Abstract
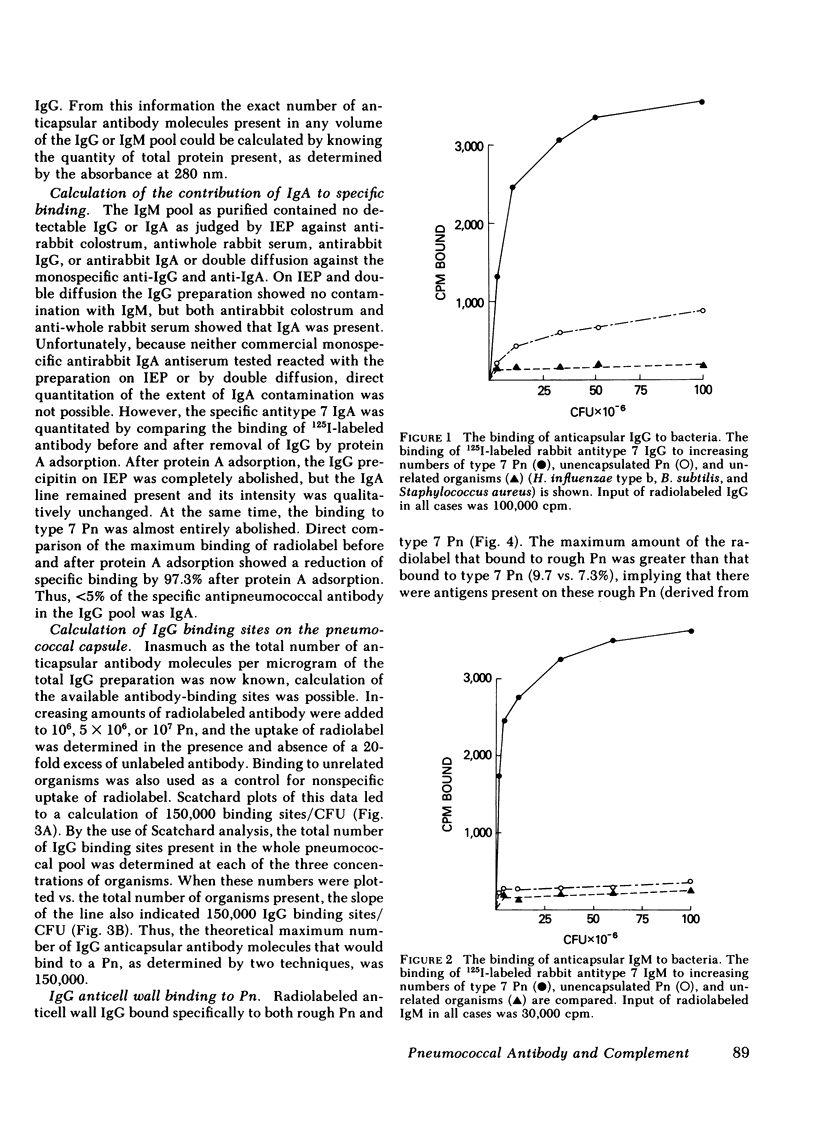
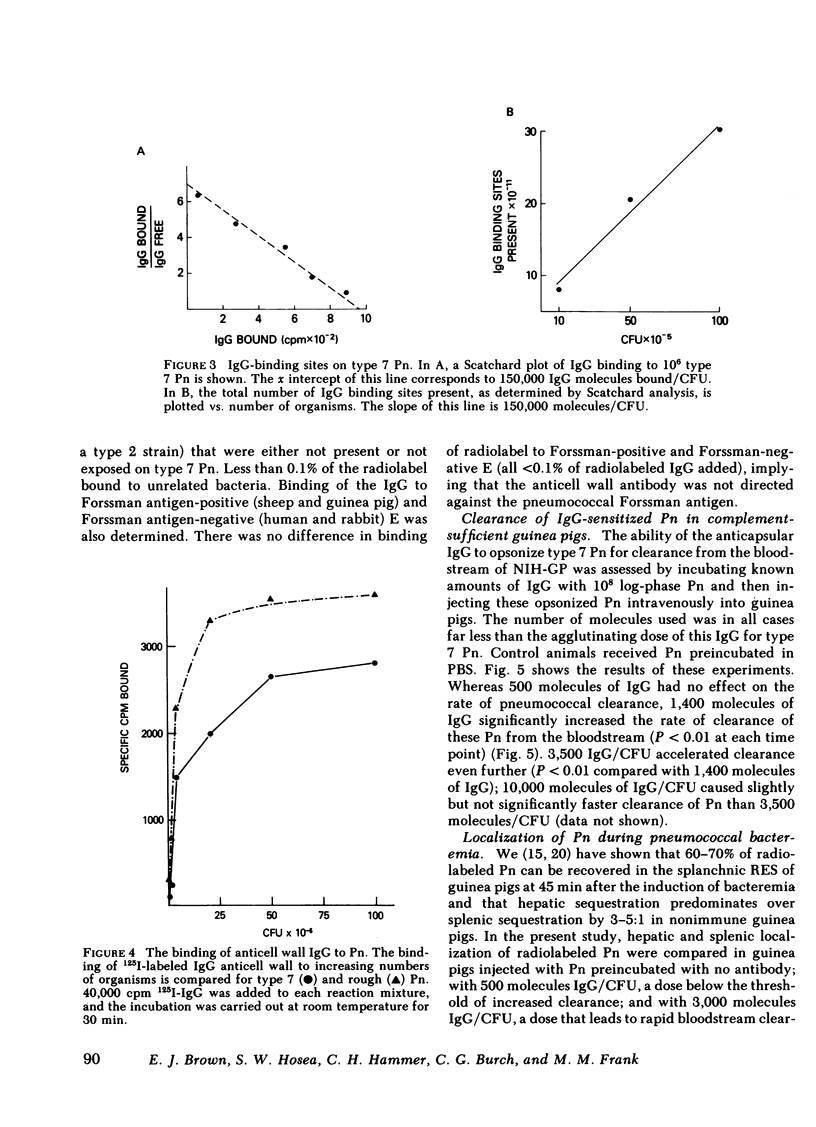
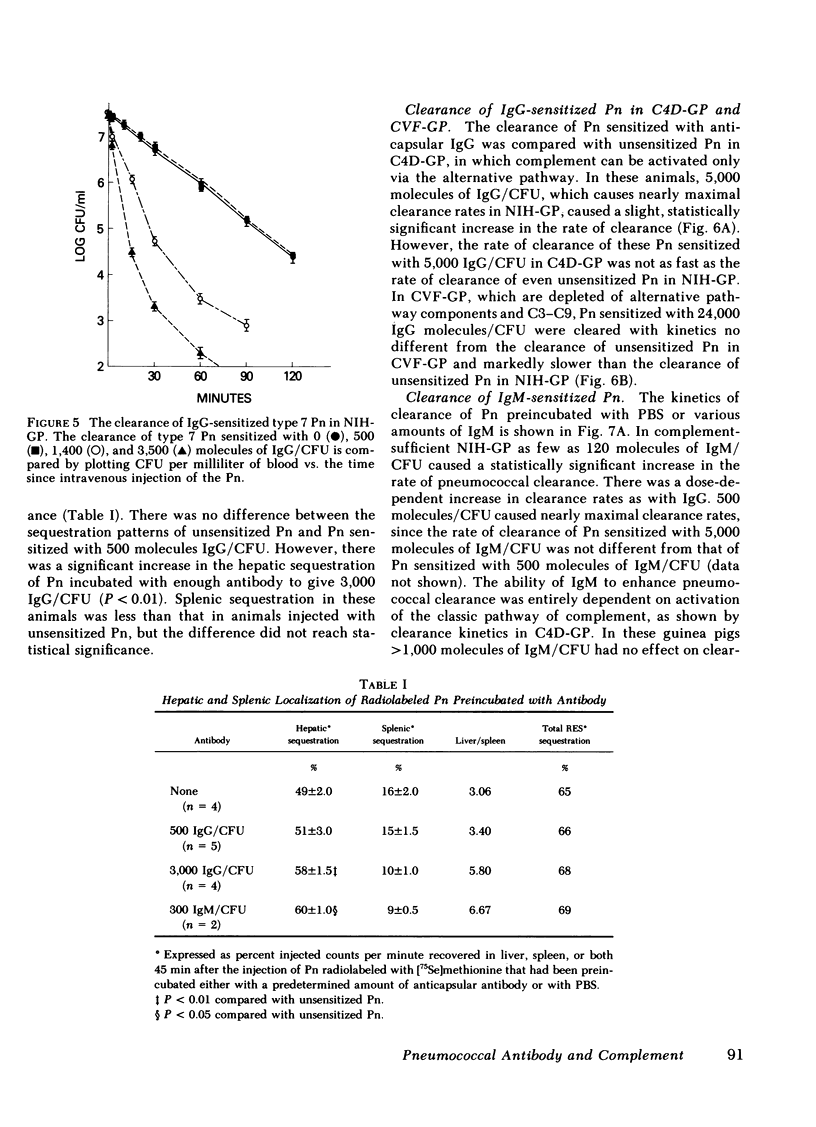
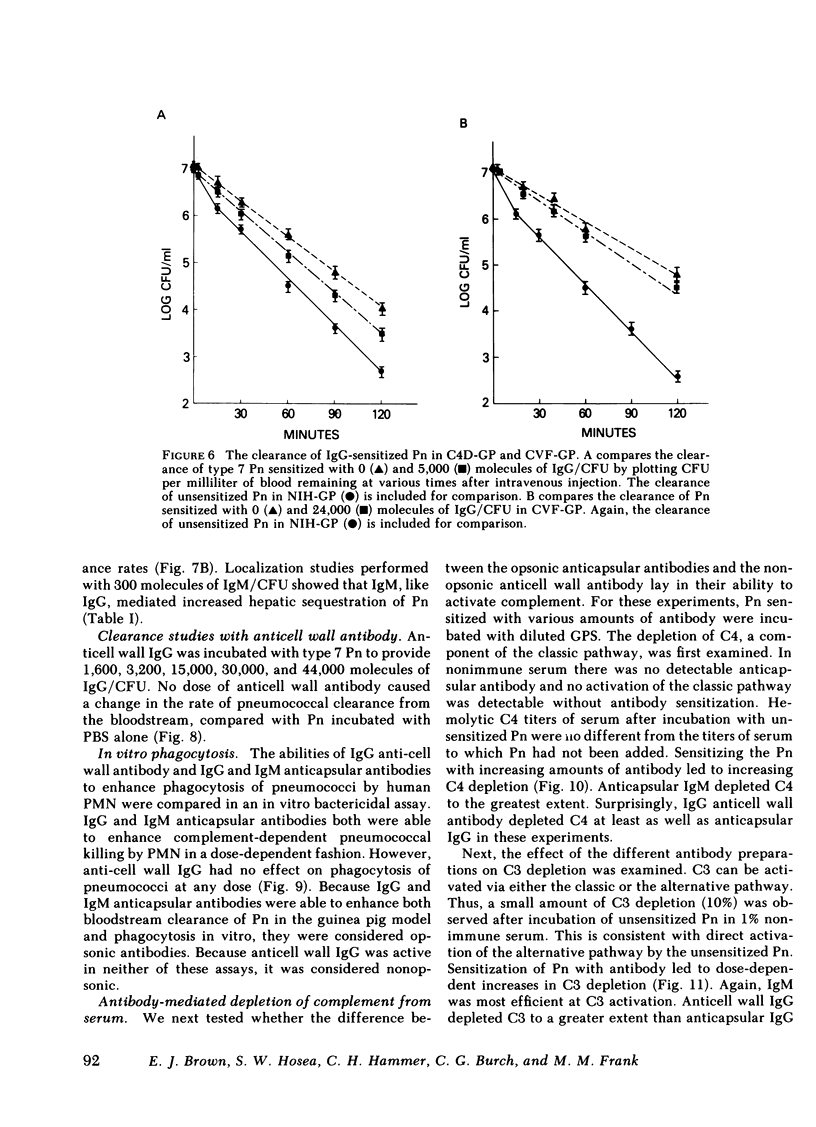
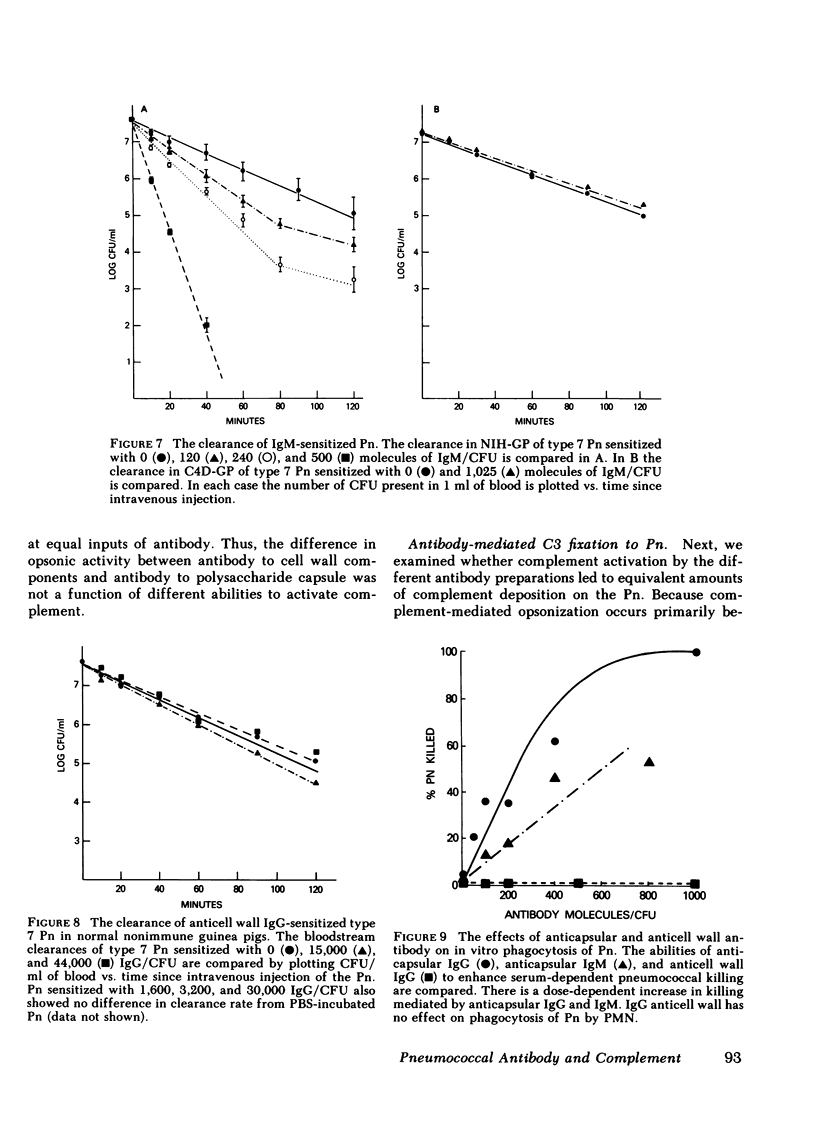
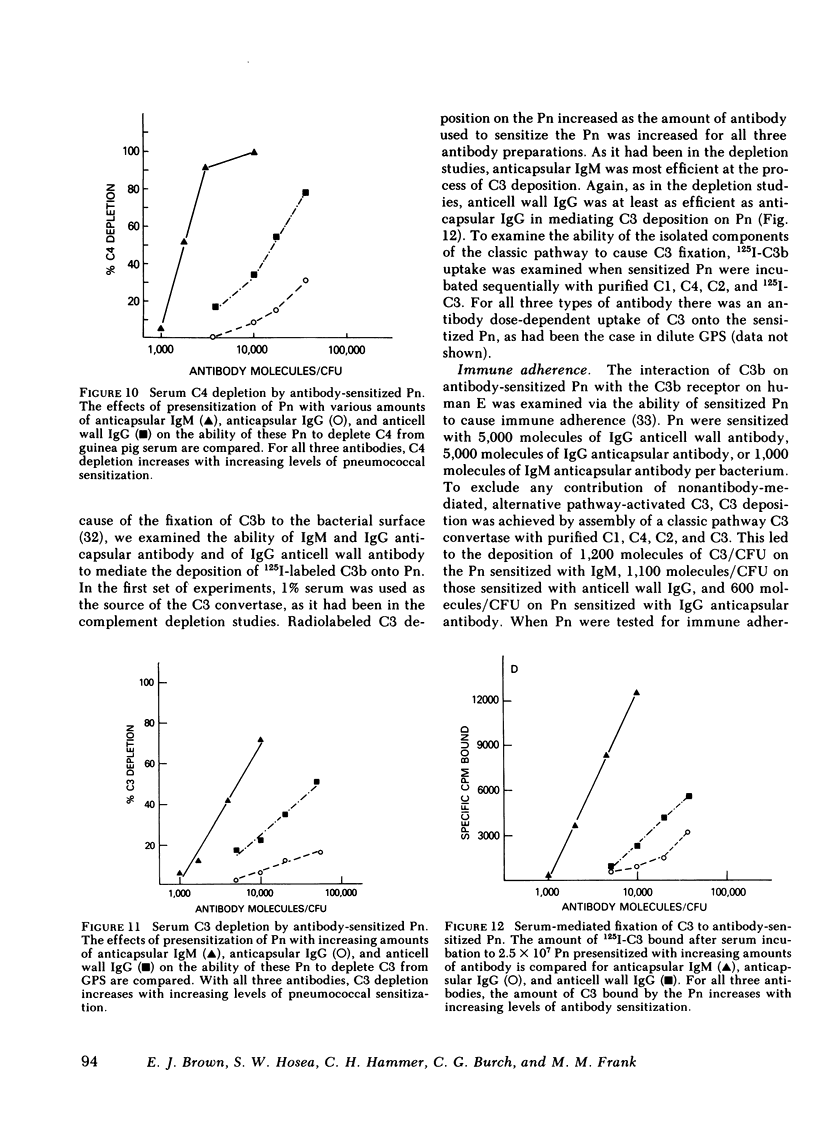
The mechanism of protection of type-specific antipneumococcal antibody and complement in bacteremia was investigated with purified rabbit antibody and a guinea pig model of pneumococcal bacteremia. IgG and IgM were isolated from the sera of rabbits immunized with type 7 pneumococci (Pn), and their binding to Pn was quantitated. The number of antibody-binding sites on the pnuemococcal capsule was also determined. Pn were incubated with various amounts of the immunoglobulin preparations before intravenous injection into nonimmune guinea pigs. Whereas 120 molecules of IgM per Pn were sufficient to enhance bloodstream clearance of Pn, 1,400 molecules of IgG per bacterium were required to produce this effect. As the amount of either IgG or IgM added to the Pn was increased, the rate of bloodstream clearance accelerated. In striking contrast, greater than 1,000 molecules of IgM had no effect on the rate of clearance in C4-deficient guinea pigs, which cannot activate complement via the classic pathway. Similarly, 5,000 molecules of IgG had only minimal effect in C4-deficient guinea pigs, and 24,000 molecules of IgG had no effect in guinea pigs depleted of complement by cobra venom factor. Thus, the in vivo opsonic effects of both IgG and IgM anticapsular antibody are mediated via their ability to activate complement. IgG anti-pneumococcal cell wall antibody, raised by intravenous injection of rabbits with unencapsulated Pn, had no effect on the rate of bloodstream clearance of Pn or on the polymorphonuclear leukocyte killing of type 7 Pn in an in vitro bacterial assay. Because the opsonic effects of anticapsular antibody required complement activation, the ability of anticell wall IgG to activate complement was compared with the two classes of anticapsular antibody. As judged by depletion of C3 and C4 from guinea pig serum, as well as by the fixation of radiolabeled C3 to Pn, IgM anticapsular antibody was the best complement activator. However, anticell wall IgG was somewhat more active than anticapsular IgG in each of these tests of complement activation and fixation. When equivalent amounts of C3 were fixed to Pn by each of the three antibodies, Pn sensitized with IgG and IgM anticapsular antibodies caused immune adherence, whereas Pn sensitized with anticell wall IgG did not. This may explain the failure of anticell wall antibody of mediate complement-dependent phagocytosis of Pn in vivo or in vitro. Although anticell wall IgG is capable of activating complement and fixing C3 to Pn, it is not opsonic; the most likely reason is that the nonopsonic antibody mediates C3 deposition in sites on the Pn that cannot interact efficiently with phagocytic cell C3 receptors.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au C. C., Einsenstein T. K. Evaluation of the role of the pneumococcal Forssman antigen (F-polysaccharide) in the cross-serotype protection induced by pneumococcal subcellular preparations. Infect Immun. 1981 Jan;31(1):169–173. doi: 10.1128/iai.31.1.169-173.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au C. C., Eisenstein T. K. Nature of the cross-protective antigen in subcellular vaccines of Streptococcus pneumoniae. Infect Immun. 1981 Jan;31(1):160–168. doi: 10.1128/iai.31.1.160-168.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian R. Maxwell Finland Lecture. Random gleanings from a life with the pneumococcus. J Infect Dis. 1975 Apr;131(4):474–484. doi: 10.1093/infdis/131.4.474. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Frank M. M. The role of complement in the localization of pneumococci in the splanchnic reticuloendothelial system during experimental bacteremia. J Immunol. 1981 Jun;126(6):2230–2235. [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Frank M. M. The role of the spleen in experimental pneumococcal bacteremia. J Clin Invest. 1981 Apr;67(4):975–982. doi: 10.1172/JCI110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman L., Green I., Frank M. Genetically controlled total deficiency of the fourth component of complement in the guinea pig. Science. 1970 Oct 2;170(3953):74–75. doi: 10.1126/science.170.3953.74. [DOI] [PubMed] [Google Scholar]
- Fujiwara M. The Forssman antigen of pneumococcus. Jpn J Exp Med. 1967 Dec;37(6):581–592. [PubMed] [Google Scholar]
- Gaither T. A., Hammer C. H., Frank M. M. Studies of the molecular mechanisms of C3b inactivation and a simplified assay of beta 1H and the C3b inactivator (C3bINA). J Immunol. 1979 Sep;123(3):1195–1204. [PubMed] [Google Scholar]
- Gigli I., Nelson R. A., Jr Complement dependent immune phagocytosis. I. Requirements for C'1, C'4, C'2, C'3. Exp Cell Res. 1968 Jul;51(1):45–67. doi: 10.1016/0014-4827(68)90158-4. [DOI] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosea S. W., Brown E. J., Frank M. M. The critical role of complement in experimental pneumococcal sepsis. J Infect Dis. 1980 Dec;142(6):903–909. doi: 10.1093/infdis/142.6.903. [DOI] [PubMed] [Google Scholar]
- Hosea S. W., Burch C. G., Brown E. J., Berg R. A., Frank M. M. Impaired immune response of splenectomised patients to polyvalent pneumococcal vaccine. Lancet. 1981 Apr 11;1(8224):804–807. doi: 10.1016/s0140-6736(81)92681-7. [DOI] [PubMed] [Google Scholar]
- Hosea S., Brown E., Hammer C., Frank M. Role of complement activation in a model of adult respiratory distress syndrome. J Clin Invest. 1980 Aug;66(2):375–382. doi: 10.1172/JCI109866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- NELSON R. A., Jr The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953 Dec 18;118(3077):733–737. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A., Ruddy S. The role of immunoglobulins in alternative complement pathway activation by zymosan. I. Human IgG with specificity for Zymosan enhances alternative pathway activation by zymosan. J Immunol. 1981 Jan;126(1):7–10. [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972 Mar;51(3):575–582. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. II. Molecular nature of IgG and IgM complement-fixing sites and effects of their interaction with serum. J Clin Invest. 1972 Mar;51(3):583–589. doi: 10.1172/JCI106847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Thompson H. C., Eisenstein T. K. Biological properties of an immunogenic pneumococcal subcellular preparation. Infect Immun. 1976 Mar;13(3):750–757. doi: 10.1128/iai.13.3.750-757.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. C., Snyder I. S. Protection against pneumococcal infection by a ribosomal preparation. Infect Immun. 1971 Jan;3(1):16–23. doi: 10.1128/iai.3.1.16-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volanakis J. E., Kaplan M. H. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971 Feb;136(2):612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Sisson S. P., Kim Y., Peterson P. K. Localization of the third component of complement on the cell wall of encapsulated Staphylococcus aureus M: implications for the mechanism of resistance to phagocytosis. Infect Immun. 1979 Dec;26(3):1159–1163. doi: 10.1128/iai.26.3.1159-1163.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Abramovitz A. S., Tomasz A. Activation of C3 via the alternative complement pathway results in fixation of C3b to the pneumococcal cell wall. J Immunol. 1980 May;124(5):2502–2506. [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S. The role of immunoglobulin in the interaction of pneumococci and the properdin pathway: evidence for its specificity and lack of requirement for the Fc portion of the molecule. J Immunol. 1974 May;112(5):1635–1642. [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S. The role of immunoglobulin in the interaction of pneumococci and the properdin pathway: evidence for its specificity and lack of requirement for the Fc portion of the molecule. J Immunol. 1974 May;112(5):1635–1642. [PubMed] [Google Scholar]


