Abstract
Purpose.
To investigate the corneal expression of toll-like receptor (TLR) 4 and determine its contribution to the immunopathogenesis of dry eye disease (DED).
Methods.
Seven to 8-week-old female C57BL/6 mice were housed in a controlled environment chamber and administered scopolamine to induce experimental DED. Mice received intravenous TLR4 inhibitor (Eritoran) to block systemic TLR4-mediated activity. The expression of TLR4 by the corneal epithelium and stroma was evaluated using real-time polymerase chain reaction and flow cytometry. Corneal fluorescein staining (CFS) was performed to evaluate clinical disease severity. The corneal expression of proinflammatory cytokines (IL-1β, IL-6, TNF, and CCL2), corneal infiltration of CD11b+ antigen-presenting cells, and lymph node frequency of mature MHC-IIhi CD11b+ cells were assessed.
Results.
The epithelial cells of normal corneas expressed TLR4 intracellularly; however, DED significantly increased the cell surface expression of TLR4. Similarly, flow cytometric analysis of stromal cells revealed a significant increase in the expression of TLR4 proteins by DED-induced corneas as compared with normal corneas. DED increased the mRNA expression of TLR4 in corneal stromal cells, but not epithelial cells. TLR4 inhibition decreased the severity of CFS and significantly reduced the mRNA expression of IL-1β, IL-6, and TNF. Furthermore, TLR4 inhibition significantly reduced the corneal infiltration of CD11b+ cells and the lymph node frequency of MHC-IIhi CD11b+ cells.
Conclusions.
These results suggest that DED increases the corneal expression of TLR4 and that TLR4 participates in the inflammatory response to ocular surface desiccating stress.
This study demonstrates that upregulation of TLR4 expression in DED participates in immunopathogenesis of DED through modulation of the innate immune responses to desiccating stress.
Introduction
Dry eye disease (DED), one of the most common ocular complaints, is an immunoinflammatory disorder of the ocular surface; however, the immunopathogenesis of DED has not yet been fully described.1,2 The 2007 Dry Eye WorkShop concluded that tear film instability and hyperosmolarity induce ocular surface inflammation.3 Recent studies have demonstrated that corneal epithelial cells respond to hyperosmolar stress by producing proinflammatory cytokines, chemokines, and matrix metalloproteinases (MMPs).4,5 Furthermore, hyperosmolar stress and proinflammatory cytokines such as interferon (IFN)-γ promote epithelial cell apoptosis.5–8
Toll-like receptors (TLRs) are pattern recognition receptors of the innate immune system that recognize highly conserved microbial structures and products.9,10 To date, 12 murine TLRs have been identified, and TLRs are expressed by a variety of cell types, including epithelial cells, dendritic cells, macrophages, and lymphocytes.10–13 TLR stimulation leads to the activation of nuclear factor-kappaB (NF-κB) that upregulates the production of proinflammatory cytokines and antimicrobial proteins.10,14 The NF-κB signaling pathway is important for the induction of innate and adaptive immune responses.10,14
TLR4 recognizes the Gram-negative bacterial cell wall component lipopolysaccharide (LPS) in association with cofactors such as CD14, LPS-binding protein (LBP), and myeloid differentiation factor-2 (MD-2).15,16 It has also been suggested that TLR4 is a receptor for endogenous ligands associated with noninfectious diseases such as myocardial ischemia-reperfusion injury and central nervous system autoimmune disease.17,18 We hypothesized that DED-induced corneal inflammation and injury may lead to the production of endogenous TLR4 ligands that activate the immune system. Therefore, we investigated the corneal expression of TLR4 and sought to determine the expression pattern of TLR4 in DED.
Materials and Methods
Animals
Seven to 8-week-old female C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were used for these experiments. The experimental protocol was approved by the Institutional Animal Care and Use Committee, and all animals were managed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Dry Eye Model
DED was induced by placing mice in a controlled environment chamber (CEC) and administering scopolamine (Sigma–Aldrich, St. Louis, MO) to maximize ocular surface dryness, as previously described.19,20 Mice placed in the CEC were exposed to a relative humidity < 25%, temperature of 20 to 22°C, and airflow of 15 L/min, 24 hours per day. Scopolamine hydrobromide (0.5 mg/0.2 mL) was injected subcutaneously in the dorsal skin of mice three times per day. Age- and sex-matched mice placed in the standard vivarium served as normal controls. Mice were euthanized on day 7 or day 9 for cellular and molecular analysis.
Corneal Fluorescein Staining
To evaluate the effects of desiccating stress on the ocular surface, corneal fluorescein staining (CFS) was performed at baseline (day 0), day 2, day 4, and day 9. One μL of 1% fluorescein (Sigma–Aldrich) was applied to the inferior-lateral conjunctival sac of the mice, and corneal fluorescein staining was examined with a slit-lamp biomicroscope under cobalt blue light 3 minutes later. Punctate staining was evaluated in a masked fashion using the National Eye Institute grading system, giving a score of 0 to 3 to each of the five areas of the cornea.21
Systemic Administration of TLR4 Inhibitor
Eritoran tetrasodium (1.1 mg) and vehicle were gifted from Eisai Research Institute (Andover, MA) and reconstituted in endotoxin-free water (Sigma–Aldrich, Monticello, IA). Mice received either 50 μL of Eritoran (5 mg/kg dissolved in vehicle) or vehicle once per day via tail vein injection beginning 1 day prior to dry eye induction.17 Mice were euthanized on day 7 or day 9 for cellular and molecular studies.
Real-Time Polymerase Chain Reaction
Total RNA was extracted from corneal tissue or bone marrow–derived dendritic cells (BMDCs) using a commercial reagent (TRIzol; Invitrogen, Carlsbad, CA) and an RNA purification kit (RNeasy Micro Kit; Qiagen, Germantown, MD). First-strand cDNA was synthesized with random hexamers using reverse transcriptase (SuperScript III; Invitrogen), and quantitative real-time polymerase chain reaction (PCR) was performed using predesigned primers (Taqman PCR Mastermix and FAM dye-labeled primers; Applied Biosystems, Foster City, CA) for IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), TNF (Mm99999068_m1), CCL2 (Mm00439620_m1), TLR4 (Mm00445273_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_g1). The GAPDH gene was used as the endogenous reference for each reaction. The results were analyzed by the comparative threshold cycle (CT) method with commercial analysis software (LightCycler, version 3; Roche Diagnostics Corp., Indianapolis, IN) and the relative expression level of each sample was expressed as fold change from wild-type DED or untreated DED group.
Western Blot Assay
Ten corneal epithelial cell sheets were isolated from each group and lysed in extraction buffer (10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 50 mM NaCl, 50 mM NaF, 1% Triton X-100, 20 μg/mL aprotinin, 2 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride) by homogenization. Lysates were centrifuged for 10 minutes at 13,000g and 4°C. Total protein (100 μg) in each group was separated by 8% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and subjected to Western blot analysis using TLR4 antibody (eBioscience Inc., San Diego, CA). Signal intensity was determined by densitometry (Quantity One; Bio-Rad, Hercules, CA) and normalized to the amount of RasGAP, as an internal control, in each sample.
Analysis of Cellular Infiltration by Immunohistochemical Staining
For whole-mount corneal staining of CD11b+ cells, corneas harvested on day 9 were fixed in acetone for 15 minutes and incubated with anti-FcR CD16/CD32 antibody (BD Pharmingen, San Diego, CA) for 45 minutes to block nonspecific staining. Corneas were immunostained with primary or isotype antibody overnight and mounted using a commercial mounting medium with DAPI (4,6 diamidino-2-phenylindole; VectaShield; Vector Laboratories, Burlingame, CA), as previously described.20 The following primary antibodies were used for immunohistochemical staining: fluorescein isothiocyanate (FITC)–conjugated rat anti–mouse CD11b (1:100; monocyte/macrophage marker; BD Pharmingen) and FITC-conjugated rat IgG2bk (isotype control; BD Pharmingen). Flat-mount corneas were examined with a confocal microscope (Leica TCS–SP5; Leica Microsystems, Wetzlar, Germany) at ×400 magnification and Z-stack images were taken through the whole thickness of the corneal stroma. FITC-CD11b+ cells in corneal stroma were counted in five to six areas in the periphery (0.5-μm area from the limbus) and two areas in the center of each cornea in a masked fashion using Z-stack images. The mean number of cells was obtained by averaging the cell number in each area examined.
Single Cell Isolation from the Cornea and Draining Lymph Nodes
Excised corneas were incubated with 20 mM EDTA at 37°C to separate the epithelial and stromal layers, and these tissues were subsequently digested in 2 mg/mL collagenase D (Roche Diagnostic Corp.) and 0.5 mg/mL DNase (Roche Diagnostic Corp.) for 2 hours at 37°C. The suspension was filtered through a 70-μm cell strainer (BD Falcon; Becton-Dickinson, Franklin Lakes, NJ). Single-cell suspensions from draining LNs were prepared with a 70-μm cell strainer. Trypan blue exclusion assay confirmed cell viability.
Flow Cytometric Analysis
Cells were incubated with Fc blocking antibody in 0.5% BSA at 4°C for 30 minutes. Cells were then immunostained with the following antibodies: FITC-conjugated anti-CD11b, Alexa Fluor 647–conjugated anti-CD11c, allophycocyanin (APC)-Cy7-conjugated anti-I-Ab, APC-conjugated anti-CD45, or PE-conjugated anti-TLR4 (UT41; eBioscience Inc.). Isotype control was stained with the appropriately matched antibodies (eBioscience Inc.). For intracellular staining of TLR4, either a cell fixation/permeabilization kit (eBioscience Inc.) or 0.5% Tween-20 was used. Cells were fixed with IC fixation buffer and then incubated with permeabilization buffer and stained with PE-conjugated anti-TLR4 antibody, per the manufacturer's recommendations. Stained cells were analyzed with a flow cytometer (LSRII; Becton-Dickinson) and a commercial program (Summit v4.3; Dako Colorado, Inc., Fort Collins, CO).
Immunohistochemical Staining of TLR4-Expressing Corneal Epithelial Cells
Corneal epithelial cell suspensions were immunostained with Alexa Fluor 488–conjugated anti-TLR4 (UT41; eBioscience Inc.) for 2 hours in 0.5% BSA at room temperature. Isotype control was stained with Alexa Fluor 488–conjugated anti-mouse IgG1 antibody (eBioscience Inc.). After washing these cells, epithelial cells were incubated with plasma membrane stains (CellMask; Invitrogen) for 5 minutes at 37°C in RPMI media (Invitrogen-Gibco), according to the manufacturer's recommendations. FITC-TLR4 positive cells in corneal epithelial cells were counted in six areas per each group under ×400 magnification in a masked fashion. For intracellular staining of TLR4, the cell fixation/permeabilization kit (eBioscience Inc.) was used as described earlier.
In Vitro Stimulation of Bone Marrow–Derived Dendritic Cells with Corneal Tissue
Bone marrow–derived dendritic cells (BMDCs) were generated using a previously described method.22 In brief, femurs and tibias were collected from freshly euthanized mice, flushed, and the resultant cells were seeded at 1 × 106/mL in RPMI 1640 (BioWitthaker, Walkersville, MD) supplemented with 10% fetal bovine serum (Gemini Bio-products, West Sacramento, CA), 1% penicillin/streptomycin (Cambrex, East Rutherford, NJ), and 20 ng/mL mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) (Biolegend, San Diego, CA) at 37°C with 5% carbon dioxide. The medium was exchanged with 10 mL medium containing 20 ng/mL GM-CSF on day 4. Loosely adherent cells were harvested on day 7 after being thoroughly washed with sterile PBS.
BMDCs (5 × 105 cells) were seeded on the bottom surface of a 24-well plate with either 100 ng/mL vehicle or Eritoran.13 Corneal epithelium and stroma harvested from naïve and dry eye–induced mice were homogenized in 500 μL of sterile fresh culture medium. Supernatants were centrifuged to remove undissolved particles and added to the top of a tissue-culture insert with 1.0 μm pores (Transwell; BD Falcon, Franklin Lakes, NJ). After BMDC pretreatment with Eritoran or vehicle for 1 hour, BMDCs were incubated with homogenized corneal tissue for 18 hours after which they were collected in TRIzol for real-time PCR analysis.
Statistical Analyses
Data are expressed as the mean ± SEM of at least three trials. The significance of the difference between groups was analyzed with the two-tailed Student's t-test using commercial analytical software (Prism, version 5.0; GraphPad, San Diego, CA). P < 0.05 was considered statistically significant.
Results
DED Increases TLR4 Expression on Corneal Epithelial and Stromal Cells
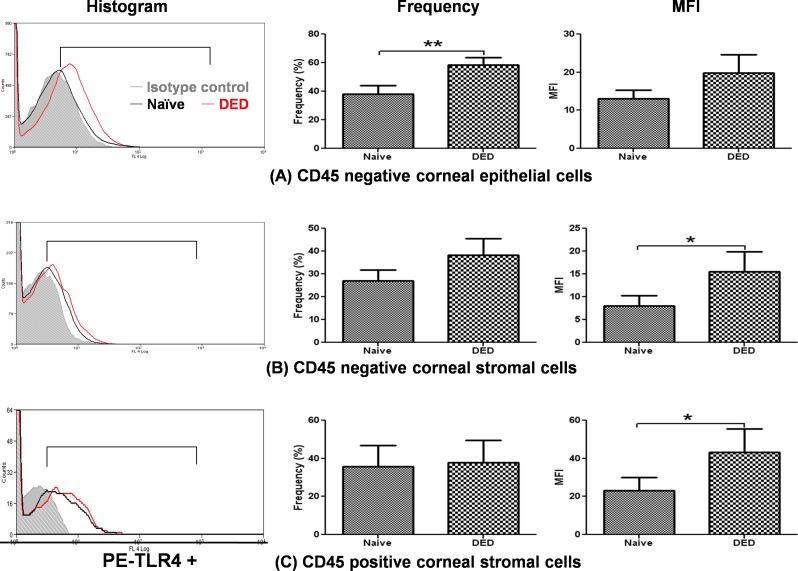
The first experiment aimed to elucidate whether TLR4 expression on the cell surface of corneal epithelial and stromal cells (including CD45 negative and positive cells) is modified in DED. Corneal epithelial and stromal cells from dry eye–induced or naïve corneas were stained with anti-CD45 and anti-TLR4 antibody. Dry eye induction significantly increased the frequency of TLR4 expression on CD45 negative epithelial cells (P = 0.0039, Fig. 1A) and increased the mean fluorescence intensity (MFI) of TLR4 expression on CD45− stromal cells (8.05 ± 2.17 in naïve vs. 15.5 ± 4.44 in DED, P = 0.048, Fig. 1B) and CD45+ stromal cells (23.07 ± 7.09 in naïve vs. 43.23 ± 12.24 in DED, P = 0.034, Fig. 1C).
Figure 1. .
Expression of TLR4 in DED corneas. Representative histograms demonstrating increased cell surface expression of TLR4 in dry eye corneas (red) as compared with normal corneas (black). After gating on CD45+ cells, flow cytometric analysis demonstrated that DED significantly increased the cell surface expression of TLR4 on corneal epithelial cells (CD45−) and stromal cells (CD45+ and CD45−) as compared with the cells of normal corneas: (A) corneal epithelial cells (CD45−); (B) corneal stromal cells (CD45−); (C) corneal stromal cells (CD45+). Data are presented as the mean ± SEM of three experiments utilizing six corneas per group (*P < 0.05; **P < 0.01).
DED Causes Corneal Epithelial Cells to Translocate Cytoplasmic TLR4 to the Cell Surface
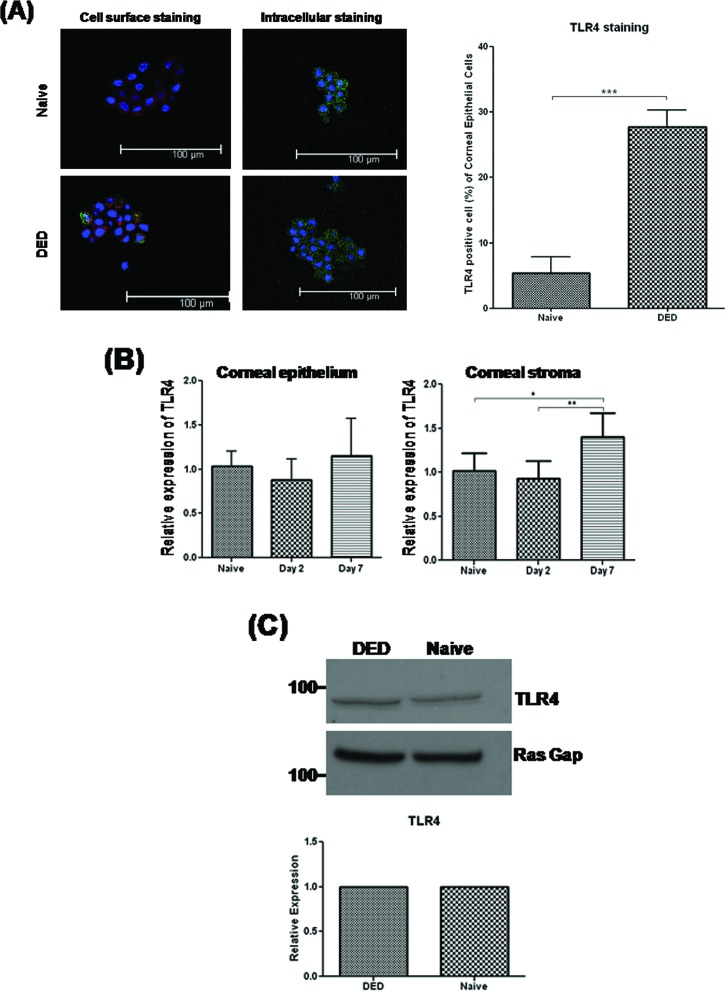
Previous studies have reported that human corneal epithelial cells express TLR4 protein intracellularly, but not on the cell surface.12 In our study, extracellular and intracellular analysis of TLR4 with flow cytometry revealed that most TLR4 was primarily located intracellularly in epithelial cells of the normal cornea (data not shown), consistent with a previous report involving human corneal epithelial cells.12 Interestingly, DED increased the expression of TLR4 mRNA in the stroma (P = 0.035) after 7 days of dry eye induction, but not in the epithelium. Furthermore, DED led to a significant increase in the cell surface expression of TLR4 by corneal epithelial cells as compared with those in the normal cornea as measured by flow cytometry and immunohistochemical examination (P = 0.0003, Fig. 2A); however, there was no increase in either the mRNA or protein levels of TLR4 in dry eye corneal epithelium (Figs. 2B, 2C), suggesting that TLR4 is located intracellularly in normal corneal epithelial cells and DED induces the translocation of cytoplasmic TLR4 to the epithelial cell surface.
Figure 2. .
Translocation of cytoplasmic TLR4 in DED corneal epithelial cells. (A) Immunohistochemical staining of cell surface and cytoplasmic TLR4 on corneal epithelial cells from naïve and DED-induced corneas with commercial-conjugated (Alexa Fluor 488) anti-TLR4 (green), commercial plasma membrane (CellMask, dark red), and DAPI nuclear staining (blue). Representative confocal images depicting increased epithelial cell surface expression of TLR4 in DED corneas as compared with naïve corneas. FITC-TLR4 positive cells in corneal epithelial cells were counted at two areas per slide for each group (two mice per group) under ×400 magnification. Data are presented as the mean ± SEM of three independent experiments for a total of six mice per group. (B) Real-time PCR analysis revealed that TLR4 mRNA expression was increased in the stroma, but not epithelium, of DED corneas on day 7. Data are presented as the mean ± SEM of three experiments with three mice per group. (C) Western blot analysis demonstrating that DED did not increase the expression of TLR4 protein relative to RasGAP, as an internal control, in the epithelium of DED corneas as compared with naïve corneas (*P < 0.05; **P < 0.01).
Systemic TLR4 Inhibition Attenuates Dry Eye–Induced Corneal Inflammation
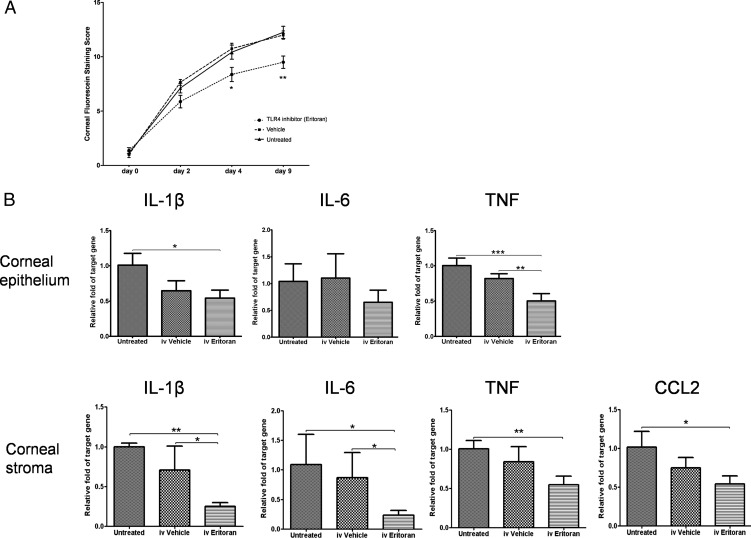
To explore the function of TLR4 in the immunopathogenesis of murine DED, we evaluated DED severity in mice treated with systemic TLR4 inhibitor. TLR4 inhibitor was injected intravenously every day beginning 1 day prior to dry eye induction. TLR4 inhibitor–treated mice displayed significantly lower CFS at day 4 (P = 0.029 vs. untreated; P = 0.012 vs. vehicle) and day 9 (P = 0.0039 vs. untreated; P = 0.0025 vs. vehicle, Fig. 3A), and reduced expression of TNF (P = 0.0020 vs. vehicle) in DED corneal epithelium and IL-1β (P = 0.027 vs. vehicle), IL-6 (P = 0.0291 vs. vehicle), TNF (P = 0.001 vs. untreated; P = 0.0501 vs. vehicle), and CCL2 (P = 0.0150 vs. untreated; P = 0.1019 vs. vehicle) in DED corneal stroma as compared with untreated and/or vehicle-treated mice at day 9 (Fig. 3B).
Figure 3. .
Systemic TLR4 inhibition decreases corneal inflammation in DED. (A) Systemic TLR4 blockade decreased the severity of CFS as compared with both the untreated and vehicle-treated groups on days 4 and 9 (n = 4 mice/8 eyes per group). Representative data from three independent repeats. (B) Real-time polymerase chain reactions revealed that intravenous treatment with TLR4 inhibitor significantly decreased the mRNA expression of IL-1β, IL-6, TNF, and CCL2 (MCP-1) as compared with untreated and vehicle-treated mice on day 9 (n = 4 corneas per group). Data are presented as the mean ± SEM of three or four experiments (*P < 0.05; **P < 0.01; ***P < 0.001).
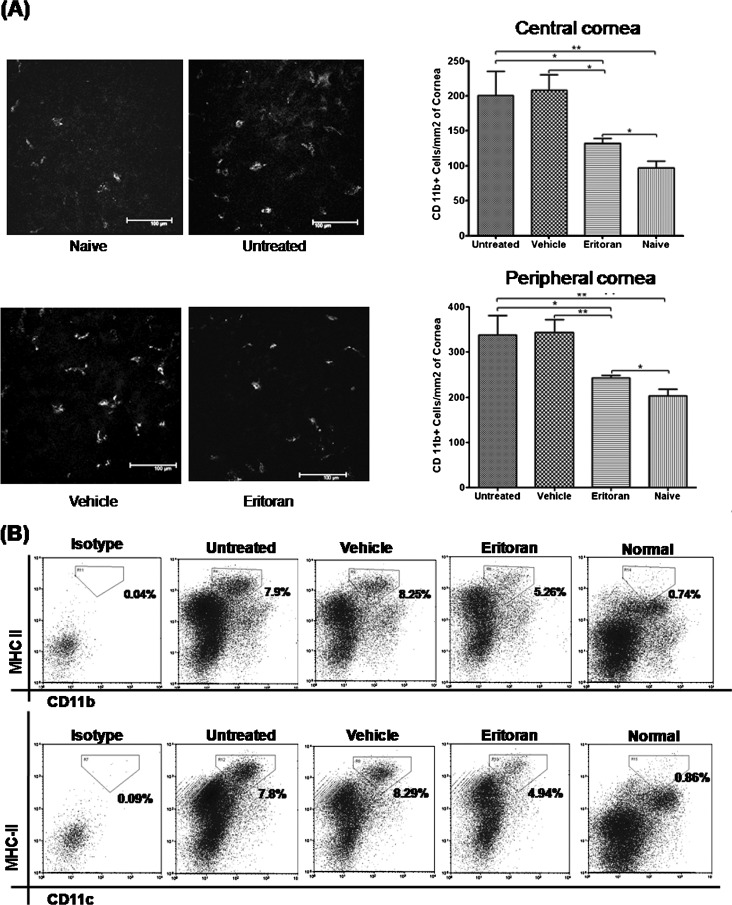
Additionally, blockade of TLR4 in wild-type DED mice significantly reduced the corneal infiltration of CD11b+ cells (P = 0.0470 vs. untreated, P = 0.0275 vs. vehicle in the central cornea; P = 0.0178 vs. untreated, P = 0.0039 vs. vehicle in the peripheral cornea; Fig. 4A) and the frequencies of MHC-IIhighCD11b+ cells and MHC-IIhighCD11c+ cells in the draining lymph nodes as compared with untreated and vehicle-treated DED controls (Fig. 4B).
Figure 4. .
Systemic TLR4 inhibition suppressed inflammatory cell activity in DED. (A) Representative confocal images showing CD11b+ cell (grayscale) infiltration of the central cornea. Intravenous TLR4 inhibition significantly decreased the number of CD11b+ cells in the periphery and center of dry eye corneas as compared with untreated and vehicle-treated corneas at day 9. Data are presented as the mean ± SEM of three or four repeated experiments, involving three to four corneas per group (*P < 0.05; **P < 0.01). (B) Representative flow data demonstrating that systemic TLR4 inhibition decreased the number of mature MHC-IIhighCD11b+ and MHC-IIhighCD11c+ APCs in the draining lymph nodes of dry eye mice, compared with untreated and vehicle-treated mice. Representative flow data from two trials with pooled cells from four mice per group.
DED Induces Corneal Expression of Endogenous TLR4 Ligands
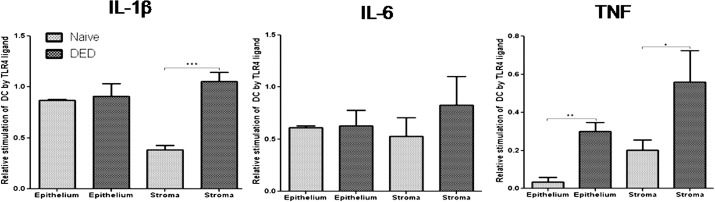
To provide evidence that an endogenous TLR4 ligand is involved in the inflammatory response to DED, we evaluated the activation of BMDCs following coculture with homogenized corneal tissues exposed to either vehicle or TLR4 inhibitor. Proinflammatory cytokine expression by DCs is an important indicator of DC activity23,24 and our data for mRNA expressions of these cytokines on BMDC after coculture are provided in Supplemental Figure S1 (see Supplementary Material and Supplementary Fig. S1, http://www.iovs.org/content/53/9/5632/suppl/DC1). BMDCs expressed significantly higher mRNA levels of IL-1β (P = 0.0003 for the corneal stroma) and TNF (P = 0.001 for the corneal epithelium; P = 0.024 for the corneal stroma) in response to homogenized dry eye corneal tissue, as compared with naïve corneal tissue (Fig. 5).
Figure 5. .
Evidence of endogenous TLR4 ligand expression in DED. Expression of IL-1β, IL-6, and TNF mRNA by bone marrow–derived dendritic cells (BMDCs) was assessed following coculture with supernatants from homogenized DED or naïve corneas (n = 8 corneas per well). Dry eye corneal tissue upregulated the expression of proinflammatory cytokines by BMDCs more than normal corneal tissue. [In vitro BMDC activation by unknown endogenous TLR4 ligands = (mRNA expression of BMDC cocultured with corneal tissue and vehicle) − (mRNA expression of BMDC cocultured with corneal tissue and TLR4 inhibitor)]. Data are presented as the mean ± SEM of three experiments for a total of 24 corneas per group (*P < 0.05; **P < 0.01; ***P < 0.001).
Discussion
Dry eye disease is a multifactorial, immune-mediated disorder of the ocular surface. Numerous studies have demonstrated that DED increases the ocular surface expression of proinflammatory cytokines such as IL-1β, IL-6, and TNF.21,25 These cytokines activate resident APCs and promote the corneal infiltration of additional CD11b+ cells.26,27 Activated APCs subsequently stimulate an adaptive immune response, as evidenced by the proliferation of CD4+ T cells in the draining lymphatics.1,28 Furthermore, APCs prime pathogenic ocular surface-specific CD4+ T cells (e.g., T helper 17 cells), supporting the theory that DED has an autoimmune component.28 Autoreactive CD4+ T cells are recruited to the ocular surface by chemokines such as CCL2, CCL5, and CXCL9-11, leading to immune-mediated ocular surface damage.7,25
TLR4 ligation results in the production of proinflammatory cytokines such as IL-1β, IL-6, and TNF that promote the activation of leukocytes and lymphocytes.10,29 TLR4 signaling is important for the activation of APC- and T-cell–mediated immunoinflammatory responses.15,16,30 The ligation of TLR4 on immature dendritic cells (DCs) leads to the downregulation of CCR6 and upregulation of CCR7, thereby enhancing the migration of DCs to draining lymph nodes.31,32 TLR4 signaling is unique in that it involves the MyD88-dependent and -independent pathways that increase the intensity and diversity of responses to LPS.13,33
There is some controversy regarding the expression of TLR4 by corneal epithelial cells. Song et al.34 have reported that human corneal epithelial cells (HCEs) express TLR4 and secrete proinflammatory mediators in response to stimulation with LPS. However, Ueta et al.12 have reported that HCEs express TLR2 and TLR4 only at the intracellular level, and the intracellular transfer of LPS in vitro does not elicit an inflammatory response. This unresponsiveness may have been caused by deficient expression of LPS cofactors such as MD-2, LBP, or CD14.15,33,35,36 MD-2–deficient mice do not experience LPS-mediated endotoxic shock, a featured they share with TLR4-deficient mice.37
In the present study, we found that TLR4 proteins are primarily expressed intracellularly in normal murine corneal epithelial cells, consistent with previous reports involving HCEs.12 DED induction increased the cell surface expression of TLR4 on corneal epithelial cells and stromal cells. Interestingly, these increases occurred without corresponding increases in the expression of TLR4 mRNA or protein. These findings suggest that the cell surface expression of TLR4 increases through the translocation of cytoplasmic TLR4. Previous studies have shown that proinflammatory cytokines such as TNF, IFN-γ, and IL-17 can increase the expression of TLR4, MD-2, and LPS-dependent cytokines by synovial tissue and intestinal epithelial cells38,39; however, Ueta et al.12 have reported that the stimulation of HCEs with IL-1β or TNF did not increase the expression of cell surface TLR2 or TLR4. Furthermore, oxidative stress causes the translocation of TLR4 in alveolar macrophages, thereby permitting the augmentation of LPS responsiveness.40 Based on the results of the present experiment, we conclude that TLR4 expression can be upregulated by extrinsic factors such as DED-associated inflammation. Further investigation will be necessary to identify which factors specifically induce the corneal epithelial and stromal cell surface expression of TLR4 in response to DED.
Systemic TLR4 blockade decreased the severity of DED as compared with both untreated and vehicle-treated mice. Previous studies have demonstrated that TLR4 deficiency diminishes immunoinflammatory responses to nonocular injury, as well as the severity of autoimmune diseases.41–45 TLR4-deficient mice express significantly lower levels of proinflammatory mediators including TNF, IL-6, CCL2, CCL5, and CXCL10 in a model of retinal ischemia.43 Furthermore, TLR4-deficient mice demonstrate decreased infiltration of inflammatory cells, particularly Th17 cells, and reduced production of IL-17 in a rheumatoid arthritis model.45 TLR4 inhibition also diminishes the production of chemokines such as IL-8/CXCL8 and CCL2, and reduces the infiltration of inflammatory cells into the cornea in response to LPS stimulation.13,20
TLR4 is an important mediator of immune responsiveness not only to LPS, but also a variety of endogenous ligands including heat shock proteins, high-mobility group box 1, and extracellular matrix degradation products.29,42,46,47 In the present study, we found that constituents of homogenized DED corneal tissue can activate BMDC, and this activation is abolished by the selective inhibition of TLR4. Recent studies have demonstrated that the degradation of extracellular matrix components including fibronectin, hyaluronic acid, and heparan sulfate generates endogenous bioactivators of TLR4. MMP-9 activity is significantly increased in DED, and MMP-9 is involved in the degradation of corneal epithelial tight junctions and basement membrane proteins.8,29,47,48 Furthermore, endogenous TLR4 ligand (e.g., S100 A8 and A9) levels are increased in the tears of patients with DED, and S100A8/A9 levels have been correlated with clinical disease severity.49,50 Therefore, we propose that DED-associated ocular surface inflammation leads to the generation of endogenous TLR4 bioactivators, although the identity of these ligands remains to be determined.
Herein, we present evidence that systemic TLR4 blockade ameliorates the clinical signs of DED in association with reduced corneal expression of proinflammatory cytokines, reduced ocular surface APC infiltration, and decreased maturation of APCs that migrate to the draining lymph nodes. Taken together, these findings suggest that TLR4 expression is upregulated in DED and TLR4 contributes to the inflammatory response to desiccating stress at the ocular surface.
Supplementary Material
Footnotes
Supported in part by National Eye Institute/National Institutes of Health Grant EY-20889.
Disclosure: H.S. Lee, Eisai Research Institute (R); T. Hattori, None; E.Y. Park, None; W. Stevenson, None; S.K. Chauhan, None; R. Dana, None
References
- 1.Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemp MA, Baudouin C, Baum J, et al. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92 [DOI] [PubMed] [Google Scholar]
- 4.Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301 [DOI] [PubMed] [Google Scholar]
- 5.Luo L, Li DQ, Pflugfelder SC. Hyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea. 2007;26:452–460 [DOI] [PubMed] [Google Scholar]
- 6.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren's and non-Sjögren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614 [PubMed] [Google Scholar]
- 7.Yoon KC, De Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569 [DOI] [PubMed] [Google Scholar]
- 8.Chotikavanich S, de Paiva CS. Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest. 2003;111:1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511 [DOI] [PubMed] [Google Scholar]
- 11.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537 [DOI] [PubMed] [Google Scholar]
- 12.Ueta M, Nochi T, Jang MH, et al. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol. 2004;173:3337–3347 [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Pearlman E. Inhibition of corneal inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564). Invest Ophthalmol Vis Sci. 2009;50:1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medzhitov R, Janeway C Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97 [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Kumar A, Wheater M, Yu FS. Lack of MD-2 expression in human corneal epithelial cells is an underlying mechanism of lipopolysaccharide (LPS) unresponsiveness. Immunol Cell Biol. 2009;87:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376 [DOI] [PubMed] [Google Scholar]
- 17.Shimamoto A, Chong AJ, Yada M, et al. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114(suppl 1):1270–1274 [DOI] [PubMed] [Google Scholar]
- 18.Kerfoot SM, Long EM, Hickey MJ, et al. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J Immunol. 2004;173:7070–7077 [DOI] [PubMed] [Google Scholar]
- 19.Barabino S, Shen L, Chen L, et al. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46:2766–2771 [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Chauhan SK, Okanobo A, Nallasamy N, Dana R. Therapeutic efficacy of topical Epigallocatechin gallate in murine dry eye. Cornea. 2011;30:1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232 [PubMed] [Google Scholar]
- 22.Schlereth S, Lee HS, Khandelwal P, Saban DR. Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. Am J Pathol. 2012;180:2351–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252 [DOI] [PubMed] [Google Scholar]
- 24.Jurewicz M, Takakura A, Augello A, et al. Ischemic injury enhances dendritic cell immunogenicity via TLR4 and NF-kappa B activation. J Immunol. 2010;184:2939–2948 [DOI] [PubMed] [Google Scholar]
- 25.Goyal S, Chauhan SK, Zhang Q, et al. Amelioration of murine dry eye disease by topical antagonist to chemokine receptor 2. Arch Ophthalmol. 2009;127:882–887 [DOI] [PubMed] [Google Scholar]
- 26.Ecoiffier T, El Annan J, Rashid S, Schaumberg D, Dana R. Modulation of integrin alpha4beta1 (VLA-4) in dry eye disease. Arch Ophthalmol. 2008;126:1695–1699 [DOI] [PubMed] [Google Scholar]
- 27.Goyal S, Chauhan SK, Dana R, et al. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. 2010;128:819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaumburg CS, Siemasko KF, de Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–3662 [DOI] [PubMed] [Google Scholar]
- 29.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen H, Tesar BM, Walker WE, Goldstein DR. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J Immunol. 2008;181:1849–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gioannini TL, Teghanemt A, Zhang D, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci USA. 2004;101:4186–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–2877 [PubMed] [Google Scholar]
- 35.Blais DR, Vascotto SG, Griffith M, Altosaar I. LBP and CD14 secreted in tears by the lacrimal glands modulate the LPS response of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:4235–4244 [DOI] [PubMed] [Google Scholar]
- 36.Tsukamoto H, Fukudome K, Takao S, Tsuneyoshi N, Kimoto M. Lipopolysaccharide-binding protein-mediated Toll-like receptor 4 dimerization enables rapid signal transduction against lipopolysaccharide stimulation on membrane-associated CD14-expressing cells. Int Immunol. 2010;22:271–280 [DOI] [PubMed] [Google Scholar]
- 37.Jiang Q, Akashi S, Miyake K, Petty HR. Cutting edge: lipopolysaccharide induces physical proximity between CD14 and Toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-κB. J Immunol. 2000;165:3541–3544 [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Cho ML, Kim JI, et al. Interleukin 17 (IL-17) increases the expression of Toll-like receptor-2, 4, and 9 by increasing IL-1beta and IL-6 production in autoimmune arthritis. J Rheumatol. 2009;36:684–692 [DOI] [PubMed] [Google Scholar]
- 39.Abreu MT, Arnold ET, Thomas LS, et al. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002;277:20431–20437 [DOI] [PubMed] [Google Scholar]
- 40.Powers KA, Szászi K, Khadaroo RG, et al. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med. 2006;203:1951–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Si R, Feng Y, et al. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem. 2011;286:31308–31319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H, Chen G, Wyburn KR, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D. Toll-like receptor 4 contributes to retinal ischemia/reperfusion injury. Mol Vis. 2010;16:1907–1912 [PMC free article] [PubMed] [Google Scholar]
- 44.Summers SA, Hoi A, Steinmetz OM, et al. TLR9 and TLR4 are required for the development of autoimmunity and lupus nephritis in pristane nephropathy. J Autoimmun. 2010;35:291–298 [DOI] [PubMed] [Google Scholar]
- 45.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun TJ, Harning EK, Giza K, et al. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J Immunol. 2011;186:563–575 [DOI] [PubMed] [Google Scholar]
- 47.Brunn GJ, Bungum MK, Johnson GB, Platt JL. Conditional signaling by Toll-like receptor 4. FASEB J. 2005;19:872–874 [DOI] [PubMed] [Google Scholar]
- 48.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723 [DOI] [PubMed] [Google Scholar]
- 49.Vogl T, Tenbrock K, Ludwig S, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049 [DOI] [PubMed] [Google Scholar]
- 50.Zhou L, Beuerman RW, Chan CM, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8:4889–4905 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.