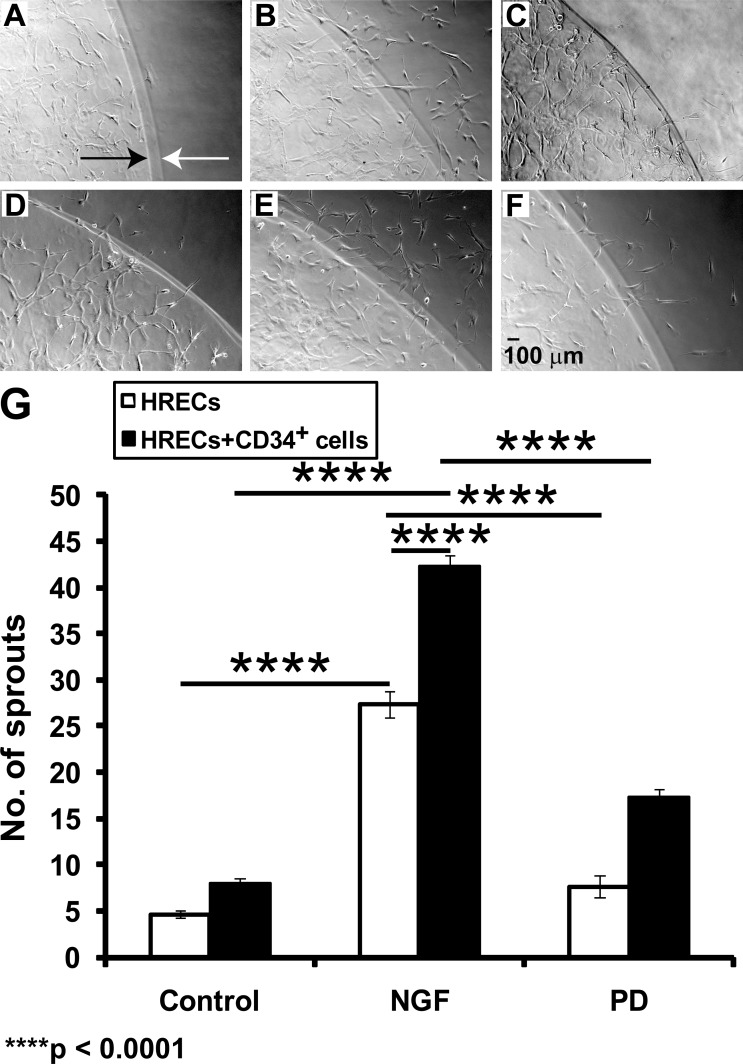
In a 3D extracellular matrix assay of in vitro angiogenesis, after 24 hours, HRECs formed a tubular network within the primary boundary and crossed into the second layer of Matrigel (Figs. 5A–F). The presence of NGF (Fig. 5B) significantly increased the numbers of sprouting tubules (t = 15.74, P < 0.0001) compared with untreated HRECs alone (Fig. 5A). This effect of NGF was decreased by the simultaneous treatment with ERK inhibitor, PD98059 (t = 13.66, P < 0.0001, Fig. 5C).
Figure 5.
NGF-treated CD34+ cells promote tubule formation in HRECs. Using a 3D model of angiogenesis, HRECs were placed on an angiogenesis assay (Matrigel) in basal medium (A), in the presence of NGF (B), and in the presence of NGF + PD98059 (C). Next, CD34+ cells that were either untreated (D), treated with NGF (E), or NGF + PD98059 (F) were mixed (Matrigel, second layer) and added on top of the first layer of HRECs (in Matrigel). After 24 hours, HRECs formed a tubular network in the inner primary boundary (shown by a black arrow) and crossed the outer second layer (of Matrigel; shown by a white arrow). Bar chart (G) showing total numbers of sprouting cells (per field) in the different groups. Data are presented as mean ± SE (n = 5). Data analysis: two-way ANOVA revealed a significant effect of the presence of NGF (F[2,12] = 433.63, P < 0.0001), the presence of CD34+ cells (i.e., coculture) (F[1,12] = 126, P < 0.0001), and the interaction of NGF and CD34+ cells in culture (F[2,12] = 16.45, P = 0.0004) on angiogenesis; each of these factors accounted for 83.54%, 12.14%, and 3.17% of the total variance, respectively. Asterisks on a bar indicate significant difference compared with corresponding control (post hoc Bonferroni multiple comparisons).
CD34+ cells promote vasculogenesis via incorporation into vessels or by providing paracrine support to the resident vasculature.21 To study the impact of NGF on the ability of CD34+ cells to interact with HRECs, CD34+ cells were either pretreated with NGF or were untreated. HRECs were first plated (on Matrigel) followed by the addition of CD34+ cells to the second layer of Matrigel 24 hours later. The presence of untreated CD34+ cells (Fig. 5D) in the second layer had no effect (t = 2.31, P > 0.05) on sprout formation in the HRECs. As shown in Figure 5E, pretreatment of CD34+ with NGF prior to coculture with HREC resulted in enhanced tubule formation by HREC in this 3D model of angiogenesis (F[2,12] = 16.45, P = 0.0004). This response (Fig. 5E) was greater than that of NGF-treated HRECs (Fig. 5B; t = 10.42, P < 0.0001). Pretreatment of CD34+ cells with PD98059 significantly reduced the sprouting response of HRECs (Fig. 5F) compared with NGF-treated CD34+ cells (Fig. 5E) (t = 17.36, P < 0.0001).
Discussion
One of the primary findings of these experiments is that physiologic concentrations of NGF increased the proliferation of CD34+ cells, which was in marked contrast to NGF's lack of effect on endothelial cells of the resident retinal vasculature. This effect occurred in a time-dependent manner. To our knowledge there are no reports to date on the effect of NGF on proliferation and migration of human CD34+ cells, although the effects on endothelial cells have been studied.10,14,15 No effect of NGF on HREC proliferation in this study corroborates these earlier reports. Although the majority of studies, including studies using choroidal cells,10 examined a higher concentration of NGF, the concentrations we used in this study were quite low but have previously been shown to influence cell proliferation.14,15
Interestingly, there are numerous reports in the literature describing concentration-specific effects of growth factors on angiogenic functions. For example, cytokines such as TGF-β stimulate a true peak of migration at an approximately 1000-fold lower concentration (1 pg/mL) than that for EGF.22 Cytokines can also show a biphasic effect.23 Stromal derived factor 1 (SDF-1) demonstrated a biphasic effect, concentrations of 0–0.1 ng/mL show an increase in migration of human CD34+ cells, then there is a decrease in migration from 0.1 to 1 ng/mL followed by a second increase in migration from 1 to 100 ng/mL.
Other examples of this biphasic response include studies by Cai et al.24 that show that VEGF elicits a biphasic proliferative response in cultured microvascular endothelial cells (MECs). Surprisingly, whereas MECs responded to VEGF concentrations up to 10 ng/mL, the response declined at 10–20 ng/mL only to increase again at VEGF concentrations at 50 ng/mL and above. Similarly, an in vivo study showed that VEGF promoted cell cycle transition from G0 to G1 only at 3 ng/mL, but at 30 ng/mL VEGF inhibited the cell cycle transition.25 These observations suggest that there is a narrow window of desensitization to VEGF that may be important in vascular homeostasis and similar events may occur with NGF.
Additionally, the observations of Takagi et al.26 showed that a significant increase in VEGF expression is associated with a transient decrease in VEGFR-2 expression and VEGF binding. These authors proposed that when angiogenesis is not immediately needed, an increase of VEGF concentration may lead to downregulation of VEGFR-2, which “buffers” the angiogenic stimulation of VEGF. Elegant studies by Rahimi et al.27 demonstrated that, although VEGFR-2 binds VEGF with a lower affinity than VEGFR-1, the cytoplasmic domain of VEGFR-2 but not VEGFR-1 is required to elicit a mitogenic signal in endothelial cells. Rahimi replaced the extracellular domain of VEGFR-2 with the extracellular domain of CSF-1R (human colony-stimulating factor 1) in porcine aortic endothelial cells and stimulated the receptor chimera with exogenous CSF-1. Their results showed that CSF-1 induced endothelial cell proliferation at CSF-1 concentrations of 0.5–2 ng/mL, but resulted in growth arrest at concentrations of 5–10 ng/mL. Like the findings described in our work, the studies of Rahimi emphasize that the proliferative effects of a growth factor may be observed at low rather than high concentrations.
Another example of a factor with highly concentration specific effects is pigment epithelium-derived factor (PEDF).28 PEDF has antiangiogenic effects at low concentration and proangiogenic effects at high concentrations.29–32 So we are not entirely surprised that the lowest concentration of NGF stimulated proliferation of CD34+ cells in our hands. Since low concentrations of NGF had the most dramatic effect on these cells, we used these concentrations of NGF throughout our studies.
Pretreatment of CD34+ cells with NGF significantly increased CXCR-4 expression, facilitating enhanced migration toward SDF-1. The maximal migratory response and the maximal effect on CXCR-4 expression occurred at the lower concentrations of NGF tested. These changes were mediated via activation of pTRK and downstream ERK signaling. The phosphorylation of TRK and ERK was rapid (1 minute) in both cell types. Although NGF had no effect on HREC proliferation, NGF did increase in vitro sprout formation by HREC, an effect that was blocked by ERK pathway inhibition. Moreover, NGF pretreatment of CD34+ cells increased their ability to assimilate with tubules formed by HRECs. This response was also dependent on ERK pathway activation.
NGF, a pleotropic molecule secreted by neurons, is involved in wound healing and tissue cicatrization (pressure ulcers).33–35 NGF is increased following ischemic or mechanical stress and has been identified as a proangiogenic agent in cerebral as well as myocardial ischemia.36,37 NGF has been shown to promote reparative angiogenesis in cutaneous wounds of type 1 diabetic mice and in ischemic hind limbs5 through activation of the NGF receptor, which influences vascular cell function and cell fate in vitro.38 Calza et al.39 showed that administration of NGF with neurotoxic 6-OHDA resulted in hypertrophy of the cervical sympathetic ganglia and hyperplasia of endothelial cells. Similarly, local delivery of NGF via silastic implants increased capillary formation in mechanically injured nerves.40 Interestingly, NGF is increased in the vitreous of patients with proliferative diabetic retinopathy, where angiogenesis typically abounds, but is alternately decreased in diabetic wound beds where angiogenesis is reduced. NGF increases expression of TrkA and improves angiogenesis in diabetic wounds, in part, by decreased expression of the p75NTR, which has been shown to increase apoptosis.6 Similarly, studies by Caporali et al.41 showed that p75NTR promotes endothelial cell apoptosis and inhibits angiogenesis. In the concentrations of NGF tested, we did not observe activation of this low-affinity receptor; however, p75NTR on ganglion cells and other neural cells likely plays a critical role in early diabetic retinopathy in animals or nonproliferative diabetic retinopathy in humans when neuronal cell apoptosis, retinal nonperfusion, and acellular capillaries exist.42
In contrast, binding of NGF to TrkA leads to its phosphorylation at Tyr490.43 Activation of TrkA results in rapid association of TrkA with phospholipase-C-gamma, phosphatidylinositiol-3 kinase, and adaptor proteins. TrkA Tyr490 phosphorylates the adaptor proteins GRB2-associated binding protein-1 and SH2-containing protein, resulting in their association with growth factor receptor–bound protein 2, a complex that enhances the rate of GDP-GTP exchange on Ras, leading to Ras activation. Activated Ras binds to Raf, which in turn phosphorylates and activates MEK (MAPK/ERK kinase). This association of NGF with TrkA initiates transcription of critical genes and promotes neurite growth.44
In this study, we observed a significant increase in vascular sprouting of HRECs in response to NGF. This effect was blocked by the ERK inhibitor PD98059, suggesting that angiogenic stimulation by NGF is mediated, in part, by the ERK pathway in HRECs. The effect of the ERK inhibitor PD98059 was also observed in CD34+ cells.
NGF can influence endothelial cell proliferation14,15 and exhibit a prosurvival effect on endothelial cells of selected vascular beds, but as we show in this report, HREC proliferation is not observed. However, enhanced tubule formation, which can occur independent of proliferation, did increase in response to NGF. NGF may exert its effects on cellular function in a number of ways, leading to angiogenesis relevant to the retina; in particular, its main function may be to enhance CD34+ cell recruitment, proliferation, and assimilation with the resident retinal vasculature.
CD34+ cells promote vasculogenesis via incorporation into vessels or by providing paracrine support to the resident vasculature.21 CD34+ cells secrete growth factors and cytokines.21 In this study, CD34+ cells alone when cocultured with HREC had a nonsignificant effect on sprouting; however, the sprout-enhancing effect of NGF was potentiated by the presence of NGF-pretreated CD34+ cells. An essential role exists for CD34+ cells to promote an environment conducive to revascularization; however, in the diabetic individual this conducive environment may lead to aberrant neovascularization. In diabetics, NGF with other growth factors such as VEGF and SDF-1 and cytokines could serve to promote sprout formation under hypoxic stress.21 Thus, increased NGF can enhance not only neuronal regeneration but also provide enhanced blood flow to the injured region by enhancing local angiogenesis. In this study, we showed that NGF increases CXCR-4 expression and migration of CD34+ cells toward SDF-1, the major homing factor for these cells. SDF-1 is a member of the CXC chemokine subfamily45 and plays a critical role in blood vessel formation.46 Blocking of SDF-1 has been shown to prevent the recruitment of EPCs to the retina and choroid after injury.47 Mice deficient in CXCR-4 have defects in the formation of blood vessels of the gastrointestinal tract,46 whereas activation of CXCR-4 facilitates EPC differentiation to endothelial cells and EPC survival.48
To our knowledge, this is the first study to demonstrate that NGF-pretreated CD34+ cells potentiate key steps relevant to angiogenesis. Our studies suggest that in the context of the ischemic retina, the signals/factors released by the ischemic injured nerves may serve as a stimulus to locally trigger pathologic angiogenesis. Thus, the increased expression of NGF may prime CD34+ cells to drive their participation in pathologic angiogenesis.
Disclosure: C.S. Jadhao, None; A.D. Bhatwadekar, None; Y. Jiang, None; M.E. Boulton, None; J.J. Steinle, None; M.B. Grant, None
Supported in part by National Institutes of Health Grants EY007739, EY012601, U01 HL087366 (MBG), and RO1 EY018358 (MEB); Thomas H. Maren Junior Investigator Award (ADB); Juvenile Diabetes Research Foundation Grant 17-2008-1044 (JJS); an Oxnard Foundation grant (JJS); a Departmental award from the Research to Prevent Blindness (Dr Barrett Haik, Chair; University of Tennessee Health Science Center [UTHSC]); and National Eye Institute Vision Core Grant PHS 3P30 EY013080 (Principal Investigator: Dianna Johnson; UTHSC).
References
- 1. Marshall SM, Flyvbjerg A. Prevention and early detection of vascular complications of diabetes. BMJ. 2006;333:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bressler NM, Edwards AR, Beck RW, et al. Exploratory analysis of diabetic retinopathy progression through 3 years in a randomized clinical trial that compares intravitreal triamcinolone acetonide with focal/grid photocoagulation. Arch Ophthalmol. 2009;127:1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aiello LP, Gardner TW, King GL, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. [DOI] [PubMed] [Google Scholar]
- 4. Lo CP, Chen CY. Neuroimaging of viral infections in infants and young children. Neuroimaging Clin N Am. 2008;18:119–132 ; viii. [DOI] [PubMed] [Google Scholar]
- 5. Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. [DOI] [PubMed] [Google Scholar]
- 6. Graiani G, Emanueli C, Desortes E, et al. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia. 2004;47:1047–1054. [DOI] [PubMed] [Google Scholar]
- 7. Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by Type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47:1055–1063. [DOI] [PubMed] [Google Scholar]
- 8. Chao MV. The p75 neurotrophin receptor. J Neurobiol. 1994;25:1373–1385. [DOI] [PubMed] [Google Scholar]
- 9. Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. [DOI] [PubMed] [Google Scholar]
- 10. Steinle JJ, Granger HJ. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Auton Neurosci. 2003;108:57–62. [DOI] [PubMed] [Google Scholar]
- 11. Park KS, Kim SS, Kim JC, et al. Serum and tear levels of nerve growth factor in diabetic retinopathy patients. Am J Ophthalmol. 2008;145:432–437. [DOI] [PubMed] [Google Scholar]
- 12. Cantarella G, Lempereur L, Presta M, et al. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–1309. [DOI] [PubMed] [Google Scholar]
- 13. Grant MB, Guay C. Plasminogen activator production by human retinal endothelial cells of nondiabetic and diabetic origin. Invest Ophthalmol Vis Sci. 1991;32:53–64. [PubMed] [Google Scholar]
- 14. Freund-Michel V, Bertrand C, Frossard N. TrkA signalling pathways in human airway smooth muscle cell proliferation. Cell Signal. 2006;18:621–627. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda T, Puro DG. Nerve growth factor: a mitogenic signal for retinal Muller glial cells. Brain Res. 1994;649:260–264. [DOI] [PubMed] [Google Scholar]
- 16. Sun Y, Tran BN, Worley LA, Delston RB, Harbour JW. Functional analysis of the p53 pathway in response to ionizing radiation in uveal melanoma. Invest Ophthalmol Vis Sci. 2005;46:1561–1564. [DOI] [PubMed] [Google Scholar]
- 17. Steinle JJ, Booz GW, Meininger CJ, Day JN, Granger HJ. Beta 3-adrenergic receptors regulate retinal endothelial cell migration and proliferation. J Biol Chem. 2003;278:20681–20686. [DOI] [PubMed] [Google Scholar]
- 18. Stitt AW, McGoldrick C, Rice-McCaldin A, et al. Impaired retinal angiogenesis in diabetes: role of advanced glycation end products and galectin-3. Diabetes. 2005;54:785–794. [DOI] [PubMed] [Google Scholar]
- 19. Zelivianski S, Spellman M, Kellerman M, et al. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer. 2003;107:478–485. [DOI] [PubMed] [Google Scholar]
- 20. Butler JM, Guthrie SM, Koc M, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scheubel RJ, Holtz J, Friedrich I, et al. Paracrine effects of CD34 progenitor cells on angiogenic endothelial sprouting. Int J Cardiol. 2010;139:134–141. [DOI] [PubMed] [Google Scholar]
- 22. Grant MB, Khaw PT, Schultz GS, Adams JL, Shimizu RW. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci. 1992;33:3292–3301. [PubMed] [Google Scholar]
- 23. Segal MS, Shah R, Afzal A, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–109. [PubMed] [Google Scholar]
- 24. Cai J, Jiang WG, Ahmed A, Boulton M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc Res. 2006;71:20–31. [DOI] [PubMed] [Google Scholar]
- 25. Kimura I, Honda R, Okai H, Okabe M. Vascular endothelial growth factor promotes cell-cycle transition from G0 to G1 phase in subcultured endothelial cells of diabetic rat thoracic aorta. Jpn J Pharmacol. 2000;83:47–55. [DOI] [PubMed] [Google Scholar]
- 26. Takagi H, King GL, Ferrara N, Aiello LP. Hypoxia regulates vascular endothelial growth factor receptor KDR/Flk gene expression through adenosine A2 receptors in retinal capillary endothelial cells. Invest Ophthalmol Vis Sci. 1996;37:1311–1321. [PubMed] [Google Scholar]
- 27. Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J Biol Chem. 2000;275:16986–16992. [DOI] [PubMed] [Google Scholar]
- 28. Tombran-Tink J. PEDF in angiogenic eye diseases. Curr Mol Med. 2010;10:267–278. [DOI] [PubMed] [Google Scholar]
- 29. Praidou A, Androudi S, Brazitikos P, Karakiulakis G, Papakonstantinou E, Dimitrakos S. Angiogenic growth factors and their inhibitors in diabetic retinopathy. Curr Diabetes Rev. 2010;6:304–312. [DOI] [PubMed] [Google Scholar]
- 30. Broadhead ML, Becerra SP, Choong PF, Dass CR. The applied biochemistry of PEDF and implications for tissue homeostasis. Growth Factors. 2010;28:280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rychli K, Huber K, Wojta J. Pigment epithelium-derived factor (PEDF) as a therapeutic target in cardiovascular disease. Expert Opin Ther Targets. 2009;13:1295–1302. [DOI] [PubMed] [Google Scholar]
- 32. Afzal A, Shaw LC, Ljubimov AV, Boulton ME, Segal MS, Grant MB. Retinal and choroidal microangiopathies: therapeutic opportunities. Microvasc Res. 2007;74:131–144. [DOI] [PubMed] [Google Scholar]
- 33. Matsuda H, Koyama H, Sato H, et al. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bernabei R, Landi F, Bonini S, et al. Effect of topical application of nerve-growth factor on pressure ulcers (Abstract). Lancet. 1999;354:307. [DOI] [PubMed] [Google Scholar]
- 35. Tuveri M, Generini S, Matucci-Cerinic M, Aloe L. NGF. a useful tool in the treatment of chronic vasculitic ulcers in rheumatoid arthritis. Lancet. 2000;356:1739–1740. [DOI] [PubMed] [Google Scholar]
- 36. Lee TH, Kato H, Chen ST, Kogure K, Itoyama Y. Expression of nerve growth factor and trkA after transient focal cerebral ischemia in rats. Stroke. 1998;29:1687–1696.discussion 1697. [DOI] [PubMed] [Google Scholar]
- 37. Hiltunen JO, Laurikainen A, Vakeva A, Meri S, Saarma M. Nerve growth factor and brain-derived neurotrophic factor mRNAs are regulated in distinct cell populations of rat heart after ischaemia and reperfusion. J Pathol. 2001;194:247–253. [DOI] [PubMed] [Google Scholar]
- 38. Kraemer R, Nguyen H, March KL, Hempstead B. NGF activates similar intracellular signaling pathways in vascular smooth muscle cells as PDGF-BB but elicits different biological responses. Arterioscler Thromb Vasc Biol. 1999;19:1041–1050. [DOI] [PubMed] [Google Scholar]
- 39. Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci U S A. 2001;98:4160–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santos PM, Winterowd JG, Allen GG, Bothwell MA, Rubel EW. Nerve growth factor: increased angiogenesis without improved nerve regeneration. Otolaryngol Head Neck Surg. 1991;105:12–25. [DOI] [PubMed] [Google Scholar]
- 41. Caporali A, Pani E, Horrevoets AJ, et al. Neurotrophin p75 receptor (p75NTR) promotes endothelial cell apoptosis and inhibits angiogenesis: implications for diabetes-induced impaired neovascularization in ischemic limb muscles. Circ Res. 2008;103:e15–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Obermeier A, Lammers R, Wiesmuller KH, Jung G, Schlessinger J, Ullrich A. Identification of Trk binding sites for SHC and phosphatidylinositol 3′-kinase and formation of a multimeric signaling complex. J Biol Chem. 1993;268:22963–22966. [PubMed] [Google Scholar]
- 44. Yoon SO, Soltoff SP, Chao MV. A dominant role of the juxtamembrane region of the TrkA nerve growth factor receptor during neuronal cell differentiation. J Biol Chem. 1997;272:23231–23238. [DOI] [PubMed] [Google Scholar]
- 45. Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–603. [DOI] [PubMed] [Google Scholar]
- 46. Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. [DOI] [PubMed] [Google Scholar]
- 47. Sengupta N, Caballero S, Mames RN, Timmers AM, Saban D, Grant MB. Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest Ophthalmol Vis Sci. 2005;46:343–348. [DOI] [PubMed] [Google Scholar]
- 48. Smadja DM, Bieche I, Uzan G, et al. PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system. Arterioscler Thromb Vasc Biol. 2005;25:2321–2327. [DOI] [PubMed] [Google Scholar]