Abstract
Estrogen receptor-α (ER) targeted therapies are routinely used to treat breast cancer. However, patient responses are limited by resistance to endocrine therapy. Breast cancer cells resistant to the pure steroidal ER antagonist fulvestrant (fulv) demonstrate increased activation of epidermal growth factor receptor (EGFR) family members and downstream ERK signaling. In this study we investigated the effects of fulv on EGFR signaling and ligand regulation in several breast cancer cell lines. EGFR/HER2/HER3 phosphorylation and ERK1,2 activation was seen after 24–48 hours after fulvestrant treatment in ER-positive breast cancer cell lines. 4-hydroxy-tamoxifen (4HT) and estradiol (E2) did not cause EGFR activation. Fulvestrant did not affect EGFR expression. Cycloheximide abolished the ability of fulv to activate EGFR suggesting autocrine production of EGFR ligands might be responsible for fulvestrant induced EGFR signaling. qRT-PCR results showed fulv differentially regulated EGFR ligands; HB-EGF mRNA was increased while amphiregulin (AREG) and epiregulin (EPR) mRNAs were decreased. Fulvestrant induced EGFR activation and upregulation of EGFR ligands was ER dependent since fulv treatment in C4-12, an ER negative cell line derivative of MCF-7 cells, did not result in EGFR activation or change in ligand mRNA levels. ER down regulation by siRNA induced similar EGFR activation and regulation of EGFR ligands as fulvestrant. Neutralizing HB-EGF antibody blocked fulv induced EGFR activation. Combination of fulv and EGFR family tyrosine kinase inhibitors (erlotinib and lapatinib) significantly decreased EGFR signaling and cell survival. In conclusion, fulvestrant activated EGFR family members accompanied by ER dependent upregulation of HB-EGF within 48 hours. EGF receptor or ligand inhibition might enhance or prolong the therapeutic effects of targeting ER by fulvestrant in breast cancer.
Keywords: fulvestrant, EGFR, EGFR ligands, breast Cancer Cell lines
Introduction
The majority of breast cancers express estrogen receptor-α (ER) and endocrine therapy is effective in a subset of these tumors. Currently several ER-targeting drugs are available [1, 2]. The two major classes are selective ER modulators (SERM) and aromatase inhibitors. Fulvestrant is a pure steroidal SERM that downregulates ER expression in model systems [3, 4]. Compared to tamoxifen, fulvestrant has no agonist activity. Despite the ability of fulvestrant to more completely inhibit ER, it is not clinically superior to tamoxifen even as first line therapy for metastatic disease [5]. While dosing of fulvestrant is important in optimizing response [6], de novo or acquired endocrine therapy resistance limits prolonged disease stabilization. Understanding how SERMs affect breast cancer cell biology could provide additional insight into improving initial therapeutic benefit and delaying or avoiding resistance.
In this study we focused on the drug fulvestrant. Compared to tamoxifen, fulvestrant binding to ER results in inhibition of receptor dimerization, rapid and strong degradation of ER, and disruption of its function [7, 8]. The potential mechanisms of fulvestrant resistance are not fully understood, but increased epidermal growth factor receptor (EGFR) signaling and/or increased EGFR family member protein expression have been implicated in resistance to ER therapies [9–11]. However, the addition of EGFR inhibitors to endocrine therapies have not shown increased clinical benefit compared to the endocrine therapy alone [11–13]. Further understanding of the crosstalk between these two systems might help define improved therapeutic strategies.
EGFR family comprises four closely related receptors: EGFR (HER1), HER2. HER3, and HER4. All HERs are tyrosine kinase receptors except for HER3. Binding of ligands to extracellular domain of EGFR, HER3, and HER4 induce receptor dimerization leading to the activation of down-stream MAPK, PI3K, STAT, and Src kinase pathways [14]. Currently 10 EGF ligands have been reported [15]. Epidermal growth factor (EGF), transforming growth factor-α (TGF-α) and amphiregulin (AREG) bind to EGFR; heparin-binding EGF-like growth factor (HB-EGF), beta-cellulin (BTC), and epiregulin (EPR) bind to both EGFR and HER4; neuregulin-1 (NRG-1) and NRG-2 bind both HER3 and HER4; and NRG-3 and NRG-4 bind to HER4. These ligands can be membrane-anchored precursor protein or soluble form ligands cleaved from the membrane by metalloproteinases. Therefore, depending on the cellular environment these ligands induce juxtacrine, autocrine, paracrine, and/or endocrine signaling [16]. EGF ligands and their receptors are involved in many aspects of cancer cell biology and have served as targets for several successful cancer therapies.
Several groups [17–21] have developed fulvestrant resistant breast cancer cell lines and reported increased EGFR/HER2 activation with subsequent downstream activation of ERK1,2 signaling. Specific EGFR tyrosine kinase inhibitors gefitinib and pan-HER inhibitor CI-1033 are effective in inhibiting fulvestrant resistant MCF-7 breast cancer cell growth [9, 21]. However, it takes several months to develop resistance to fulvestrant. During this period, cells may undergo many selective changes that were not present de novo.
The purpose of this study was to investigate the effects of fulvestrant on EGFR signaling and ligand regulation in wild-type, hormone sensitive, ER-positive breast cancer cells for a better understanding of the potential mechanisms of fulvestrant resistance. We have found that fulvestrant, but not tamoxifen or estradiol, up-regulated HB-EGF to activate EGFR signaling. Increased HB-EGF expression was mediated by ER expression suggesting a specific role for fulvestrant in regulating ER-mediated transcription of EGF family ligands.
Materials and Methods
Cells and reagents
Human breast cancer cell lines. MCF-7L was from Dr. C. Kent Osborne (Baylor College of Medicine, Houston, Texas), MCF-7 ATCC and T47D were obtained from ATCC and are routinely cultured in our lab [22, 23]. C4-12 [24], an ER negative cell line derived from MCF-7L was kindly provided by Dr. Adrian V. Lee (Department of Pharmacology and Chemical Biology, University of Pittsburgh Cancer Institute, Magee Women Research Institute, Pittsburgh, Pennsylvania, USA). C4-12 cells were maintained in phenol red-free αMEM and supplemented with 5% charcoal/dextran-treated FBS.
Fulvestrant (ICI 182,780) was purchased from Tocris Bioscience (Bristol, UK). The working concentration for all the experiments was 100 nM. Estradiol (E2), 4-hydroxy-Tamoxifen (4HT), Cycloheximide (CHX) and anti-actin antibody were purchased from Sigma (ST. Louis, MO). Experimental concentrations used were 10 nM for E2 and 100 nM for 4HT. Erlotinib and lapatinib were purchased from BioVision Research Products (Mountain View, CA). Neutralizing HB-EGF antibody was purchased from R&D Systems, Inc. (Minneapolis, MN). Anti Phospho-Tyr antibody (PY20) was a product of BD Biosciences (San Jose, CA). pEGFR-Y1068, EGFR, pHER2 –Y1228, HER2, pHER-3-Y1289, HER3, pERα (ser167), pERα (ser 118), ERα, PARP, pAKT, AKT, pERK1,2 and ERK1,2 antibodies were from Cell Signaling Technology, Inc. (Danvers, MA). Human ESR1 siRNA was from Dharmacon, (Lafayette, CO) and Control siRNA was from Santa Cruz, CA.
Cell lysate preparation
Cells were serum-starved 24 hour before any treatment. At the end of the treatment, cells were washed once in ice cold phosphate buffered saline (PBS, Biofluids, Rockville, MD). Cells were then lysed with 200 μl per 6cm plate with TNESV buffer (50mM-Tris-pH 7.4, 1% NP-40, 2mM EDTA,100 mM NaCl, 10mM sodium orthovanadate, 1mM phenylmethylsulfonylfluoride, 20 μg/ml leupeptin, 20 μg/ml aprotinin). Protein concentration of the cleared lysates was determined by the copper-bicinchoninic acid method with a Pierce Laboratory kit (Rockford, IL).
Immunoblotting
After SDS-PAGE proteins were transferred overnight to nitrocellulose membranes (Bio Rad, Hercules, CA). All blotting steps were carried out at room temperature with gentle rocking. The membranes were blocked in 5% non-fat dry milk in Tris-buffered saline -Tween 20 (TBST: 0.15M CaCl2, 0.01M Tris HCl- pH 7.4, 0.05% Tween 20). For antiphosphotyrosine blotting with PY-20, membranes were washed five times over 30 min with TBST after blocking, incubated with a 1:2000 dilution of PY-20 in TBST for 1hr, washed, and binding was detected by chemiluminescence as described below. The other immunoblots were performed with overnight incubation at 4°C in primary antibody. Blots were then washed and incubated with a 1:2000 dilution of HRP linked anti-rabbit or mouse secondary antibody in blocking buffer for one hour, followed by further washing. Enhanced chemiluminescence was performed according to manufacturer’s instructions (Pierce, Rockford, IL).
Immunoprecipitation
500 μg protein cell lysate was used for immunoprecipitation with 1:100 EGFR, HER2 or HER3 antibodies in 0.5 ml TNSEV buffer for 2 hours at 4°C. Then 25 ml protein-A agarose beads was added with gentle rock at 4°C overnight. Beads were washed 5 times and pull down proteins were used for immunoblotting with PY20 or phospho-specific receptor antibodies.
Live Cell Quantification
Cells in serum-containing medium were seeded as triplicate sets into 24-well plates with 100,000 cells per well. Cells were switched to serum-free medium for 24 hours, then treated with or without fulvestrant in the presence or absence of erlotinib (10 μM) or lapatinib (1 μM). Live cell number was quantified at day 3 after treatment by the MTT assay. Briefly, 60 μl of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide solution in PBS was added to each well. After incubation for 3 hours at 37 °C, wells were aspirated, and formazan crystals were lysed with 500 μl of solubilization solution (95% DMSO + 5% Improved Minimal Essential Medium). Absorbance was measured with a plate reader at 570 nm by using a 670 nm differential filter.
Reverse transcription-quantitative real-time polymerase chain reaction (qRT-PCR)
MCF-7L, T47D, MCF-7 ATCC and C4-12 cells were treated with E2, 4HT and fulvestrant for 1, 4, 24 and 48 hours. mRNA were purified with TriPure Isolation Reagent according to the manufacturer’s instructions (Roche Diagnostics, Indianapolis, IN). A total of 2 μg of RNA was reverse transcribed using the Transcriptor first strand cDNA synthesis kit (Roche), and quantitative PCR was performed using the FastStart universal SYBR Green kit according to the manufacturer’s recommended protocol (Roche). All primers were synthesized at BioMedical Genomics Center at University of Minnesota.
Statistical analysis
Statistical significance between two groups was tested using Student’s t test. Error bars represent SD and asterisks denote statistical significance (p<0.05).
Results
Fulvestrant induced EGFR family member activation and downstream MAPK signaling
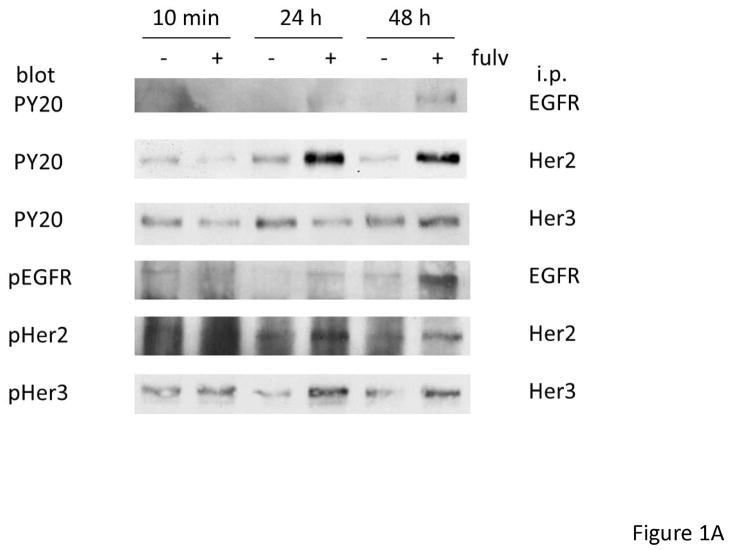
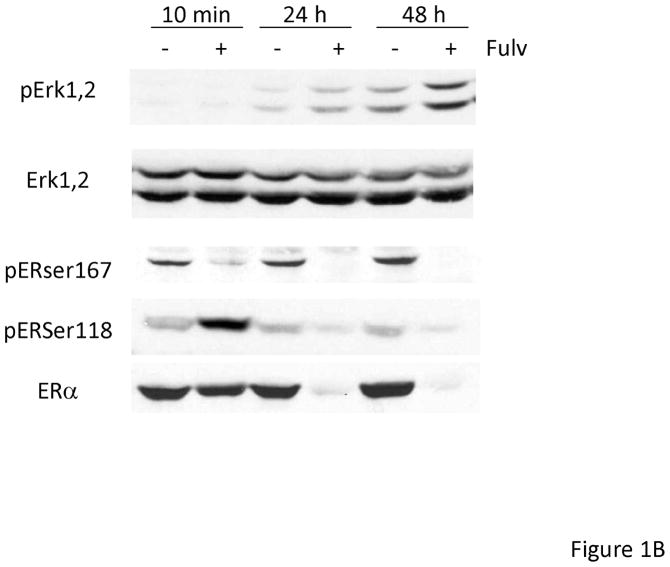
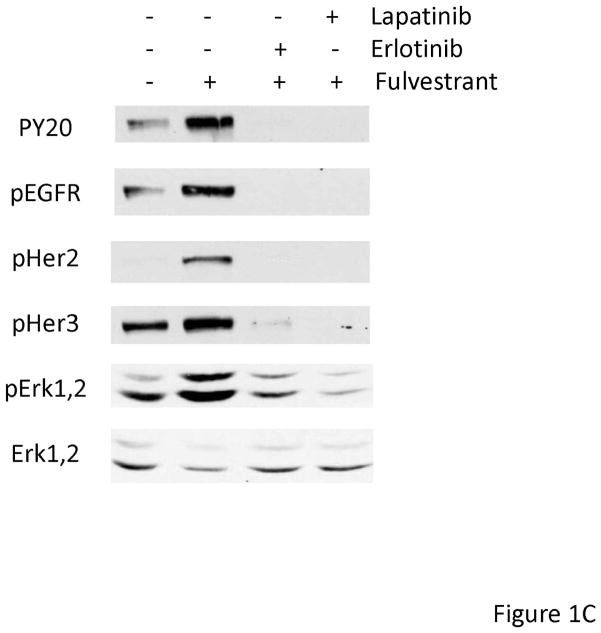
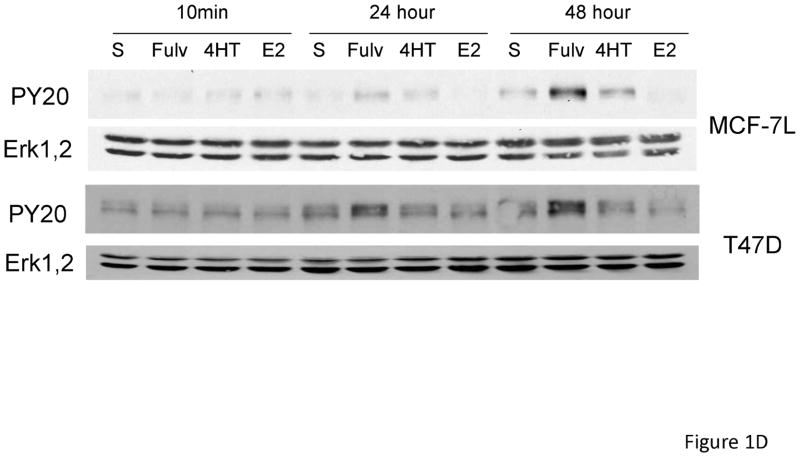
In order to determine if EGFR family members were activated upon fulvestrant treatment in naive breast cancer cells, we first treated MCF-7L cells with 100 nM fulvestrant. To identify specific EGFR family members we immunoprecipitated (i.p.) cell lysates with specific antibodies followed by anti-phosphotyrosine immunoblotting. Figure 1A, shows that short term fulvestrant treatment (10 min) did not induce EGFR receptor activation while prolonged exposure (24 to 48 hours) resulted in EGFR, HER2 and HER3 phosphorylation. Anti-phosphotyrosine total cell lysate immunoblotting was consistent with the results from immunoprecipitation of specific EGFR family members (Figure 1C and data not shown). Bands detected by anti-phophotyrosine blotting were consistent with the molecular weight of the EGFR family members. ER phosphorylation was decreased when ER was down regulated by fulvestrant (Figure 1B). Under prolonged serum-free conditions, ERK1,2 activation was detected likely due to stress induced under these culture conditions. However, fulvestrant further induced a higher level of activation (Figure 1B). We did not see significant changes in pAKT level suggesting PI3K signaling pathway was not induced by fulvestrant (data not shown). As shown in Figure 1C, EGFR and down-stream ERK1,2 signaling were blocked by the EGFR family member tyrosine kinase inhibitors erlotinib and lapatinib. Since EGFR and down-stream MAPK or PI3K signaling pathway were also reported to play roles in tamoxifen resistant breast cancer cells [25], we then treated MCF-7L and T47D cells with 4-hyrdoxy tamoxifen (4HT) and estradiol (E2) to determine if this fulvestrant activation of EGFR family members was specific. As shown in Figure 1D, only fulvestrant treatment induced activation of EGFR phosphorylation. It is notable that estradiol (E2) was unable to mimic the effects of fulvestrant suggesting that steroidal ligand binding to the ER alone was not sufficient to initiate EGFR family member signaling.
Figure 1.
Fulvestrant induced EGFR family member and MAPK signaling. Cells were starved in SFM for 24 hours prior to treatment. MCF-7L cells were then treated with (+) or without (−) 100 nM of fulvestrant (Fulv) for 10 minutes, 24 hours and 48 hours. (A) 500 μg cell lysates were used for receptor immunoprecipitation as indicated and immunoblotted with the indicated antibodies. (B) 50 μg cell lysates were used for immunoblotting with pErk1,2, total Erk1,2, phospho ERα and ERRα antibodies. (C) MCF-7L cells were treated for 48 hours with 100nM fulvestrant in combination with 1 μM lapatinib/10 μM erlotinib as indicated. 50 μg of cell lysates were used for immunoblotting for receptor phosphorylation and Erk1,2 phosphorylation. (D) MCF-7L and T47D cells were treated with 100nM fulvestrant, 100 nM 4-hydroxytamoxifen (4HT) and 10 nM estradiol (E2) respectively for 10 minutes, 24 hours and 48 hours. 50 μg cell lysates were used for phosphor-tyrosine blot (PY20) indicating receptor phosphorylation. Total Erk1,2 was used as loading control.
Fulvestrant induced upregulation of mRNA of EGFR ligand HB-EGF
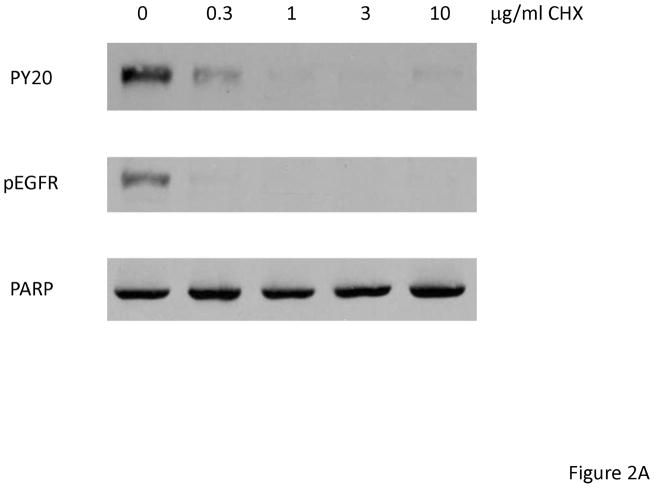
Fulvestrant-induced EGFR activation only occurred after 24 hours suggesting that juxtacrine or autocrine expression of EGFR ligands might be induced by fulvestrant. To determine if mRNA translation was needed, we treated MCF-7L cells with cycloheximide, an inhibitor of translation, and fulvestrant for 48 hours. As it is shown in Figure 2A, cycloheximide abolished the ability of fulvestrant to activate EGFR suggesting autocrine production of EGFR ligands might be induced by fulvestrant. PARP cleavage was not seen after cycloheximide, suggesting that apoptosis was not induced (data not shown).
Figure 2.
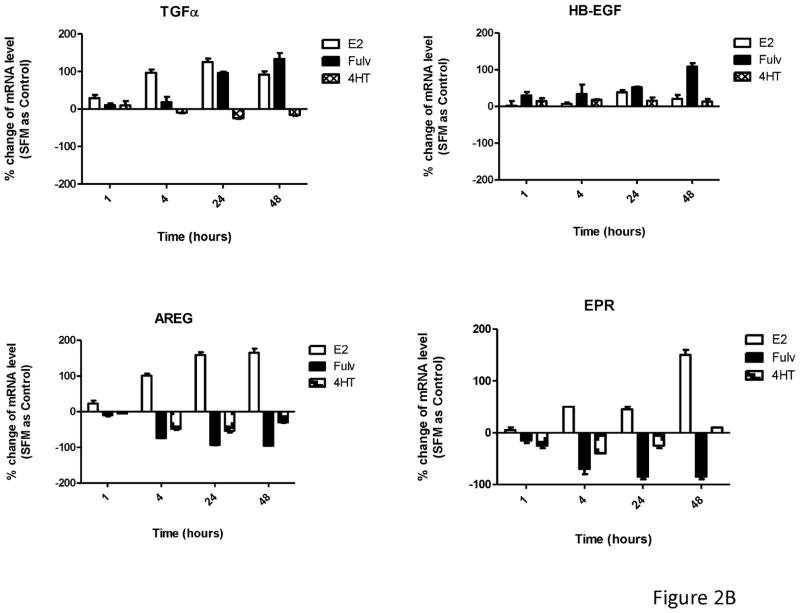
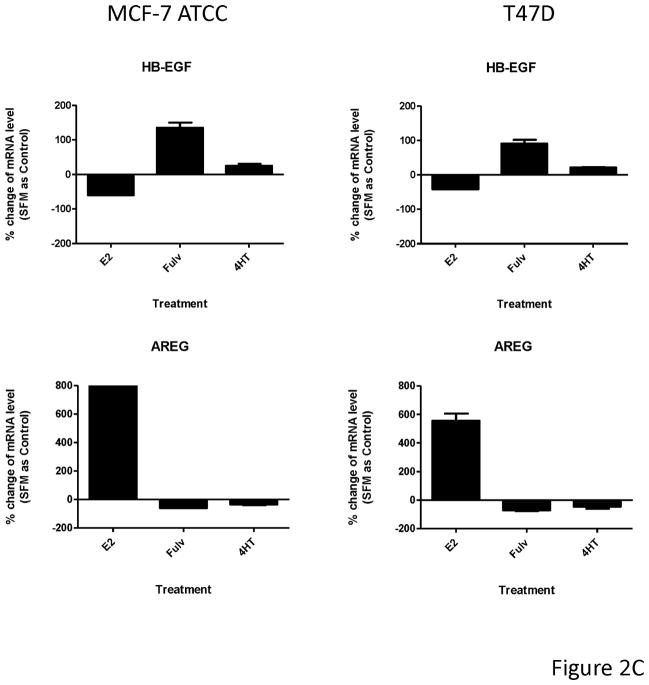
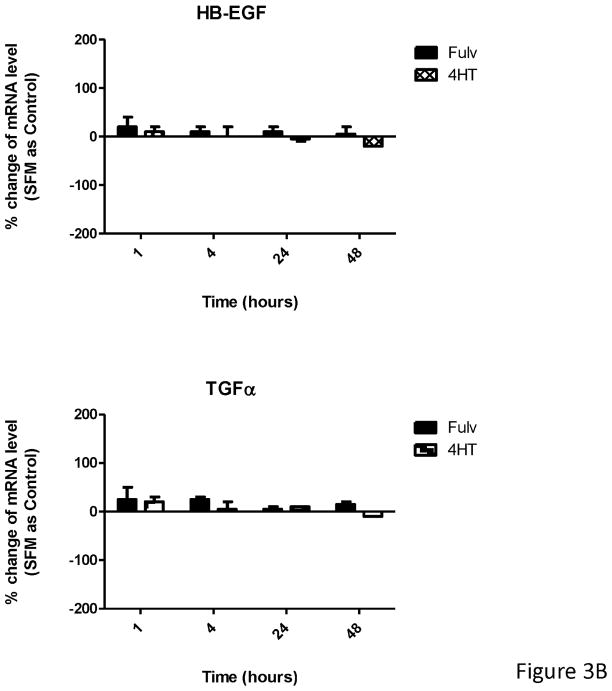
Fulvestrant regulated EGFR ligand mRNA expression. (A) MCF-7L cells were treated for 48 hours with 100 nM fulvestrant in the absence or presence of different concentrations of cycloheximide (CHX, 0.3 to 10 μg/ml), an inhibitor of translation. Cell lysates were blotted for receptor activation (PY20 and pEGFR). PARP blot was used as loading control and the indication of cell healthy status. (B) MCF-7L cells were treated with and without 10 nM E2, 100nM fulvestrant and 100 nM 4HT for 1, 4, 24, and 48 hours. qRT-PCR was performed by using specific primers for different ligands. % change of mRNA levels was shown in the figure by using SFM (without any treatment) as control. (C) MCF-7 ATCC and T47D cells were treated with and without 10 nM E2, 100nM fulvestrant and 100 nM 4HT for 48 hours. qRT-PCR was performed by using specific primers for HB-EGF and AREG ligands, respectively. % change of mRNA levels was shown in the figure by using SFM (without any treatment) as control.
To detect specific EGFR ligands, we used qRT-PCR at 1, 4, 24 and 48 hours after fulvestrant, 4HT, and E2 treatment. As shown in Figure 2B, both E2 and fulvestrant increased TGFα mRNA levels starting at 24 hours and were sustained through 48 hours. HB-EGF mRNA level was significantly increased only after 48 hours of fulvestrant treatment which correlated with the time course of EGFR activation. 4-HT did not affect mRNA levels of these ligands. In contrast, amphiregulin (AREG) and epiregulin (EPR) mRNA levels were substantially reduced 48 hours after fulvestrant treatment. A similar trend was seen for AREG and EPR mRNA levels in 4HT treated cells but to a much lesser extent. These results demonstrated that ER ligands specifically and differentially regulated EGFR ligand gene expression with fulvestrant causing upregulation of HB-EGFR and down-regulation of AREG and EPR messages. The similar observation was seen in MCF-7 ATCC and T47D cells (Figure 2C). We also examined cells for expression of epidermal growth factor, neuregulin-1, betacellulin, and epigen and saw no expression or regulation (data not shown).
Fulvestrant induced EGFR family member activation required ER
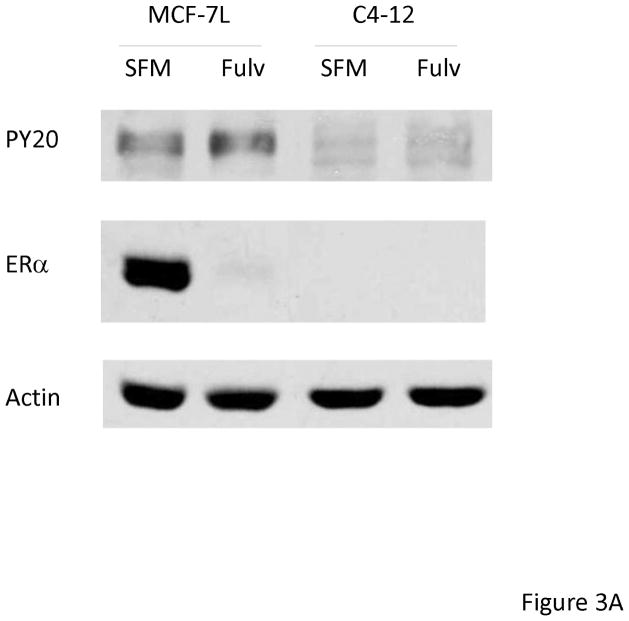
To further determine if the fulvestrant effects were ER-dependent we used an ER negative breast cancer cell line (C4-12) derived from MCF-7L cells [24]. MCF-7L and C4-12 cells were treated with and without fulvestrant for 48 hours. As shown in Figure 3A, fulvestrant did not induce EGFR phosphorylation in C4-12 cells. Next, we did qRT-PCR after 48 hours treatment of fulvestrant and 4HT. Again, HB-EGF and TGFα mRNA levels remained unchanged by both fulvestrant and 4HT (Figure 3B). These results suggested that ER is necessary and required for fulvestrant induced EGFR activation.
Figure 3.
Fulvestrant induced EGFR family member activation was ER dependent. MCF-7L and C4-12 (an ER negative cell line derived from MCF-7L cells) were starved in SFM for 24 hours, then cells were treated with (Fulv) or without (SFM) 100 nM fulvestrant for 48 hours. (A) 50 μg cell lysates were used for immunoblotting of ERα and receptor phosphorylation by using anti phosphotyrosine (PY20) antibody. Actin blot was used as loading control. (B) C4-12 cells were used for HB-EGF and TGFα mRNA quantification. % change of mRNA levels was shown in the figure by using SFM as control.
Fulvestrant induced EGFR phosphorylation required HB-EGF function
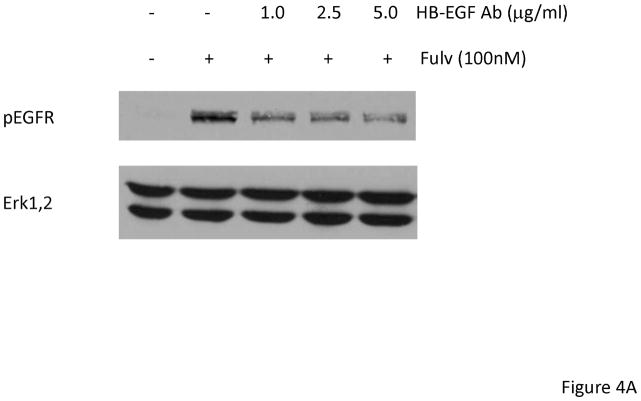
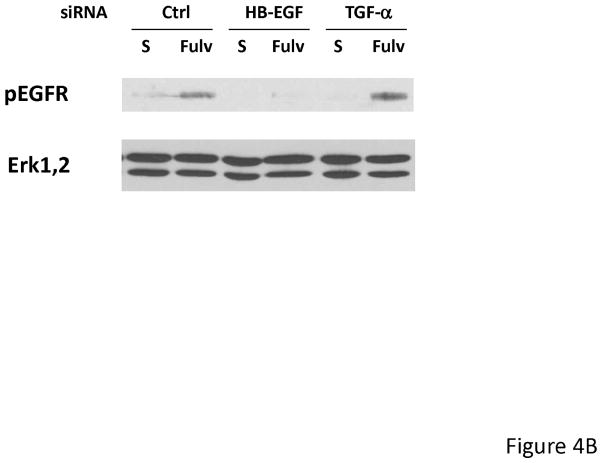
To determine a functional role for HB-EGF, MCF-7L cells were treated with 1.0 to 5.0 μg/ml neutralizing HB-EGF antibody together with fulvestrant for 24 hours. As shown in Figure 4A, EGFR phosphorylation was partially inhibited by neutralizing HB-EGF antibody. We also used HB-EGF siRNA to downregulate expression. Figure 4B shows that HB-EGF downregulation inhibited the ability to activate EGFR while TGFα siRNA did not. In addition, HB-EGF siRNA alone and in combination with fulvestrant further inhibited monolayer growth while TGFα siRNA did not (data not shown). These findings were consistent with the idea that upregulation of HB-EGF mRNA by fulvestrant resulted in stimulation of EGFR family members and decreased response to fulvestrant.
Figure 4.
Fulvestrant induced EGFR phosphorylation was partially inhibited by neutralizing HB-EGF antibody and HB-EGF siRNA. (A) MCF-7L cells were treated with 100 nM fulvestrant in the presence or absence of 1.0 to 5.0 mg/ml neutralizing HB-EGF antibody for 24 hours. 50 μg cell lysates were used to do immunoblotting of pEGFR. Total Erk1,2 blot was used as loading control. (B) MCF-7L cells were cultured in 6 well plates and transfected with 25 nM Control (Ctrl), HB-EGF and TGFα siRNA. 2 days after transfection, cells were washed and treated without 100 nM fulvestrant (Fulv) in SFM. 48 hours later, cell lysates were immunoblotted for pEGFR and total Erk1,2.
Combination treatment of fulvestrant with EGFR inhibitors erlotinib or lapatinib significantly decreased cell growth
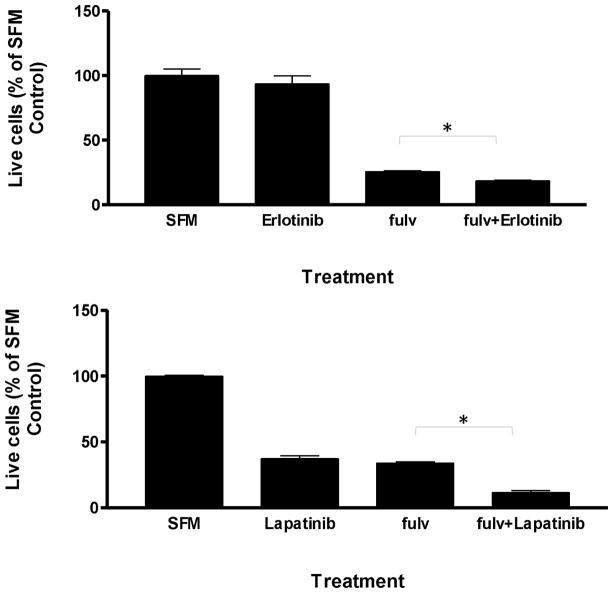
To further explore the biological role of fulvestrant induced EGFR activation in MCF-7L cell biology, we examined the effects of EGFR TKIs on cell survival. Figure 5 shows that in serum-free conditions, fulvestrant treatment reduced MCF-7 cell numbers compared to control. Erlotinib (10 μM) or lapatinib (1 μM) with fulvestrant further reduced cell numbers beyond fulvestrant alone. Therefore, while fulvestrant down regulated ER and inhibited cell growth and survival, suppression of EGFR family signaling further reduced cell numbers. Thus, EGFR family signaling might play a role in the development of fulvestrant resistance.
Figure 5.
Combination treatment of fulvestrant with erlotinib or lapatinib significantly decreased cell survival. MCF-7L cells were seeded at 1×105/well. Next day cells were starved overnight and then treated as indicated (fulvestrant 100nM, erlotinib 10 M, lapatinib 1 μM) for 3 days. Percentages of surviving cells were shown by using non-treated cells (SFM) as 100% control. * p< 0.05
ER down regulation by siRNA also induced EGFR activation and ligand expression in MCF-7L cells
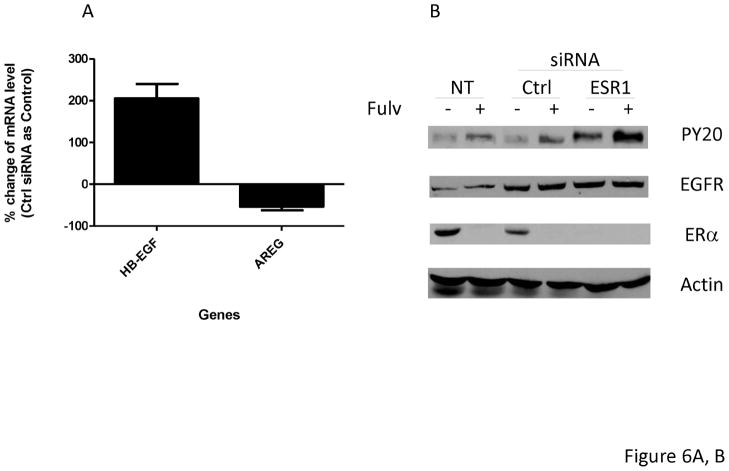
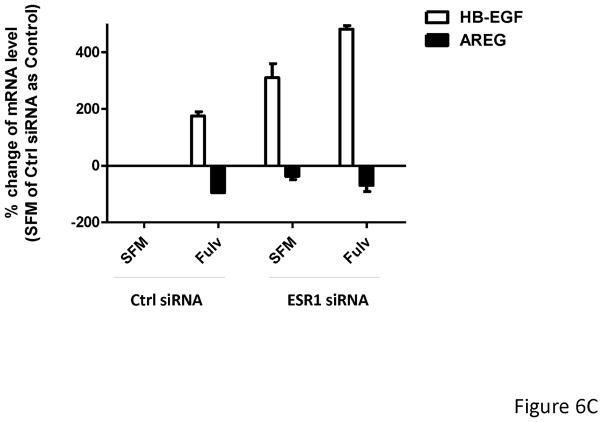
Fulvestrant down regulates ER protein levels. Since ER can function as a transcriptional activator or repressor, we decreased ER expression using ESR1 siRNA to mimic fulvestrant’s effects on ER levels. As shown in Figure 6A, similar to fulvestrant treatment, cells exposed to an ESR1 siRNA for 48 hours resulted in increased HB-EGF mRNA and decreased AREG mRNA levels in MCF-7L cells when compared to control siRNAs. EGFR phosphorylation was increased in ESR1 siRNA alone and in combination with fulvestrant (Figure 6B). As shown in Figure 6C, fulvestrant treatment upregulated HB-EGF and down regulated AREG in both control siRNA transfected (Ctrl) cells and ESR1 siRNA transfected (ESR1) MCF-7L. Increased HB-EGF mRNA levels in ESR1 transfected MCF-7L cell after fulvestrant treatment suggests down regulation of ER by siRNA and fulvestrant treatment both had effect on EGFR ligands upregulation.
Figure 6.
ERα down regulation by siRNA also induced EGFR activation and regulation of ligands in MCF-7L cells.
MCF-7L cells were transfected with 25 nM control siRNA (Ctrl) or ESR1 siRNA (ESR1) for 48 hours. Then (A) mRNA was extracted from cells. qRT-PCR was performed by using HB-EGF and AREG primers. % change of mRNA levels was shown in the figure by using Ctrl siRNA as control. (B) Transfected cells and non-transfected cells (NT) were further treated with −/+ 100 nM fulvestrant for 48 hours. Cell lysates were used for Western Blotting for PY20 (receptor activation) and total EGFR and ERα Actin blot was used as loading control. (C) All cells were treated with −/+ 100 nM of fulvestrant for 48 hours. Then qRT-PCR was performed by using HB-EGF and AREG specific primers. % change of mRNA levels was shown in the figure by using Ctrl siRNA transfected cells (SFM) as control.
Discussion
It is well documented that the existence of crosstalk between ER and EGFR in a bidirectional way in breast cancer cells via ER genomic and nongenomic functions in an estradiol dependent or independent manner. [26, 27]. Clinical and experimental [8, 11] observations suggest that breast tumors exhibit an inverse relationship between ER and EGFR expression, but detailed studies are lacking on tumor tissue from before treatment and after resistance has developed. Fulvestrant, as a pure ER antagonist, not only blocks ER function, but also rapidly degrades ER as a selective estrogen receptor degrader (SERD). Since it is well known that estradiol regulates EGFR family ligands, we evaluated the effects of fulvestrant on this growth factor system. Prolonged exposure to fulvestrant, but not estradiol or tamoxifen, resulted in activation of all EGFR family members. Total EGFR and TGFα levels remained unchanged consistent with previously published data [28, 29].
It has been reported that estradiol and SERMs including tamoxifen, raloxifene, and fulvestrant differentially regulate gene expression in breast cancer and endometrial cancer cells [30–33]. Among those genes are EGFR ligands amphiregulin (AREG) and TGFα. We have shown that fulvestrant induced activation of EGFR system (Figure 1) and conditioned media collected from fulvestrant treated cells activated EGFR in non-fulvestrant treated breast cancer cells (data not shown). These data suggested EGFR ligands were upregulated by fulvestrant and we used qRT-PCR technology to show HB-EGF and AREG were specifically regulated by fulvestrant, with an increase of HB-EGF and decrease of AREG mRNA levels in ER-positive breast cancer cells.
This effect required ER expression as downregulation of ER by siRNA blunted the fulvestrant effect. Hutcheson et al. [34] reported recently that in ER positive breast cancer cells, fulvestrant treatment sensitized cells to heregulin β1. However, we did not observe NRG1 gene expression, which may due to the gene silencing by methylation commonly observed in breast cancer cells [35]. Our data do not support a role for NRG1 in fulvestrant induced EGFR activation. Our qRT-PCR data suggested differential regulation of EGFR ligands HB-EGF and AREG by fulvestrant treatment contributed to EGFR family member activation and was dependent on ER expression. HB-EGF neutralizing antibody partially blocked EGFR phosphorylation (Figure 4) further support this notion. Results from monolayer cell growth analysis (Figure 5) suggested breast cancer cells use EGFR signaling to mainly support cell survival after loss of ER. It is notable that estradiol does not result in EGFR activation further highlighting the importance of ER downregulation in activating this signaling pathway. The time course of ER loss may also be important. The C4-12 cells, which were derived from MCF-7 and have lost ER, do not have upregulation of this signaling pathway. These cells were created by clonal selection under estrogen deprived conditions over many months [10]. Thus, acute downregulation of ER by a drug has different effects that loss of ER over time estrogen deprivation.
Preclinical data implicate EGFR system signaling as mechanism of endocrine resistance. However, clinical trials combining fulvestrant or aromatase inhibitors with EGFR tyrosine kinase inhibitor gefitinib in phase II clinical trials showed disappointing results [11, 13]. It is notable that the dose of fulvestrant used in these early trials was low compared to the recently approved dosing and schedule [6]. In addition, a lack of selection of patients with activated EGFR signaling could contribute to these negative results for the combination trials. It is likely that only a minority of patients demonstrate ER expression and EGFR activation. Our results showed that decreased levels of ER protein resulted in EGFR activation; EGFR inhibitors erlotinib and lapatinib effectively inhibited cell growth beyond that achieved by fulvestrant alone. This is consistent with the report that low ER levels, but not EGFR, correlated with benefit from gefitinib [12]. Therefore sequential or concurrent treatment of an EGFR inhibitor with fulvestrant might further enhance clinical benefit in tumors with activated EGFR signaling.
All the EGFR family members are transcriptionally repressed by estrogen [36] and in MCF-7 breast cancer cells, stably overexpressed EGFR or constitutively active HER2, Raf, or MAP/extracellular signal regulated kinase kinase, resulted in cell lines exhibiting hyperactivation of MAPK, estrogen-independent growth, and the reversible down-regulation of ERα expression [37]. As mentioned before, one of the major actions for fulvestrant is rapidly down regulating ER. Stope et al. have reported that ERα attenuates TGFβ signaling in breast cancer cells independent from agonistic and antagonistic ligands [38]. E-cadherin transcription has been reported been activated by unliganded ERα and suppressed by estrogen-activated ERα [39]. The transfection of ERα cDNA into MDA-MB-231 cells also resulted in down-regulation of Toll-like receptor-9 (TLR9) expression [40]. Furthermore, Luqmani et al [41] used siRNA to target ERα in MCF-7 human breast cancer cells and observed an array of gene expression changes. Our data support the idea that loss of unliganded ER results in changes in gene expression as ER siRNA increased EGFR phosphorylation via changes in HB-EGF without affecting total EGFR levels. These data suggested that while fulvestrant induced potent growth-inhibitory effect in ER positive breast cancer cells, it also simultaneously induced EGFR pathway through down regulation of ER and upregulation of EGFR ligands that override the initial response to fulvestrant and might ultimately result in a resistance phenotype.
In conclusion, fulvestrant as an endocrine therapy for ER positive breast cancer effectively inhibits ER dependent cell growth and cell survival with important clinical activity, but also results in enhanced EGFR signaling and EGFR ligand expression in an ER-dependent manner. As Sonne-Hansen et al reported [21], breast cancer cells can switch between ERα and EGFR signaling. SERMs effect ER-mediated gene transcription. While growth regulatory genes may be suppressed, other genes are de-repressed and their expression is induced. Some of these genes may contribute to the invasive cell phenotype as reported by Borley et al. [42]. Therefore, combined treatment against both signaling pathways could enhance the benefit of fulvestrant. Our experiments suggest that blockade of EGFR family members might affect primary or secondary resistance which could be explored directly in patients receiving fulvestrant.
Acknowledgments
This work was supported by Public Health Service grants from the NCI CA74285 and Cancer Center Support Grant P30 CA77398.
Financial Support: Public Health Service grants CA74285, CA89652, and Cancer Center Support Grant P30 CA77398
Footnotes
Conflict of Interest – The authors declare that they have no conflict of interest.
References
- 1.Barrios C, Forbes JF, Jonat W, Conte P, Gradishar W, Buzdar A, Gelmon K, Gnant M, Bonneterre J, Toi M, Hudis C, Robertson JF. The sequential use of endocrine treatment for advanced breast cancer: where are we? Ann Oncol. 2012;23:1378–1386. doi: 10.1093/annonc/mdr593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali S, Buluwela L, Coombes RC. Antiestrogens and their therapeutic applications in breast cancer and other diseases. Annu Rev Med. 2011;62:217–232. doi: 10.1146/annurev-med-052209-100305. [DOI] [PubMed] [Google Scholar]
- 3.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 4.Pink JJ, Jordan VC. Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Research. 1996;56:2321–2330. [PubMed] [Google Scholar]
- 5.Howell A, Robertson JF, Abram P, Lichinitser MR, Elledge R, Bajetta E, Watanabe T, Morris C, Webster A, Dimery I, Osborne CK. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22:1605–1613. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 6.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, Verhoeven D, Pedrini JL, Smirnova I, Lichinitser MR, Pendergrass K, Garnett S, Lindemann JP, Sapunar F, Martin M. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 7.Dowsett M, Cuzick J, Wale C, Howell T, Houghton J, Baum M. Retrospective analysis of time to recurrence in the ATAC trial according to hormone receptor status: an hypothesis-generating study. J Clin Oncol. 2005;23:7512–7517. doi: 10.1200/JCO.2005.01.4829. [DOI] [PubMed] [Google Scholar]
- 8.Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004;90(Suppl 1):S2–6. doi: 10.1038/sj.bjc.6601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osipo C, Meeke K, Cheng D, Weichel A, Bertucci A, Liu H, Jordan VC. Role for HER2/neu and HER3 in fulvestrant-resistant breast cancer. Int J Oncol. 2007;30:509–520. [PubMed] [Google Scholar]
- 10.Emde A, Mahlknecht G, Maslak K, Ribba B, Sela M, Possinger K, Yarden Y. Simultaneous Inhibition of Estrogen Receptor and the HER2 Pathway in Breast Cancer: Effects of HER2 Abundance. Transl Oncol. 2011;4:293–300. doi: 10.1593/tlo.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith IE, Walsh G, Skene A, Llombart A, Mayordomo JI, Detre S, Salter J, Clark E, Magill P, Dowsett M. A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007;25:3816–3822. doi: 10.1200/JCO.2006.09.6578. [DOI] [PubMed] [Google Scholar]
- 12.Osborne CK, Neven P, Dirix LY, Mackey JR, Robert J, Underhill C, Schiff R, Gutierrez C, Migliaccio I, Anagnostou VK, Rimm DL, Magill P, Sellers M. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011;17:1147–1159. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson RW, O’Neill A, Vidaurre T, Gomez HL, Badve SS, Sledge GW. A randomized trial of combination anastrozole plus gefitinib and of combination fulvestrant plus gefitinib in the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-1997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 15.Kataoka H. EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. J Dermatol Sci. 2009;56:148–153. doi: 10.1016/j.jdermsci.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 17.McClelland RA, Barrow D, Madden TA, Dutkowski CM, Pamment J, Knowlden JM, Gee JM, Nicholson RI. Enhanced epidermal growth factor receptor signaling in MCF7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ICI 182,780 (Faslodex) Endocrinology. 2001;142:2776–2788. doi: 10.1210/endo.142.7.8259. [DOI] [PubMed] [Google Scholar]
- 18.Massarweh S, Osborne CK, Jiang S, Wakeling AE, Rimawi M, Mohsin SK, Hilsenbeck S, Schiff R. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006;66:8266–8273. doi: 10.1158/0008-5472.CAN-05-4045. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Cheng D, Weichel AK, Osipo C, Wing LK, Chen B, Louis TE, Jordan VC. Cooperative effect of gefitinib and fumitremorgin c on cell growth and chemosensitivity in estrogen receptor alpha negative fulvestrant-resistant MCF-7 cells. Int J Oncol. 2006;29:1237–1246. [PubMed] [Google Scholar]
- 20.Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E, Laenkholm AV, Rasmussen LM, Riese DJ, 2nd, de Cremoux P, Stenvang J, Lykkesfeldt AE. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat. 2009;114:263–275. doi: 10.1007/s10549-008-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonne-Hansen K, Norrie IC, Emdal KB, Benjaminsen RV, Frogne T, Christiansen IJ, Kirkegaard T, Lykkesfeldt AE. Breast cancer cells can switch between estrogen receptor alpha and ErbB signaling and combined treatment against both signaling pathways postpones development of resistance. Breast Cancer Res Treat. 2010;121:601–613. doi: 10.1007/s10549-009-0506-y. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Kamaraju S, Hakuno F, Kabuta T, Takahashi S, Sachdev D, Yee D. Motility response to insulin-like growth factor-I (IGF-I) in MCF-7 cells is associated with IRS-2 activation and integrin expression. Breast Cancer Res Treat. 2004;83:161–170. doi: 10.1023/b:brea.0000010709.31256.c6. [DOI] [PubMed] [Google Scholar]
- 23.Byron SA, Horwitz KB, Richer JK, Lange CA, Zhang X, Yee D. Insulin receptor substrates mediate distinct biological responses to insulin-like growth factor receptor activation in breast cancer cells. Br J Cancer. 2006;95:1220–1228. doi: 10.1038/sj.bjc.6603354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oesterreich S, Zhang P, Guler RL, Sun X, Curran EM, Welshons WV, Osborne CK, Lee AV. Re-expression of estrogen receptor alpha in estrogen receptor alpha- negative MCF-7 cells restores both estrogen and insulin-like growth factor-mediated signaling and growth. Cancer Res. 2001;61:5771–5777. [PubMed] [Google Scholar]
- 25.Ghayad SE, Cohen PA. Inhibitors of the PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent Pat Anticancer Drug Discov. 2010;5:29–57. doi: 10.2174/157489210789702208. [DOI] [PubMed] [Google Scholar]
- 26.Schiff R, Osborne CK. Endocrinology and hormone therapy in breast cancer: new insight into estrogen receptor-alpha function and its implication for endocrine therapy resistance in breast cancer. Breast Cancer Res. 2005;7:205–211. doi: 10.1186/bcr1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the Estrogen Receptor and the HER Tyrosine Kinase Receptor Family: Molecular Mechanism and Clinical Implications for Endocrine Therapy Resistance. Endocr Rev. 2008;29:217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen SS, Egeblad M, Jaattela M, Lykkesfeldt AE. Acquired antiestrogen resistance in MCF-7 human breast cancer sublines is not accomplished by altered expression of receptors in the ErbB-family. Breast Cancer Res Treat. 1999;58:41–56. doi: 10.1023/a:1006232830161. [DOI] [PubMed] [Google Scholar]
- 29.McClelland RA, Gee JM, Francis AB, Robertson JF, Blamey RW, Wakeling AE, Nicholson RI. Short-term effects of pure anti-oestrogen ICI 182780 treatment on oestrogen receptor, epidermal growth factor receptor and transforming growth factor-alpha protein expression in human breast cancer. Eur J Cancer. 1996;32A:413–416. doi: 10.1016/0959-8049(95)00517-x. [DOI] [PubMed] [Google Scholar]
- 30.Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 2004;64:1522–1533. doi: 10.1158/0008-5472.can-03-3326. [DOI] [PubMed] [Google Scholar]
- 31.Tee MK, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell. 2004;15:1262–1272. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vendrell JA, Magnino F, Danis E, Duchesne MJ, Pinloche S, Pons M, Birnbaum D, Nguyen C, Theillet C, Cohen PA. Estrogen regulation in human breast cancer cells of new downstream gene targets involved in estrogen metabolism, cell proliferation and cell transformation. J Mol Endocrinol. 2004;32:397–414. doi: 10.1677/jme.0.0320397. [DOI] [PubMed] [Google Scholar]
- 33.Gielen SC, Burger CW, Kuhne LC, Hanifi-Moghaddam P, Blok LJ. Analysis of estrogen agonism and antagonism of tamoxifen, raloxifene, and ICI182780 in endometrial cancer cells: a putative role for the epidermal growth factor receptor ligand amphiregulin. J Soc Gynecol Investig. 2005;12:e55–67. doi: 10.1016/j.jsgi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Hutcheson IR, Goddard L, Barrow D, McClelland RA, Francies HE, Knowlden JM, Nicholson RI, Gee JM. Fulvestrant-induced expression of ErbB3 and ErbB4 receptors sensitizes oestrogen receptor-positive breast cancer cells to heregulin beta1. Breast Cancer Res. 2011;13:R29. doi: 10.1186/bcr2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chua YL, Ito Y, Pole JC, Newman S, Chin SF, Stein RC, Ellis IO, Caldas C, O’Hare MJ, Murrell A, Edwards PA. The NRG1 gene is frequently silenced by methylation in breast cancers and is a strong candidate for the 8p tumour suppressor gene. Oncogene. 2009;28:4041–4052. doi: 10.1038/onc.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revillion F, Pawlowski V, Lhotellier V, Louchez MM, Peyrat JP. mRNA expression of the type I growth factor receptors in the human breast cancer cells MCF-7: regulation by estradiol and tamoxifen. Anticancer Res. 2003;23:1455–1460. [PubMed] [Google Scholar]
- 37.Creighton CJ, Hilger AM, Murthy S, Rae JM, Chinnaiyan AM, El-Ashry D. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res. 2006;66:3903–3911. doi: 10.1158/0008-5472.CAN-05-4363. [DOI] [PubMed] [Google Scholar]
- 38.Stope MB, Popp SL, Knabbe C, Buck MB. Estrogen receptor alpha attenuates transforming growth factor-beta signaling in breast cancer cells independent from agonistic and antagonistic ligands. Breast Cancer Res Treat. 2010;120:357–367. doi: 10.1007/s10549-009-0393-2. [DOI] [PubMed] [Google Scholar]
- 39.Cardamone MD, Bardella C, Gutierrez A, Di Croce L, Rosenfeld MG, Di Renzo MF, De Bortoli M. ERalpha as ligand-independent activator of CDH-1 regulates determination and maintenance of epithelial morphology in breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:7420–7425. doi: 10.1073/pnas.0903033106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandholm J, Kauppila JH, Pressey C, Tuomela J, Jukkola-Vuorinen A, Vaarala M, Johnson MR, Harris KW, Selander KS. Estrogen receptor-alpha and sex steroid hormones regulate Toll-like receptor-9 expression and invasive function in human breast cancer cells. Breast Cancer Res Treat. 2012;132:411–419. doi: 10.1007/s10549-011-1590-3. [DOI] [PubMed] [Google Scholar]
- 41.Luqmani YA, Al Azmi A, Al Bader M, Abraham G, El Zawahri M. Modification of gene expression induced by siRNA targeting of estrogen receptor alpha in MCF7 human breast cancer cells. Int J Oncol. 2009;34:231–242. [PubMed] [Google Scholar]
- 42.Borley AC, Hiscox S, Gee J, Smith C, Shaw V, Barrett-Lee P, Nicholson RI. Anti-oestrogens but not oestrogen deprivation promote cellular invasion in intercellular adhesion-deficient breast cancer cells. Breast Cancer Res. 2008;10:R103. doi: 10.1186/bcr2206. [DOI] [PMC free article] [PubMed] [Google Scholar]