Summary
Cigarette smoking has deleterious effects on the musculo-skeletal system. The loss of bone mineral content and increased incidence of fractures are the best known negative consequences. The pathogenesis is complex, due to direct toxic effects on osteoblasts/osteoclasts activity of nicotine, and indirect actions on sex and adrenocortical hormones, vitamin D, intestinal calcium absorption, vessels and oxygen supply. Smoking may favour the onset or aggravate the progression of rheumatoid arthritis and back pain. Negative influences have been observed on muscle and on tendons. Moreover, smoking habit is associated to a number of short term post-operative complications and higher resource consumption.
Smoking cessation is highly advisable with positive effects on the bone metabolism on the long term. More positive and immediate results can be obtained in patients submitted to orthopedic surgery: the healing process is improved, the frequency of complications is reduced, and the length of hospital stay is shortened.
Keywords: arthritis, muscle, nicotine, osteoporosis, smoking, tendon
Introduction
Cigarette smoking (CS) is the largest cause of preventable deaths in the world.
Notwithstanding the overwhelming relevance of lung cancer, chronic bronchitis and coronary heart disease, CS is also an important risk factor for a plethora of conditions, which jeopardize the duration and quality of life.
A large amount of clinical and experimental research shows that CS has deleterious effects on the musculoskeletal system, and worsens the prognosis of several orthopaedic disorders and surgical procedures. The number of cigarette smokers is still high all over the world, but the clinical relevance of this collateral, but important, problem is underestimated.
Aim of our paper is to summarize the present knowledge on this topic, and to alert physicians about the need of considering the effects of smoking on the locomotor system, which have a major health and economic impact1.
A search of English-language articles was performed in PubMed, Web of Knowledge (WOK) and EMBASE using the key search terms “cigarette smoking”, “smoking”, “nicotine” combined with “bone mineral content”, “osteoporosis”, “fractures”, “wound healing”, “orthopaedic surgery”, “back pain”, “rheumatoid arthritis”, “osteoarthritis”, “muscles”, “tendons”, independently. Bibliographies were hand searched to include any applicable studies that were not captured by our search. Articles were eligible if they provided specific information related to the correlation between cigarette smoking and musculo-skeletal disorders.
Bone metabolism and osteoporosis
Epidemiology
The first suggestion of an association between tobacco smoking and osteoporosis dates back to 19762. Afterwards, the effect of smoking on Bone Mineral Content (BMC) has been confirmed by several epidemiological studies.
In a cohort study, Gerdhem & Obrant3 found significantly lower values of BMC in the femoral neck, tibia and calcaneus, and, at a lesser extent, in the lumbar spine and bones of the hand. These results were confirmed in a large epidemiologic survey on 14000 subjects, from 19500 randomly selected, submitted to the evaluation of BMC by DEXA at proximal femur site. Cotinine, a marker for tobacco exposure, was simultaneously measured, and a significant inverse relationship between cotinine and BMC was observed in both genders4.
A five year longitudinal study investigated the development of bone density in a population of 833 young men (18–20 years), using high resolution peripheral Quantitative Computed Tomography. Men who had started smoking had considerably smaller increases in total body and lumbar spine BMC, and substantially greater decreases at the total hip and femoral neck, than men who were non smokers at both baseline and follow-up visits5.
Epidemiological surveys on post-menopausal women show that smokers loose significantly more cortical bone than non smoking counterparts6. A cross-sectional study of bone density at the lumbar spine and the femoral neck in 41 pairs of female twins, discordant for smoking habits, showed that women, who had smoked one pack of cigarettes each day throughout adulthood, by the time of menopause had an average deficit of 5–10% in bone density, sufficient to increase the risk of fracture7.
Passive smoking has attenuated but yet evident effects on bone mineral density, as shown by Kim et al.8 who found a clear positive association between secondhand smoke exposure and lumbar and femoral neck osteoporosis in 925 postmenopausal never-smoking Korean women.
In conclusion, summing up the results of epidemiologic studies, smoking has a central role in the development of bone loss at all skeletal sites. Bone loss shows a positive relationship with the daily number of cigarette smoked and years of exposure, and is independent from sex, age, weight, BMI, and several unhealthy lifestyle habits, frequently seen in smokers (e.g. lack of physical activity and sun exposure, low calcium intake and alcohol and caffeine use)9.
Pathogenesis
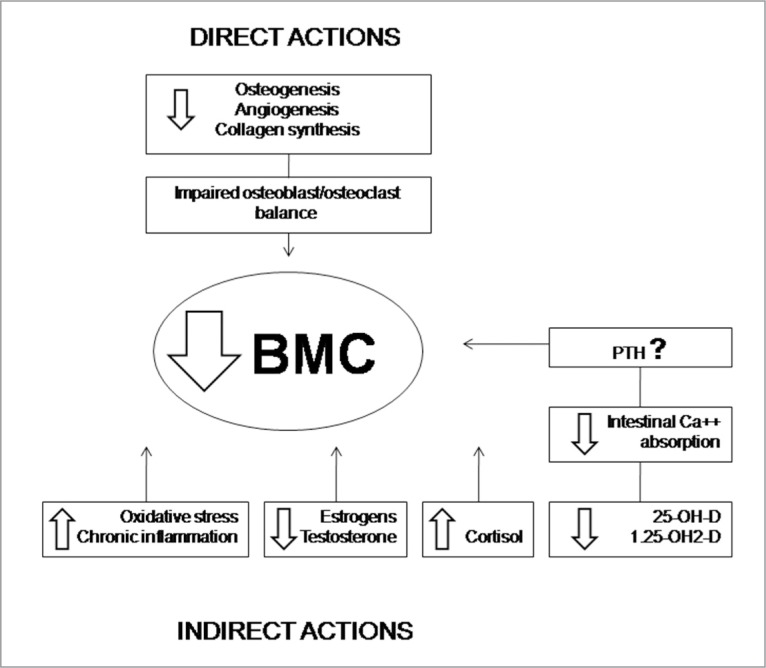
The pathogenesis of bone mass loss is complex and only partially clarified. Alterations of bone metabolism may occur directly by a toxic activity on bone cells, and indirectly by involvement of sexual, calciotropic, and adrenocortical hormones, vascular system and oxygen supply (Fig. 1).
Figure 1.
Pathogenetic mechanisms leading to decreased. BMC in smokers. PTH = parathermone
Direct activity
Several in vitro studies show that nicotine may affect bone metabolism in a biphasic manner, stimulating at low doses, at concentrations analogous to those acquired by light smokers, and depressant at high doses, with concentrations analogous to those acquired by heavy smokers. Moreover, there are suggestions that bone metabolism may be also influenced by other components of smoke, which contains thousand of harmful activities.
In Walker’s et al.10 experiments, performed on human bone cells (isolated from biopsies), toxic, anti-proliferative effects were observed at high nicotine levels (>1 mmol/L), while stimulatory effects were found at very low levels (0.01–10 micromol/L).
The positive effects from low level treatment correlated with an up-regulated expression of the AP-1 transcription factor, c-fos, while western analysis of proteins highlightened an increase in osteopontin, a bone matrix protein implicated in regulating re-adsorption10.
These results were confirmed by Rothem et al.11 on human osteosarcoma cells, and by Gullihorn et al.12in vitro cultures of osteoblast-like cells.
Indirect activity
CS is provided of anti-estrogenic activity as smokers women show lower levels of estrogens, and an earlier menopause than their non smoking counterparts. Moreover, smoking may nullify the protective skeletal effects of usual doses of estrogen replacement, and higher doses must be used to achieve clinical effects comparable to those observed in non-smokers6. Smoking may influence estrogens production and metabolism by different mechanisms: 1) inhibiting the enzyme aromatase, which is essential for estradiol synthesis; 2) increasing 2-α-hydroxylation, with irreversible conversion of estrone to the inactive metabolite 2-methoxyestrone; and 3) increasing sex-hormone binding globulin levels, with subsequent reduction of active free estradiol13.
Also the role of testosterone on bone health, proven by the presence of specific receptors in bone, and indirectly by the conversion through aromatization to estrogens14, must be taken into account.
Indeed, low serum testosterone levels are associated with significant deterioration of trabecular bone, and increased risk of osteoporotic fractures15.
It is supposed that smoking may alter vitamin D metabolism influencing the activity of 25-hydroxylase in the liver16. Serum levels of 25-hydroxyvitamin-D, as well as of 1.25-dihydroxyvitamin-D, are significantly reduced in current smokers. As consequence, intestinal calcium absorption is decreased, as shown by the reduced serum levels after an oral calcium load16.
The effects of CS on serum parathyroid hormone are controversial: some authors have found increased levels of parathyroid hormone, and have put emphasis on the role of secondary hyperparathyroidism, but other studies have shown suppressed parathyroid hormone levels despite low vitamin D levels. The underlying mechanisms for these differences remain unknown13. Hypercortisolism, which is frequently present in smokers, may alter directly the osteoblastic and osteoclastic activity, and indirectly influences bone metabolism, impairing gastrointestinal and renal tubular calcium re-absorption13.
Finally, several systemic effects of CS may contribute to reduce BMC: among them, chronic inflammation (as shown by increased IL-6 levels), increased oxidative stress, vascular damage, and reduced oxygen delivery to tissues13.
Fractures
As consequence of osteoporosis the incidence of fractures is increased. The risk, adjusted for confounding variables, is higher in men than in women. It is estimated that smoking increases the lifetime risk of developing a vertebral fracture by 32% in men and 13% in women, and a hip fracture by 40% and 31%, respectively17. However, smoking cessation reduces the risk of hip fracture in men after 5 years, while the deleterious effects seem to be more long lasting in female ex-smokers17.
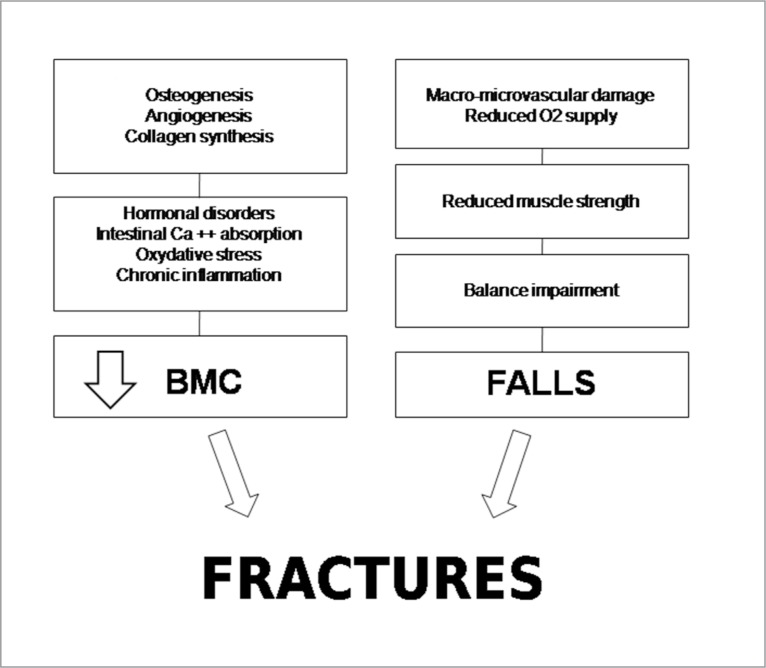
The reduced BMC is the major determinant of fractures, but it must be remembered that the systemic effects of CS, such as the negative influence on vascular system, oxygen supply, muscle strength and the overall reduced performance, may influence balance, increasing the risk of falls in smokers (Fig. 2).
Figure 2.
Pathogenetic mechanisms favouring fractures in smokers.
Post-surgery outcomes
Fractures healing
Fracture healing is a complex process, which has been studied in a variety of mammalian models. It can be divided into a number of discreet phases (haematoma and inflammatory response, callus formation, ossification, and remodelling), involving a cascade of synthesis and activation of matrix proteins, growth factors, cytokines, and angiogenic stimulators that coordinate restoration of mechanical stability at the fracture site.
Smoking delays fracture healing. In a study performed on 146 consecutive closed and grade I open tibia shaft fractures, treated with cast immobilization, external fixation, or intramedullary rod fixation, the median time to clinical healing for smokers was significantly greater than for non smokers (269 days vs 136 days)18. CS also adversely affects graft incorporation at different sites (humerus, tibia and femur), using a composite graft of liophilized cancellous allogenic chips and demineralised bone matrix19.
Clinical studies are consistent with experimental observations. At 7 days post-injury, mice exposed to CS had a smaller fracture callus with less cartilage matrix and a more immature morphology of proliferating cells; at 14 days they showed more chondrogenesis, but less percentage of bone, compared to controls, and at day 28 a larger callus in the fracture site20.
Yamano et al.21 showed that nicotine inhibits bone wound healing around titanium implants in rats. In this experiment, expression levels of osteopontin, type II collagen, bone morphogenic protein-2 and bone sialoprotein were significantly down-regulated in the nicotine-delivered group compared with controls.
Several factors potentially undermine the normal fracture repair. As previously seen, nicotine is provided by a direct toxic activity, which inhibits the proliferation of a variety of cells essential for healing. Besides that, nicotine is a potent vasoconstrictor, that reduces blood flow, essential for the injured tissues; it also increases platelet adhesiveness, favouring microvascular occlusion. The limited blood supply, the carbon monoxide increased concentrations, and the toxic effects of hydrogen cyanide on the mitochondrial respiratory chain, are responsible for tissue hypoxia. This in turn is a major factor in the impaired production of cartilaginous callus, since oxygen is essential for the physiologic hydroxylation of proline and lysine, a critical step in type II collagen synthesis22.
Chondrocyte implantation
The deleterious effects of CS are also manifest in the outcome of autologous chondrocyte implantation for the treatment of full thickness chondral defects of the knee. In the Jaiswal’s et al. study23, the mean Modified Cincinatti Knee score was significantly lower in smokers (n=48) than in non smokers (n= 66), both before and after implantation (p< 0.05). Graft failures were only seen in smokers (p= 0.016). A strong negative correlation between the number of cigarettes smoked and the outcome following surgery was reported.
Wound healing
Fibroblast and mesenchymal stem cells are crucial mediators of wound repair, producing different cytokines which have an important role in the granulation tissue formation. CS negatively influences the production of Growth Factors, leading to poor healing and to chronically unhealed wounds.
The delayed healing and post-operative short term complications (i.e osteomyelitis, pulmonary infections, cardiac failure or rhythm disorders), requiring post-operative intensive care, frequently observed in smokers submitted to surgical orthopaedic procedures are also responsible of longer hospital stay, and higher resource consumption24.
Back pain
Smoking has been associated with back pain, independently from confounding factors such as age, sex, physical activity, life style habits, and coughing due to respiratory diseases, which may contribute to exacerbate the symptom25.
Based on the observation that smoking is associated with higher levels of negative emotional symptoms, it was suggested that persons with chronic back pain may be motivated to smoke because they perceive smoking will help them effectively cope with pain and related emotional distress. However, this hypothesis has been rejected by studies26,27 which have shown that this association is evident also after controlling for the presence of any lifetime anxiety and mood disorders.
On the contrary, experimental studies strongly support an organic pathogenesis. In animals, submitted to passive smoking (equivalent to 20 cigarettes/day for 8 weeks), cell necrosis and fibrosis in the nucleus pulposus, chondrocytes degeneration, reduced collagen synthesis, misalignement of collagen layers, and increased degradation of extracellular matrix proteins were observed28.
In mice exposed to tobacco smoke by direct inhalation to model long term smoking in humans, both a reduced proteoglycan synthesis and increased degradation of a key disc extracellular matrix protein, aggrecan, within its interglobular domain, were found. Cleavage of aggrecan interglobular domain is extremely detrimental as this results in the loss of the entire glycosaminoglycan attachment region of aggrecan, which is vital for attracting water necessary to counteract compressive forces29.
Based on these experimental observations, the most widely accepted explanations for the association between smoking and disc degeneration include an adverse toxic activity of nicotine itself, increased degradation of collagen, and decreased blood and oxygen supply, resulting from the vascular damage, and/or vasoconstriction of the vascular network surrounding the intervertebral discs.
Osteoarthritis
The association between osteoarthritis (OA) and CS has been object of several epidemiological surveys. Felson et al.30 evaluating 1415 members of the Framingham Heart Study cohort, after 36 years follow-up, found that subjects who had been smokers at baseline had a lower rate of OA (28%) than did non smokers (37.5%), after adjusting for age, sex and weight. These results were confirmed in a retrospective study by Cerhan et al.31 Subsequently, Felson et al.32 performed a longitudinal study of knee OA, evaluating the Kellgren-Lawrence score of OA on weight bearing knee. This report revealed that smokers had a lower risk, compared to non smokers (R= 0.4, CI 0.2–0.8). These literature data, suggesting that smoking would well have a protective effect against OA, could be comforted by in vitro studies, which show an anabolic action of nicotine on chondrocytes33.
However, the protective effect remains modest and non significant, and may be reported to inaccurate methods of evaluation and selection bias, resulting from studies not primarily designed to investigate smoking outcomes.
More recently, longitudinal research performed by means of MRI has shown significant loss of knee cartilage at the medial tibiofemoral joint and the patellofemoral joint in smokers. Davies-Tuck et al.34 studied, in a 2 years follow-up, the changes in the tibial and patellar cartilage in a cohort of 271 middle-aged adults. Being a smoker (former or current) was associated with increased annual loss of medial tibial and patella cartilage volume. Patients with bone marrow lesions at baseline were at higher risk of medial cartilage loss, suggesting that cartilage loss may be partially mediated by bone marrow lesions, which may represent one step in the continuum from a healthy to a diseased joint.
Rheumatoid arthritis
Many environmental factors have been associated with an increased risk of developing rheumatoid arthritis (RA), but so far smoking is the sole widely studied and accepted. It results from meta-analyses that smoking contributes up to 25% of the population burden of RA, and there is evidence that the risk is associated with a long duration but merely a moderate intensity of smoking35.
The risk is present in both sexes, but is stronger in males and especially strong for seropositive RA35.
Epidemiological investigations have shown that smoking may be a specific risk factor only for the anti-citrullinated peptide antibody positive (ACPA+) RA through an interaction with the shared epitope. Ever-smokers with two copies of the shared epitope have a relative risk of 21 for ACPA+ RA as compared with non smokers with no shared epitope36. The observed differential effect may be because smoking acts as an inflammatory mediator or be related to an interaction between smoking and genotype. CS can influence the development of more aggressive joint damage, and is additionally associated with a higher prevalence of extra-articular manifestations, including sub-cutaneous nodules and interstitial lung disease37. In observational cohort studies, heavy smokers had less improvement in disease activity after treatment to both biologic and non-biologic disease-modifying anti-rheumatic drugs38. However, these conclusions have not been confirmed in a randomized, placebo double blind controlled study, where serum cotinine, a metabolite of nicotine, was measured as an objective marker of tobacco exposure39.
Muscles
The age related loss of muscle mass and strength is significantly influenced by CS. In the Amsterdam Growth and Health Longitudinal Study, four repeated measurements of the quadriceps strength, at the ages of 21, 27, 32 and 36 years, were performed. In men and women, an inverse relationship between tobacco smoking and quadriceps strength was found: smoking 100 g of tobacco/week resulted in a reduction of 2.9% in men and of 5.0% in women, independently of lifestyle covariates40.
Clinical and experimental studies have shown that CS induced skeletal muscle damage is due to impaired muscle metabolism, increased inflammation and oxidative stress, over-expression of atrophy related genes and activation of various intracellular signaling pathways41.
Among them, ubiquity in specific protease-19 (USP-19) is up regulated in the skeletal muscle in some degradative conditions, such as diabetes, dexamethasone treatment, and cancer. An over expression of USP-19 has been also observed in rats exposed to chronic CS. This finding suggests that USP-19 may be a potential therapeutic target using specific inhibitors42.
Besides the reduction of the muscle mass and strength, CS is associated to a higher risk of muscle pain. A survey performed on a large general population (about 13000 subjects) showed, after adjustment for potential confounders, that current and ex smokers had higher risk than lifetime non smokers for musculoskeletal pain (neck, upper and lower limbs)43. The increased musculoskeletal pain could arise from a pharmacological effect of tobacco smoke; however, on the contrary, it cannot be excluded that people with a low pain threshold are more likely to take up and continue smoking26.
Tendons
Cohort studies show that smoking is associated with persistent shoulder pain and tendinopathy, mainly on the dominant side, and is an important risk factor for the development of rotator cuff tears44. In a study including 408 patients who underwent arthroscopic repair of cuff tears, a positive relationship between the daily average number of cigarettes, and the total number of cigarettes smoked in life, and the increasing severity of tears was found45.
CS is also a strong risk factor for distal biceps tendon rupture. Indeed, smokers have a 7.5 times greater risk of distal biceps tendon rupture compared to non smokers46, and patients who sustain bilateral distal biceps tendon ruptures are usually middle-aged men with higher rates of nicotine and anabolic steroid use than the general population47.
Negative effects of nicotine have been shown also on tendon healing. In experimental models of transected tendons (Achilles and rotator cuff), animals receiving subcutaneous administration of nicotine tartrate, compared to controls, showed an increased gap in repair site, associated to fibroblast degeneration and irregular fibrils organization48.
Conclusions
The present review provides a clear demonstration of the deleterious effects of CS on the musculo-skeletal system. The relationships of CS with the reduction of BMC, osteoporosis and fractures are the best known, but there is evidence of negative influence on OA, RA, intervertebral discs degeneration, decreased muscle mass and strength, muscle pain, tendons degeneration and ruptures. Moreover, smoking habit delays fracture and tendon healing and is associated to a number of post operative short term complications, responsible of longer hospital stay, and higher resource consumption. Therefore, smoking cessation is highly advisable to prevent musculo-skeletal diseases. Long term positive effects can be seen on the bone metabolism, as shown by the increase of BMC at different sites, and by the reduced incidence of fractures49. More evident results can be obtained from smoking cessation in the short term, in patients submitted to orthopaedic surgery, being the healing process improved, the frequency of complications reduced, and the length of hospital stay shortened50.
Even though the cessation of smoking is routinely advised peri-operatively, temporal guidelines are vague and inconclusive. Where applicable, twelve hours is certainly a minimum, as this is the amount of time required to clear the human body of carbon oxide. Five/seven days smoke-free prior to surgery have a favourable outcome on wound healing, but twenty days pre-operatively and post-operatively, or longer, are considered better options.
Several orthopaedic surgeons in their practice urge their patients to stop smoking before elective bone graft surgery is done. They feel that the incidence of delayed healing of bone graft procedures is so high that it would not be worth proceeding with surgery while the patient is still smoking. There is a need of controlled studies to state whether this assumption, based on anecdotic observations, can be considered scientifically proved.
References
- 1.Collins SE, Eck S, Torchalla I, Schröter M, Batra A. Understanding treatment-seeking smokers' motivation to change: Content analysis of the decisional balance worksheet. Addict Behav. 2012 Aug 31;38(1):1472–1480. doi: 10.1016/j.addbeh.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniell HW. Osteoporosis of the slender smoker. Arch Intern Med. 1976;136:298–304. doi: 10.1001/archinte.136.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Gerdhem P, Obrant KJ. Effects of cigarette-smoking on bone mass as assessed by dual-energy X-ray absorptiometry and ultrasound. Osteoporos Int. 2002;13(12):932–936. doi: 10.1007/s001980200130. [DOI] [PubMed] [Google Scholar]
- 4.Benson BW, Shulman JD. Inclusion of tobacco exposure as a predictive factor for decreased bone mineral content. Nicotine Tob Res. 2005;7(5):719–724. doi: 10.1080/14622200500259119. [DOI] [PubMed] [Google Scholar]
- 5.Rudäng R, Darelid A, Nilsson M, et al. Smoking is associated with impaired bone mass development in young adult men: A five year longitudinal study. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1674. [DOI] [PubMed] [Google Scholar]
- 6.Noale M, Maggi S, Crepaldi G. Osteoporosis among Italian women at risk: the osteolab study. J Nutr Health Aging. 2012;16(6):529–533. doi: 10.1007/s12603-011-0359-z. [DOI] [PubMed] [Google Scholar]
- 7.Bączyk G, Opala T, Kleka P, Chuchracki M. Multifactorial analysis of risk factors for reduced bone mineral density among postmenopausal women. Arch Med Sci. 2012;8(2):332–341. doi: 10.5114/aoms.2012.28562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KH, Lee CM, Park SM, et al. Secondhand smoke exposure and osteoporosis in never-smoking postmenopausal women: the Fourth Korea National Health and Nutrition Examination Survey. Osteoporos Int. 2012 doi: 10.1007/s00198-012-1987-9. [DOI] [PubMed] [Google Scholar]
- 9.Kim BS, Kim SJ, Kim HJ, et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. 2012;90(3–4):109–115. doi: 10.1016/j.lfs.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Walker LM, Preston MR, Magnay JL, Thomas PB, El Haj AJ. Nicotinic regulation of c-fos and osteopontin expression in human-derived osteoblast-like cells and human trabecular bone organ culture. Bone. 2001;28(6):603–608. doi: 10.1016/s8756-3282(01)00427-6. [DOI] [PubMed] [Google Scholar]
- 11.Rothem DE, Rothem L, Soudry M, Dahan A, Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab. 2009;27(5):555–561. doi: 10.1007/s00774-009-0075-5. [DOI] [PubMed] [Google Scholar]
- 12.Gullihorn L, Karpman R, Lippiello L. Differential effects of nicotine and smoke condensate on bone cell metabolic activity. J Orthop Trauma. 2005;19(1):17–22. doi: 10.1097/00005131-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Yoon V, Maalouf NM, Sakhaee K. The effects of smoking on bone metabolism. Osteoporos Int. 2012;23(8):2081–2092. doi: 10.1007/s00198-012-1940-y. [DOI] [PubMed] [Google Scholar]
- 14.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. Clin Invest. 2000;106(12):1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier C, Nguyen TV, Handelsman DJ, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008 doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 16.Need AG, Kemp A, Giles N, Morris HA, Horowitz M, Nordin BE. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int. 2002;13(1):83–88. doi: 10.1007/s198-002-8342-9. [DOI] [PubMed] [Google Scholar]
- 17.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005 Feb;16(2):155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz MA, Finnegan M, Natarajan R, Champine J. Effect of smoking on tibial shaft fracture healing. Clin Orthop Relat Res. 1999;(365):184–200. doi: 10.1097/00003086-199908000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Ziran BH, Hendi P, Smith WR, Westerheide K, Agudelo JF. Osseous healing with a composite of allograft and demineralized bone matrix: adverse effects of smoking. Am J Orthop (Belle Mead NJ) 2007;36(4):207–209. [PubMed] [Google Scholar]
- 20.El-Zawawy HB, Gill CS, Wright RW, Sandell LJ. Smoking delays chondrogenesis in a mouse model of closed tibial fracture healing. J Orthop Res. 2006;24(12):2150–2158. doi: 10.1002/jor.20263. [DOI] [PubMed] [Google Scholar]
- 21.Yamano S, Berley JA, Kuo WP, Gallucci GO, Weber HP, Sukotjo C. Effects of nicotine on gene expression and osseointegration in rats. Clin Oral Implants Res. 2010;21(12):1353–1359. doi: 10.1111/j.1600-0501.2010.01955.x. [DOI] [PubMed] [Google Scholar]
- 22.Williams G, Daly M, Proude EM, et al. The influence of alcohol and tobacco use in orthopaedic inpatients on complications of surgery. Drug Alcohol Rev. 2008;27(1):55–64. doi: 10.1080/09595230701711108. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal PK, Macmull S, Bentley G, Carrington RW, Skinner JA, Briggs TW. Does smoking influence outcome after autologous chondrocyte implantation?: A case-controlled study. J Bone Joint Surg Br. 2009;91(12):1575–1578. doi: 10.1302/0301-620X.91B12.22879. [DOI] [PubMed] [Google Scholar]
- 24.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 25.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 26.Williams LJ, Pasco JA, Jacka FN, Dodd S, Berk M. Pain and the relationship with mood and anxiety disorders and psychological symptoms. J Psychosom Res. 2012;72(6):452–456. doi: 10.1016/j.jpsychores.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Zvolensky MJ, Jenkins EF, Johnson KA, Goodwin RD. Personality disorders and cigarette smoking among adults in the United States. J Psychiatr Res. 2011;45(6):835–841. doi: 10.1016/j.jpsychires.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemoto Y, Matsuzaki H, Tokuhasi Y, et al. Hystological changes in intervertebral discs after smoking and cessation: experimental study using a rat passive smoking model. J Orthop Sci. 2006;11(2):191–197. doi: 10.1007/s00776-005-0987-4. [DOI] [PubMed] [Google Scholar]
- 29.Vo N, Wang D, Sowa G, et al. Differential effects of nicotine and tobacco smoke condensate on human annulus fibrosus cell metabolism. J Orthop Res. 2011;29(10):1585–1591. doi: 10.1002/jor.21417. [DOI] [PubMed] [Google Scholar]
- 30.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40(4):728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 31.Cerhan JR, Wallace RB, el-Khoury GY, Moore TE. Risk factors for progression to new sites of radiographically defined osteoarthritis in women. Rheumatol. 1996;23(9):1565–1578. [PubMed] [Google Scholar]
- 32.Felson DT, Anderson JJ, Naimark A, Hannan MT, Kannel WB, Meenan RF. Does smoking protect against osteoarthritis? Arthritis Rheum. 1989;32(2):166–172. doi: 10.1002/anr.1780320209. [DOI] [PubMed] [Google Scholar]
- 33.Ying X, Cheng S, Shen Y, et al. Nicotine promotes proliferation and collagen synthesis of chondrocytes isolated from normal human and osteoarthritis patients. Mol Cell Biochem. 2012;359(1–2):263–269. doi: 10.1007/s11010-011-1020-1. [DOI] [PubMed] [Google Scholar]
- 34.Davies-Tuck ML, Wluka AE, et al. Smoking is associated with increased cartilage loss and persistence of bone marrow lesions over 2 years in community-based individuals. Rheumatology (Oxford) 2009;48(10):1227–1231. doi: 10.1093/rheumatology/kep211. [DOI] [PubMed] [Google Scholar]
- 35.Lahiri M, Morgan C, Symmons DP, Bruce IN. Modifiable risk factors for RA: prevention, better than cure? Rheumatology (Oxford) 2012;51(3):499–512. doi: 10.1093/rheumatology/ker299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLADR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 37.Nyhäll-Wåhlin BM, Petersson IF, Nilsson JA, Jacobsson LT, Turesson C, BARFOT study group High disease activity disability burden and smoking predict severe extra-articular manifestations in early rheumatoid arthritis. Rheumatology (Oxford) 2009;48(4):416–420. doi: 10.1093/rheumatology/kep004. [DOI] [PubMed] [Google Scholar]
- 38.Saevarsdottir S, Wedrén S, Seddighzadeh M, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the Epidemiological Investigation of Rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum. 2011;63(1):26–36. doi: 10.1002/art.27758. [DOI] [PubMed] [Google Scholar]
- 39.Maska LB, Sayles HR, O'Dell JR, et al. Serum cotinine as a biomarker of tobacco exposure is not associated with treatment response in early rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012 doi: 10.1002/acr.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kok MO, Hoekstra T, Twisk JW. The longitudinal relation between smoking and muscle strength in healthy adults. Eur Addict Res. 2012;18(2):70–75. doi: 10.1159/000333600. [DOI] [PubMed] [Google Scholar]
- 41.Rom O, Kaisari S, Aizenbud D, Reznick AZ. Identification of possible cigarette smoke constituents responsible for muscle catabolism. J Muscle Res Cell Motil. 2012 doi: 10.1007/s10974-012-9299-4. [DOI] [PubMed] [Google Scholar]
- 42.Liu Q, Xu WG, Luo Y, et al. Cigarette smoke-induced skeletal muscle atrophy is associated with up-regulation of USP-19 via p38 and ERK MAPKs. J Cell Biochem. 2011;112(9):2307–2316. doi: 10.1002/jcb.23151. [DOI] [PubMed] [Google Scholar]
- 43.Palmer KT, Syddall H, Cooper C, Coggon D. Smoking and musculoskeletal disorders: findings from a British national survey. Ann Rheum Dis. 2003;62(1):33–36. doi: 10.1136/ard.62.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bodin J, Ha C, Sérazin C, et al. Effects of Individual and Work-related Factors on Incidence of Shoulder Pain in a Large Working Population. J Occup Health. 2012 doi: 10.1539/joh.11-0262-oa. [DOI] [PubMed] [Google Scholar]
- 45.Carbone S, Gumina S, Arceri V, Campagna V, Fagnani C, Postacchini F. The impact of preoperative smoking habit on rotator cuff tear: cigarette smoking influences rotator cuff tear sizes. J Shoulder Elbow Surg. 2012;21(1):56–60. doi: 10.1016/j.jse.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 46.Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275–283. [PubMed] [Google Scholar]
- 47.Schneider A, Bennett JM, O'Connor DP, Mehlhoff T, Bennett JB. Bilateral ruptures of the distal biceps brachii tendon. J Shoulder Elbow Surg. 2009;18(5):804–807. doi: 10.1016/j.jse.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 48.Duygulu F, Karaoğlu S, Zeybek ND, Kaymaz FF, Güneş T. The effect of subcutaneously injected nicotine on achilles tendon healing in rabbits. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):756–761. doi: 10.1007/s00167-006-0046-5. [DOI] [PubMed] [Google Scholar]
- 49.Okoli CT, Kelly T, Hahn EJ. Secondhand smoke and nicotine exposure: a brief review. Addict Behav. 2007;32(10):1977–1988. doi: 10.1016/j.addbeh.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 50.Lindström D, Sadr Azodi O, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg. 2008;248(5):739–745. doi: 10.1097/SLA.0b013e3181889d0d. [DOI] [PubMed] [Google Scholar]