Abstract
Pacemaker neurons in neonatal spinal nociceptive circuits generate intrinsic burst firing and are distinguished by a lower “leak” membrane conductance compared with adjacent nonbursting neurons. However, little is known about which subtypes of leak channels regulate the level of pacemaker activity within the developing rat superficial dorsal horn (SDH). Here we demonstrate that a hallmark feature of lamina I pacemaker neurons is a reduced conductance through inward-rectifying potassium (Kir) channels at physiological membrane potentials. Differences in the strength of inward rectification between pacemakers and nonpacemakers indicate the presence of functionally distinct Kir currents in these two populations at room temperature. However, Kir currents in both groups showed high sensitivity to block by extracellular Ba2+ (IC50 ∼ 10 μm), which suggests the presence of “classical” Kir (Kir2.x) channels in the neonatal SDH. The reduced Kir conductance within pacemakers is unlikely to be explained by an absence of particular Kir2.x isoforms, as immunohistochemical analysis revealed the expression of Kir2.1, Kir2.2, and Kir2.3 within spontaneously bursting neurons. Importantly, Ba2+ application unmasked rhythmic burst firing in ∼42% of nonbursting lamina I neurons, suggesting that pacemaker activity is a latent property of a sizeable population of SDH cells during early life. In addition, the prevalence of spontaneous burst firing within lamina I was enhanced in the presence of high internal concentrations of free Mg2+, consistent with its documented ability to block Kir channels from the intracellular side. Collectively, the results indicate that Kir channels are key modulators of pacemaker activity in newborn central pain networks.
Introduction
Spontaneous activity is essential for the proper maturation of neuronal circuits in the CNS, via its wide-ranging effects on neurotransmitter phenotype (Borodinsky et al., 2004), axonal pathfinding (Hanson et al., 2008), synapse formation (Gonzalez-Islas and Wenner, 2006), and the synchronization of firing across networks (Tritsch et al., 2007). Spontaneous network activity during early development can reflect a variety of underlying mechanisms, such as transient synaptic connections, gap junction coupling, GABAergic depolarizations, and the presence of pacemaker neurons (Blankenship and Feller, 2010), which have been previously defined as cells exhibiting intrinsic, oscillatory burst firing (Ramirez et al., 2004).
Within the neonatal spinal cord, pacemaker-like cells have been described in the ventral horn (Tazerart et al., 2007, 2008), where they are proposed to contribute to rhythmogenesis in locomotor networks (Brocard et al., 2010). Pacemaker neurons were also recently identified within lamina I of the newborn superficial dorsal horn (SDH) (Li and Baccei, 2011), which receives direct projections from nociceptive Aδ- and C-fiber sensory neurons (Light and Perl, 1979; Sugiura et al., 1986) and represents a key component of the ascending pain pathway. Pacemakers constitute ∼25–30% of the lamina I population during the first days of life (Li and Baccei, 2011) and are similar to intrinsically bursting neurons in other regions of the CNS (Del Negro et al., 2002) in that they are distinguished by a high ratio of persistent, voltage-gated Na+ conductance to “leak” membrane conductance. This has been attributed to a significantly higher membrane resistance compared with adjacent nonbursting lamina I neurons (Li and Baccei, 2011). Leak conductance potently regulates neuronal excitability across the CNS and can result from any channel being open at the resting membrane potential (Goldstein et al., 2001). This raises the possibility that altered leak channel function and/or expression may predispose a subset of neurons to function as pacemakers within the immature SDH.
Inward-rectifying potassium (Kir) channels are strong candidates to modulate intrinsic burst firing within the newborn spinal cord. Low Kir conductance in neurons can drive spontaneous firing by depolarizing the membrane potential above the threshold to recruit persistent voltage-gated Na+ currents (Leao et al., 2012), which are essential for rhythmic bursting within lamina I (Li and Baccei, 2011). In addition, the block of Kir currents by metabotropic inputs unmasks endogenous burst firing in the majority of deep dorsal horn neurons from the adult spinal cord (Derjean et al., 2003). Unfortunately, little is known about the underlying basis for leak membrane conductance in developing SDH neurons. As a result, the degree to which Kir channels regulate spontaneous pacemaker activity within newborn spinal pain circuits remains unknown.
Here we demonstrate that a reduced conductance through “classical” Kir channels near physiological potentials is a critical determinant of intrinsic burst firing in spinal lamina I neurons during early life. This implies that the number of functional pacemaker neurons within the developing SDH network is not constant. It is instead dependent on Kir function, which may be modulated by both the extracellular and the intracellular environments.
Materials and Methods
All experiments adhered to animal welfare guidelines established by the University of Cincinnati Institutional Animal Care and Use Committee.
Preparation of spinal cord slices.
Sprague Dawley rats of either sex were deeply anesthetized with sodium pentobarbital (30 mg/kg) at postnatal days 2 (P2) to P5 and perfused with ice-cold dissection solution consisting of (in mm) 250 sucrose, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 6 MgCl2, 0.5 CaCl2, and 25 glucose, continuously bubbled with 95% O2/5% CO2. The lumbar spinal cord (L2–L6) was isolated and immersed in low-melting-point agarose (3% in the above solution; Invitrogen), and parasagittal slices (350–400 μm) were cut using a Vibroslice tissue slicer (HA-752; Campden Instruments). The slices were placed in a chamber filled with oxygenated dissection solution for 30 min and allowed to recover in an oxygenated artificial CSF (aCSF) solution containing (in mm) 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 1.0 MgCl2, 2.0 CaCl2, and 25 glucose, pH 7.2, for ≥1 h at room temperature.
Patch-clamp recordings.
After recovery, slices were transferred to a submersion-type recording chamber (RC-22; Warner Instruments) and mounted on the stage of an upright microscope (BX51WI; Olympus). Slices were then perfused at room temperature with oxygenated aCSF at a rate of 1.5–3 ml/min.
Patch electrodes were constructed from thin-walled, single-filamented borosilicate glass (1.5 mm outer diameter; World Precision Instruments) using a microelectrode puller (P-97; Sutter Instruments). Pipette resistances ranged from 4 to 6 MΩ, and seal resistances were >1 GΩ. In the majority of experiments, patch electrodes were filled with a solution containing the following (in mm): 130 K-gluconate, 10 KCl, 10 HEPES, 10 Na-phosphocreatine, 4 MgATP, and 0.3 Na2-GTP, pH 7.2 (305 mOsm). To determine the extent to which bursting activity within lamina I depended on the intracellular levels of free Mg2+, some experiments used either a “high-Mg2+” internal solution [consisting of (in mm) 130 K-gluconate,10 KCl, 10 HEPES, 10 Na-phosphocreatine, 0.3 Na2-GTP, 1.45 K2ATP, and 2.55 MgCl2] or a “low-Mg2+” solution [composed of (in mm) 90 K-gluconate,10 KCl, 10 HEPES, 10 Na-phosphocreatine, 0.3 Na2-GTP, 21.6 K2ATP, and 1.56 MgCl2]. Using a Mg-ATP calculator (version 1.3; MaxChelator) (Schoenmakers et al., 1992), the solutions were designed to produce free [Mg2+]internal of 1 mm (high Mg2+) or 10 μm (low Mg2+), while maintaining a constant [Mg-ATP]internal of 1.55 mm to minimize potential differences in ion channel phosphorylation and “rundown” between intracellular solutions (Pearson and Dolphin, 1993).
Dorsal horn neurons were visualized with infrared differential interference contrast, and patch-clamp recordings were obtained from L4–L5 cells located across the mediolateral extent of the spinal cord using a Multiclamp 700B amplifier (Molecular Devices). Sampled cells were categorized as lamina I neurons if they resided within 40 μm of the edge of the dorsal white matter (Lorenzo et al., 2008). Approximately 1 min after establishment of the whole-cell configuration, the spontaneous firing patterns of dorsal horn neurons were classified at the resting membrane potential (Vrest) as “silent,” “irregular,” “tonic,” or “bursting” (Li and Baccei, 2011). Membrane resistance (Rm) was measured using the hyperpolarization produced by negative current injections (5–10 pA) from Vrest.
Inward-rectifying K+ (Kir) currents were isolated as described previously (Derjean et al., 2003). Briefly, neurons were voltage clamped at −55 mV in the presence of 10 μm NBQX, 25 μm AP-5, 10 μm gabazine, and 0.5 μm strychnine to block fast synaptic transmission in the slice. Negative voltage ramps (from −55 to −155 mV) were applied at a rate of 0.2 mV/ms. BaCl2 (0.2–200 μm) was bath applied to block Kir (Coetzee et al., 1999), and the Ba2+-sensitive component of the current was subsequently isolated via electronic subtraction (see Fig. 2). Conductance (gBa-sensitive) was calculated as g = I/(Vm − Erev), at two different membrane potentials that were equidistant (25 mV) from the reversal potential (Derjean et al., 2003). To estimate the degree of Kir inward rectification, a ratio of these two conductances was calculated as follows: g(E-25)/g(E+25). The potential contribution of constitutively active G-protein-coupled Kir channels (GIRKs) to the above ramp currents was investigated by bath-applying the selective GIRK antagonist tertiapin-Q (100 nm). Bovine serum albumin (0.1 mg/ml) was included in the solution to reduce nonspecific binding of the peptide to the perfusion tubing. At this concentration, tertiapin-Q was found to partially block the outward currents evoked by the GABAB receptor (GABABR) agonist baclofen (data not shown), confirming that the peptide adequately penetrated the slice under our experimental conditions. To examine the expression of hyperpolarization-activated cation currents (Ih) in lamina I neurons, hyperpolarizing voltage steps (from −70 to −150 mV in 10 mV increments) were delivered from a holding potential of −60 mV. Alternatively, in current-clamp mode, hyperpolarizing current steps (0–20 pA) were administered from the resting potential to detect the presence of a depolarizing “sag” in the membrane potential region, which is characteristic of Ih expression (Yoshimura and Jessell, 1989).
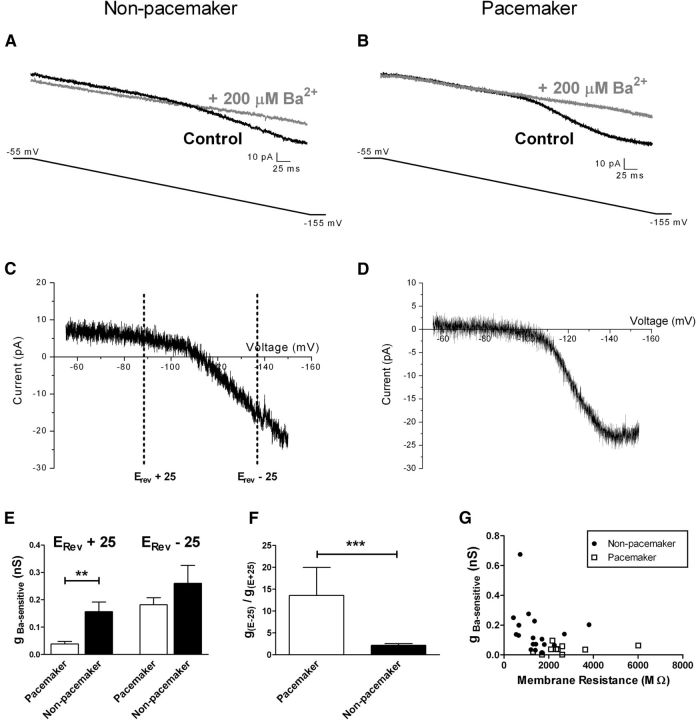
Figure 2.
Low Kir conductance in spontaneously bursting neurons within the newborn SDH. A, Representative currents recorded in nonpacemaker neurons during negative voltage ramps from a holding potential of −55 to −155 mV before (control; black) and after (gray) the bath application of 200 μm BaCl2. The illustrated example corresponds to a silent lamina I neuron at P3. B, Representative traces obtained using the same protocol as in A, administered to a lamina I pacemaker neuron. Note the overlap in traces during the early portion of the negative voltage ramp. C, D, Example of Ba2+-sensitive currents obtained by electronic subtraction (black minus gray) plotted as a function of membrane voltage for nonpacemaker (C) and pacemaker (D) neurons within lamina I of the neonatal spinal cord. Inward rectification was observed in all cases as the slope was greater at potentials negative to the observed reversal potential (Erev). C includes representative voltages (equidistant from Erev) used to calculate the Ba2+-sensitive conductance (gBa-sensitive). E, Bursting lamina I neurons possessed significantly lower gBa-sensitive compared with adjacent nonbursting cells at potentials positive to Erev (**p = 0.002, Mann–Whitney test; left) but not at potentials more negative than Erev (p = 0.981; right). F, The degree of inward rectification of Ba2+-sensitive K+ currents [measured as g(Erev -25)/g(Erev +25)] was significantly greater in the population of pacemaker neurons within lamina I (***p = 0.0007, Mann–Whitney test). G, Across the general population of neonatal lamina I neurons, there was a significant inverse correlation between the level of Ba2+-sensitive conductance and the membrane resistance (r = −0.398; p = 0.036; Spearman's test), suggesting an important contribution of gBa-sensitive to resting leak conductance within these cells.
Membrane voltages were adjusted for liquid junction potentials (∼14 mV) calculated using JPCalc software (P. Barry, University of New South Wales, Sydney, Australia; modified for Molecular Devices) unless otherwise specified. Currents were filtered at 4–6 kHz through a −3 dB, four-pole low-pass Bessel filter; digitally sampled at 20 kHz; and stored on a personal computer (ICT) using a commercially available data acquisition system (Digidata 1440A with pClamp 10.2 software; Molecular Devices).
Biocytin staining and immunohistochemistry.
Rat pups (P1–P5) of either sex were deeply anesthetized with sodium pentobarbital (30 mg/kg, i.p.), perfused with aCSF solution, and decapitated. The lumbar spinal cord was isolated with dura and pia matter removed from the dorsal surface. The intact cord was transferred to the recording chamber and perfused at room temperature with oxygenated aCSF at 1.5–3 ml/min. Whole-cell patch-clamp recordings were obtained from lamina I neurons under infrared LED illumination (Safronov et al., 2007; Szucs et al., 2009) using the standard K-gluconate intracellular solution (see above) with the addition of 0.3% biocytin. Following the classification of spontaneous firing pattern (Fig. 1), nonbursting neurons were removed from the spinal cord under visual control via strong negative pressure applied to the electrode, thus ensuring that cells later identified with biocytin staining corresponded to pacemakers. Meanwhile, neurons exhibiting spontaneous burst firing were dialyzed with biocytin for 30–40 min. Approximately two to six pacemaker neurons were labeled in this manner per spinal cord.
Figure 1.
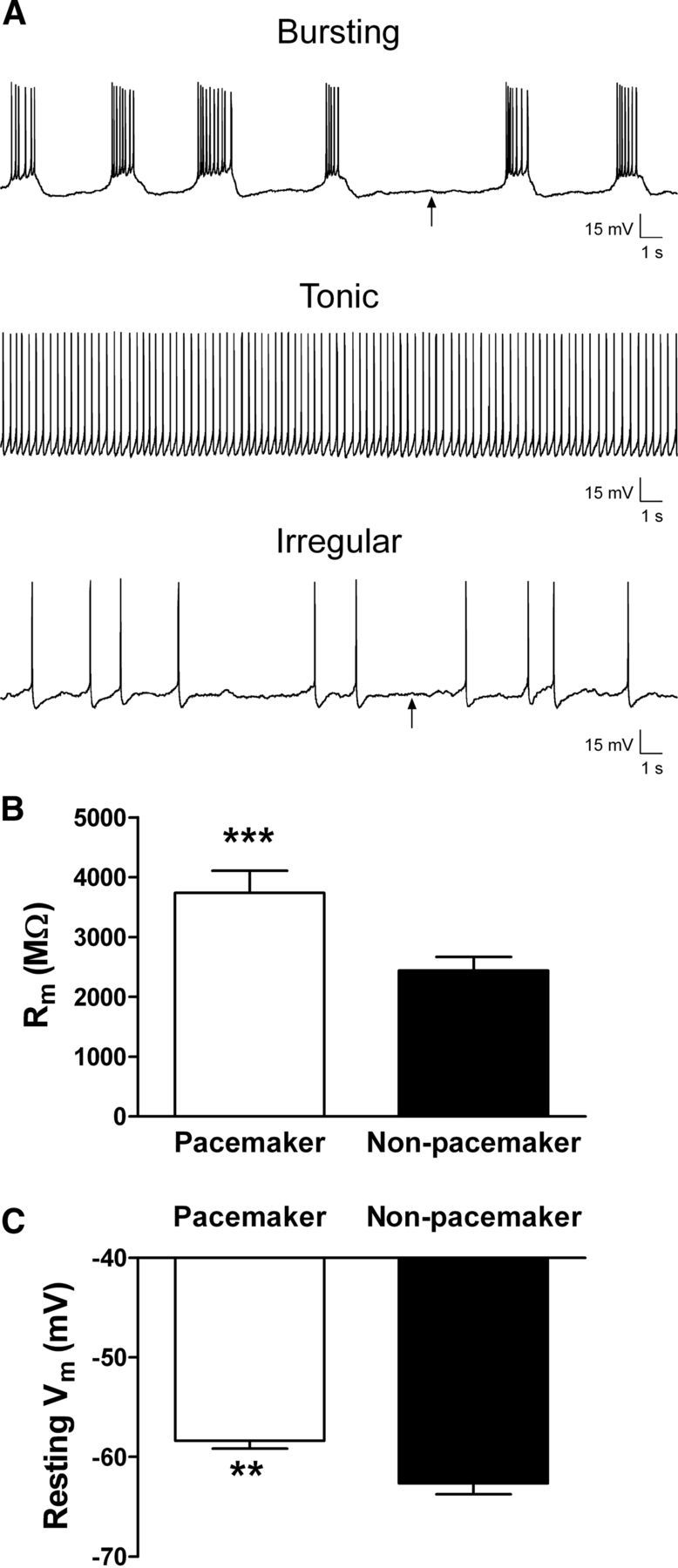
Lamina I pacemaker neurons are distinguished by high membrane resistance and depolarized resting potentials. A, Spontaneous firing patterns were classified as rhythmic bursting (top), tonic (middle), irregular (bottom), or silent (data not shown). Arrows indicate approximate regions used to measure the resting membrane potential in a spontaneously active neuron. B, Membrane resistance (Rm) was significantly higher in the pacemaker population of lamina I neurons compared with adjacent nonbursting neurons (***p = 0.0001, Mann–Whitney test). C, Pacemaker neurons also exhibited a significantly more depolarized resting potential (n = 15) compared with nonpacemaker neurons (n = 22; **p = 0.007, Mann–Whitney test). Tonically firing neurons were excluded from the analysis because of difficulties in accurately measuring their resting potential.
The spinal cords were then fixed overnight at 4°C in 4% paraformaldehyde, followed by cryoprotection in 30% sucrose in 0.1 m PBS overnight. Cords were rapidly frozen, and parasagittal sections were cut at 10 μm on a cryostat, mounted onto Superfrost Plus slides (Fisher Scientific), and left to dry overnight on a slide warmer at 39°C. Sections were washed three times for 5 min in 0.1 m PBS, permeabilized with 0.3% Tween 20 in 0.1 m PBS for 10 min, and incubated with avidin conjugated to Rhodamine Red (1:1000; Invitrogen) for 2 h at room temperature. The slides were then washed an additional three times for 5 min in 0.1 m PBS and loosely coverslipped with an excess of Vectashield mounting medium (Vector Laboratories), and biocytin-filled neurons were visualized on a Microshot-SA fluorescence microscope (Nikon). Slides containing labeled neurons were then subjected to immunostaining for Kir2 channels as described below.
Slides containing labeled cells (or those selected for peptide preabsorption control experiments) were additionally washed six times for 10 min and blocked in 10% normal goat serum/0.3% Tween 20 in 0.1 m PBS for 1 h. Primary antibodies raised in rabbit against Kir2.1 or Kir2.3 (1:200; Alomone Labs) or Kir2.2 (1:250; Epitomics) were applied in dilution buffer containing 2% normal goat serum, 0.06% Tween 20, and 0.1 m PBS and incubated for 48 h at 4°C. For each antibody used, a preabsorption control was prepared by incubating an undiluted antibody with its corresponding peptide (Alomone Labs) in a 1:5 to 1:10 ratio for 1 h before antibody dilution. Tissue was then washed three times for 5 min in 0.3% Tween 20 in 0.1 m PBS and incubated for 2 h at room temperature in a goat anti-rabbit secondary antibody conjugated to Alexa 488 (1:1000) diluted in 2% normal goat serum in 0.1 m PBS. Sections were washed six times for 5 min and coverslipped with Vectashield. Images were acquired on a LSM510 inverted confocal microscope (Zeiss) with a 63× Real Zoom oil-immersion objective and processed in Zen imaging software. Cells were imaged at an optical thickness of 0.5 μm to visualize the presence of Kir protein located on the cell surface. Cells were determined to express Kir2.1, Kir2.2, or Kir2.3 protein if Alexa 488-positive puncta colocalized with avidin-conjugated rhodamine fluorescence.
Data analysis and statistics.
Electrophysiological data were analyzed using Clampfit (Molecular Devices) and Origin (OriginLab Corporation) software. Neurons were classified as bursting (i.e., pacemakers) if they exhibited slow oscillations in membrane potential that gave rise to multiple action potentials (Li and Baccei, 2011). Nonparametric tests were used in cases in which the distribution of data failed the D'Agostino & Pearson normality test (Prism; GraphPad Software) or when the number of observations was insufficient (n < 24) to definitively conclude that data were distributed in a Gaussian manner. p < 0.05 was considered significant. n refers to the number of neurons sampled in a given group. Data are expressed as means ± SEM.
Results
Pacemaker neurons are distinguished by low conductance through Ba2+-sensitive Kir channels
To identify which subtypes of leak conductance are important for regulating pacemaker activity in the newborn superficial dorsal horn, we obtained in vitro whole-cell patch-clamp recordings from lamina I neurons in rat spinal cord slices prepared at P2–P5. The pattern of spontaneous activity at room temperature was classified as bursting, tonic, irregular (Fig. 1A) or silent (data not shown), as described previously (Li and Baccei, 2011). The observed rhythmic burst firing was previously shown to reflect the intrinsic membrane properties of the lamina I neuron, thus constituting “pacemaker” activity within the superficial dorsal horn (Li and Baccei, 2011). Pacemaker neurons exhibited a significantly higher membrane resistance (i.e., lower resting leak conductance) compared with adjacent nonbursting lamina I neurons (n = 33–40 in each group; p = 0.0001, Mann–Whitney test; Fig. 1B). This was accompanied by a significantly more depolarized resting potential (Vrest) within the pacemaker population (p = 0.007, Mann–Whitney test; Fig. 1C). Since membrane resistance in SDH neurons decreases with elevated temperature (Graham et al., 2008), we obtained additional recordings from neonatal lamina I neurons at 32–34°C and found that 9 of the 20 neurons sampled (45%) exhibited spontaneous pacemaker activity. Critically, this demonstrates that the intrinsic burst firing is not an artifact related to recording at room temperature.
Kir channels are known to be important for the maintenance of Vrest in both neurons and muscle through their contribution to the leak membrane conductance (Hibino et al., 2010). Therefore, we hypothesized that the distinct passive membrane properties (Fig. 1B,C) of pacemaker neurons reflect, at least in part, a reduced Kir conductance near the resting potential. After the classification of the spontaneous firing pattern, voltage-clamp experiments were performed in which lamina I neurons were hyperpolarized from −55 to −155 mV (at a rate of 0.2 mV/ms) in the presence of a mixture of antagonists to block fast synaptic transmission in the slice (see Materials and Methods). BaCl2 (200 μm) was subsequently added to the bath. Electronic subtraction of the Ba2+-sensitive component revealed a current with a mean reversal potential (Erev) of −97.7 ± 2.5 mV (n = 28), which was close to the predicted equilibrium potential for K+ ions under our experimental conditions (−101.4 mV). Measurements of conductance (see Materials and Methods) at two potentials equidistant from Erev (Fig. 2C) (Derjean et al., 2003) consistently demonstrated inward rectification, as evidenced by a higher conductance at more negative membrane potentials (Vm), which is consistent with the isolation of Kir current using this protocol.
The effects of extracellular Ba2+ application on the current evoked by the negative voltage ramp clearly differed between pacemaker and nonpacemaker neurons within lamina I of the neonatal spinal cord. In nonpacemakers, Ba2+ blocked a portion of the recorded current throughout the voltage ramp, thus lowering the overall slope of the observed current (Fig. 2A,C). Meanwhile, in pacemaker neurons from the same slices, Ba2+ evoked only a minimal block of the current between −55 and −85 mV (Fig. 2B,D), suggesting a reduced Kir conductance in this population at these physiological membrane potentials. Indeed, the conductance of the Ba2+-sensitive component (gBa-sensitive) was significantly lower in the bursting group (0.039 ± 0.009 nS; n = 10) compared with nonbursting lamina I neurons (0.156 ± 0.036 nS; n = 18; p = 0.002, Mann–Whitney test; Fig. 2E, left) at Vm positive to the reversal potential (i.e., Erev + 25). However, the level of gBa-sensitive was not statistically different between groups when measured at more negative potentials (i.e., Erev − 25; p = 0.981; Fig. 2E, right). As a result, Kir currents exhibited a greater degree of inward rectification within the pacemaker population, as the ratio of gBa-sensitive measured at the more negative Vm relative to that measured at the positive Vm was significantly elevated in the bursting neurons (p = 0.0007, Mann–Whitney; Fig. 2F). These data indicate that the lower gBa-sensitive that characterizes pacemakers at physiological membrane potentials cannot be simply explained by a lower number of Kir channels and in turn suggests that bursting neurons express distinct Kir currents compared with adjacent nonbursting neurons within lamina I of the neonatal spinal cord.
Importantly, gBa-sensitive measured at positive membrane potentials (i.e., Erev + 25) was inversely correlated with membrane resistance (Rm) across the lamina I population (r = −0.398; n = 28; p = 0.036; Spearman's test; Fig. 2G). This supports the notion that Ba2+-sensitive leak conductance is an important determinant of Rm in the developing superficial dorsal horn.
Bursting lamina I neurons express classical Kir channels during early life
Classical Kir channels (Kir2.x) are known to be constitutively active near the resting potential, exhibit strong inward rectification, and are highly sensitive to block by external Ba2+ (Hibino et al., 2010). However, it remains unclear whether functional Kir2.x channels are expressed within the developing dorsal horn. To better characterize the Kir channels found in neonatal lamina I neurons, we examined the sensitivity of Kir currents to increasing concentrations of extracellular Ba2+ (Fig. 3A). In both pacemaker and nonpacemaker populations within lamina I, the currents evoked by the negative voltage ramp were highly sensitive to external Ba2+ (IC50 at Vm = −140 mV; bursting: 8.62 ± 1.7 μm, n = 6; nonbursting: 13.9 ± 2.3 μm, n = 8; p = 0.109, unpaired t test; Fig. 3B). In addition, the sensitivity to Ba2+ block was lower at more depolarized potentials (Fig. 3C), which is consistent with the known properties of classical Kir2.x channels (Liu et al., 2001; Young et al., 2009).
Figure 3.
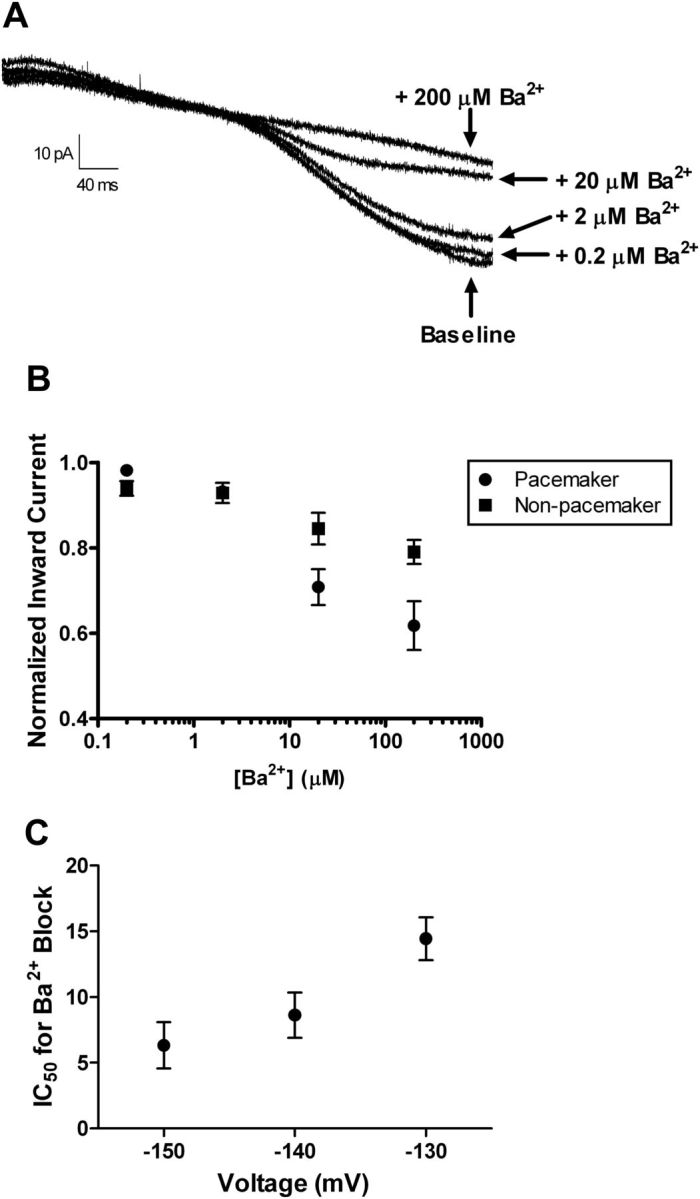
High Ba2+ sensitivity of Kir currents in neonatal lamina I neurons. A, Representative currents evoked during voltage ramps from −55 to −155 mV in an individual lamina I pacemaker neuron at baseline and in the presence of increasing concentrations of external BaCl2 (0.2–200 μm). B, Dose–response relationships suggest that a fraction of the recorded current (measured at −140 mV) was highly sensitive to Ba2+ block in both pacemaker and nonpacemaker populations within lamina I of the immature spinal cord. C, The measured IC50 for the block by external Ba2+ was voltage dependent, consistent with the presence of classical Kir2.x channels. Data are pooled from bursting and nonbursting groups of lamina I cells.
However, it should be noted that GIRK channels (Kir3.x) also exhibit strong inward rectification and sensitivity to external Ba2+ (Coetzee et al., 1999). GIRK channels can be constitutively active within some types of central neurons (Takigawa and Alzheimer, 2002; Chen and Johnston, 2005) and thus modulate their resting leak conductance. Importantly, we observed no evidence of tonic GIRK channel activity in neonatal lamina I neurons regardless of the pattern of spontaneous activity, as leak conductance in both bursting (n = 6; Fig. 4A,B) and nonbursting (n = 4) cells was unaffected by the bath application of the selective GIRK antagonist tertiapin-Q (Jin and Lu, 1998). This strongly suggests that GIRK channels do not contribute to resting leak conductance within the immature SDH and therefore are not responsible for the Ba2+-sensitive K+ currents isolated in our experiments (Fig. 2).
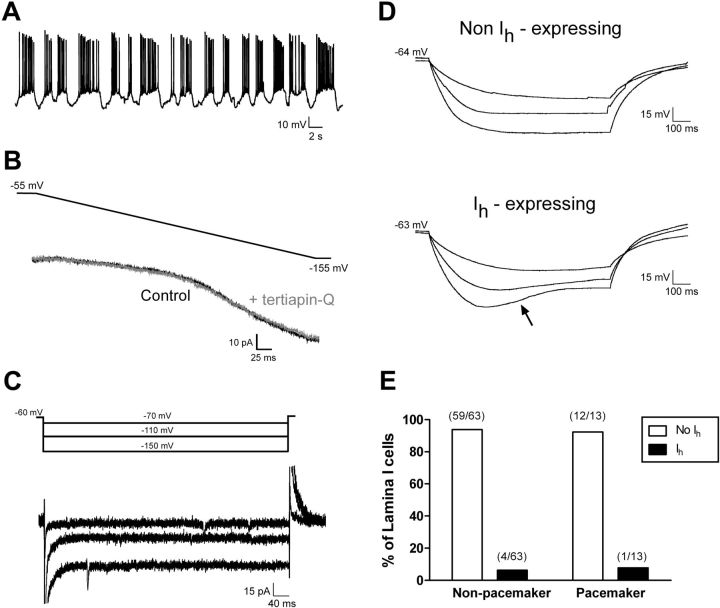
Figure 4.
GIRKs and hyperpolarization-activated cation channels do not contribute to resting leak conductance within immature SDH neurons. A, Example of spontaneous, rhythmic burst firing in a neonatal lamina I cell. B, Current evoked by a negative voltage ramp in the absence (black) or presence (gray) of the selective GIRK antagonist tertiapin-Q (100 nm). C, The majority of pacemaker neurons exhibited no evidence of the time-dependent inward current at hyperpolarized Vm that would indicate the presence of hyperpolarization-activated cation currents (Ih; same cell as in A). D, Current-clamp recordings from a representative pacemaker neuron showing an absence of the depolarizing sag in Vm in response to hyperpolarizing current injections (top), in contrast to the clear sag (arrow) witnessed in a minority of lamina I neurons at the same age (bottom). E, Group data demonstrating that the majority of pacemaker and nonpacemaker neurons in lamina I of the neonatal spinal cord lack hyperpolarization-activated cation current (Ih).
Alternatively, the strong inward rectification observed in pacemaker neurons during the negative voltage ramp (Fig. 2D) could potentially reflect the onset of hyperpolarization-activated cation currents (Ih), which have been documented in the rodent superficial dorsal horn (Grudt and Perl, 2002). However, this seems unlikely given the high sensitivity of the observed currents to external Ba2+ (Fig. 3A,B), since Ih is reported to be insensitive to Ba2+ at submillimolar concentrations (Robinson and Siegelbaum, 2003). Nonetheless, to further address this issue, voltage-clamp and current-clamp protocols were used to detect the presence of Ih in identified pacemaker neurons within lamina I during early life (see Materials and Methods). In 12 of 13 pacemaker neurons examined, negative voltage steps between −70 and −150 mV failed to evoke a slowly activating inward current (Fig. 4C). In addition, injections of hyperpolarizing current into bursting neurons did not produce the depolarizing sag in the membrane potential that is characteristic of Ih expression (Fig. 4D). Similarly, the vast majority of nonbursting neurons (59 of 63; p = 1.0 compared with bursting cells, Fisher's exact test) also failed to show evidence of Ih expression (Fig. 4E), suggesting that Ih is not an important contributor to leak conductance in lamina I during early life. Overall, these results further support the idea that the strongly rectifying, highly Ba2+-sensitive currents in newborn lamina I cells correspond to K+ flux through Kir2.x channels.
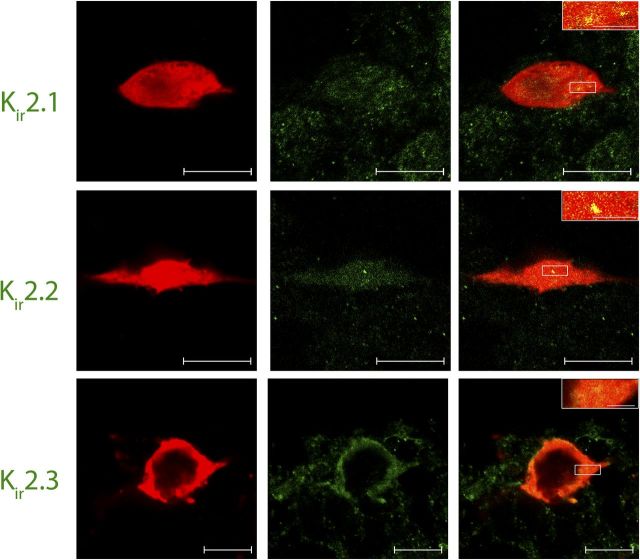
The low Kir conductance that distinguishes spinal pacemakers (Fig. 2) could reflect the absence of particular Kir2.x isoforms within this population. To investigate this possibility, we characterized the pattern of Kir2.x expression within identified pacemaker neurons using immunohistochemistry. Whole-cell patch-clamp recordings (with biocytin included in the intracellular solution) were obtained from bursting lamina I neurons in an intact spinal cord preparation (Safronov et al., 2007; Szucs et al., 2009), which has the advantages of better preserving anatomical structures within the dorsal horn and facilitating tissue sectioning for subsequent immunohistological analysis of the recorded neuron. The results clearly indicate that the population of lamina I pacemaker neurons expresses multiple subtypes of classical Kir2.x channels. Kir2.1 immunoreactivity was observed in every lamina I neuron exhibiting spontaneous, rhythmic burst firing (13 of 13 cells examined; Fig. 5, top). Similarly, Kir2.2-positive puncta were found in nine of nine pacemaker cells sampled (Fig. 5, middle). Finally, the vast majority of bursting neurons (10 of 12) also demonstrated immunoreactivity for Kir2.3 (Fig. 5, bottom). In all cases, preabsorption of the Kir2 primary antibody with the relevant antigen, or omission of the primary antibody, failed to produce measurable signals within the SDH (data not shown). Collectively, the data suggest that Kir2.x channels are well positioned to regulate the firing of lamina I neurons within the newborn spinal cord.
Figure 5.
Lamina I pacemaker neurons express classical Kir2.1–2.3 channels. Confocal images (0.5 μm optical thickness) of representative biocytin-filled pacemaker neurons (red) processed for Kir immunohistochemistry (green) with Kir2.1 (top row), Kir2.2 (middle row), or Kir2.3 (bottom row) antibodies. Merged images demonstrate that immunoreactive puncta for all three Kir2 isoforms (yellow; inset) were localized to identified pacemaker neurons within lamina I of the neonatal spinal cord (right). Scale bars: 10 μm; inset, 2 μm.
Block of Kir channels unmasks rhythmic burst firing in neonatal lamina I neurons
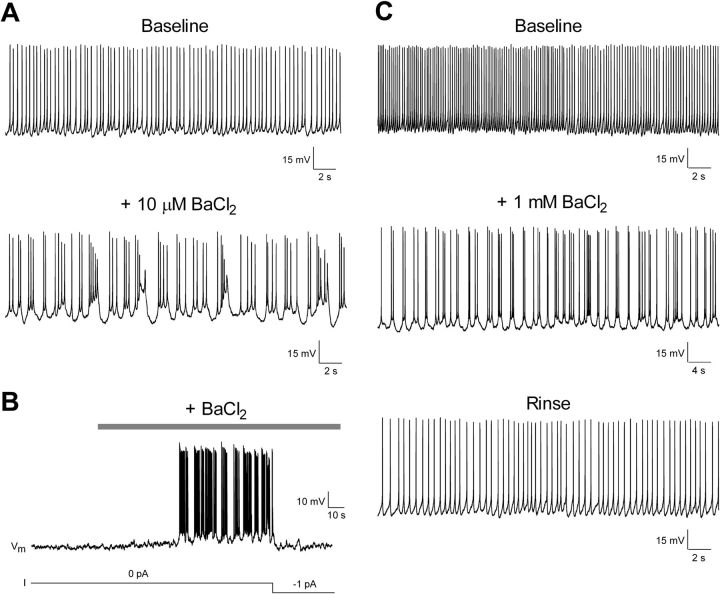
The observation that lamina I pacemaker neurons are distinguished by their high membrane resistance (Fig. 1B) and reduced gBa-sensitive near the resting membrane potential (Fig. 2E) suggests Kir channels modulate the generation of pacemaker activity within the developing SDH. To further examine this possibility, BaCl2 was bath applied (10 μm to 1 mm) to nonbursting neurons within lamina I of the neonatal spinal cord. External Ba2+ evoked a depolarizing shift in membrane potential in these cells (3.04 ± 0.73 mV; n = 33). More importantly, in a significant proportion of neurons that initially showed either a tonic (3 of 7 cells examined) or irregular (5 of 14 cells) pattern of spontaneous activity, the administration of extracellular Ba2+ promoted a switch to rhythmic burst firing that was often reversible after washout (Fig. 6A,C). In addition, in 6 of the 12 silent lamina I neurons sampled, Ba2+ application evoked a slow membrane depolarization with subsequent generation of spontaneous bursting (Fig. 6B). In many cases, this burst firing could be silenced by the injections of small amounts (≤2 pA) of hyperpolarizing current through the patch electrode (Fig. 6B), indicating that even minor shifts in gBa-sensitive may have profound consequences for the pattern of spontaneous activity within this population. The recruitment of burst firing is unlikely to be solely explained by the depolarization produced by Ba2+, as our previous work demonstrated that directly depolarizing nonpacemaker neurons via the patch electrode only evoked bursting in ∼12% of cells at P2–P3 (Li and Baccei, 2011). Collectively, these results suggest that the propensity of a given lamina I neuron to generate pacemaker activity is tightly controlled by the level of leak conductance through Ba2+-sensitive Kir channels.
Figure 6.
Block of Kir channels can recruit new pacemaker neurons within newborn spinal pain circuits. A, Example of neonatal lamina I neuron exhibiting a tonic pattern of spontaneous firing in the presence of aCSF (baseline). Bath application of a low concentration of Ba2+ (10 μm) revealed the ability of this neuron to generate burst firing. B, In a silent lamina I cell, external Ba2+ application (200 μm) evoked a slow depolarizing shift in membrane potential, which eventually triggered pacemaker activity. The injection of a small hyperpolarizing current through the patch electrode (bottom) was sufficient to silence this rhythmic burst firing. C, Example of a lamina I neuron that switched to rhythmic burst firing in the presence of external Ba2+ and reverted back to a tonic mode of action potential discharge after washout.
Strong inward rectification is not an innate property of Kir channels, but rather arises from the voltage-dependent block of the channel pore by intracellular free Mg2+ (Matsuda et al., 1987; Lu and MacKinnon, 1994) and internal free polyamines (Lopatin et al., 1994; Fakler et al., 1995). Therefore, if rhythmic bursting in lamina I neurons is indeed regulated by Kir channels, one would predict that the prevalence of pacemaker activity within the neonatal SDH could be significantly altered by manipulating the intracellular concentrations of these free cations. To further investigate this issue, whole-cell patch-clamp recordings from neonatal lamina I neurons were alternately obtained using intracellular recording solutions designed to contain either low (∼10 μm) or high (∼1 mm) levels of free Mg2+ via manipulations of the internal ATP concentration (see Materials and Methods). Although our intracellular recording solution did not contain polyamines, endogenous polyamines can be very slow to “wash out” during dialysis in the whole-cell configuration (Shin et al., 2005; Fleidervish et al., 2008). In addition, since ATP also strongly binds to polyamines (Watanabe et al., 1991), altering the internal levels of ATP in our sampled neurons will also modify the intracellular concentration of free polyamines, such that the high [Mg2+]free solution should also contain elevated levels of free polyamines. Importantly, a significantly higher percentage (18 of 38; 47%) of lamina I neurons exhibited rhythmic burst firing when the high [Mg2+]free internal solution was used, compared with neurons from the same spinal cord slices dialyzed with the low internal [Mg2+]free solution (7 of 38; 18%; p = 0.014, Fisher's exact test; Fig. 7A). This was accompanied by a significantly higher membrane resistance under high [Mg2+]free conditions (p = 0.013, Mann–Whitney test; Fig. 7B). It should be noted that these experiments cannot conclusively determine whether the increased prevalence of bursting seen with the high-Mg2+/ low-ATP internal solution was attributable to elevations in free Mg2+ or free polyamines, as the intracellular polyamine concentration remains unknown. Nonetheless, these data provide further support for the notion that Kir channels are key modulators of intrinsic burst firing within developing spinal nociceptive circuits.
Figure 7.
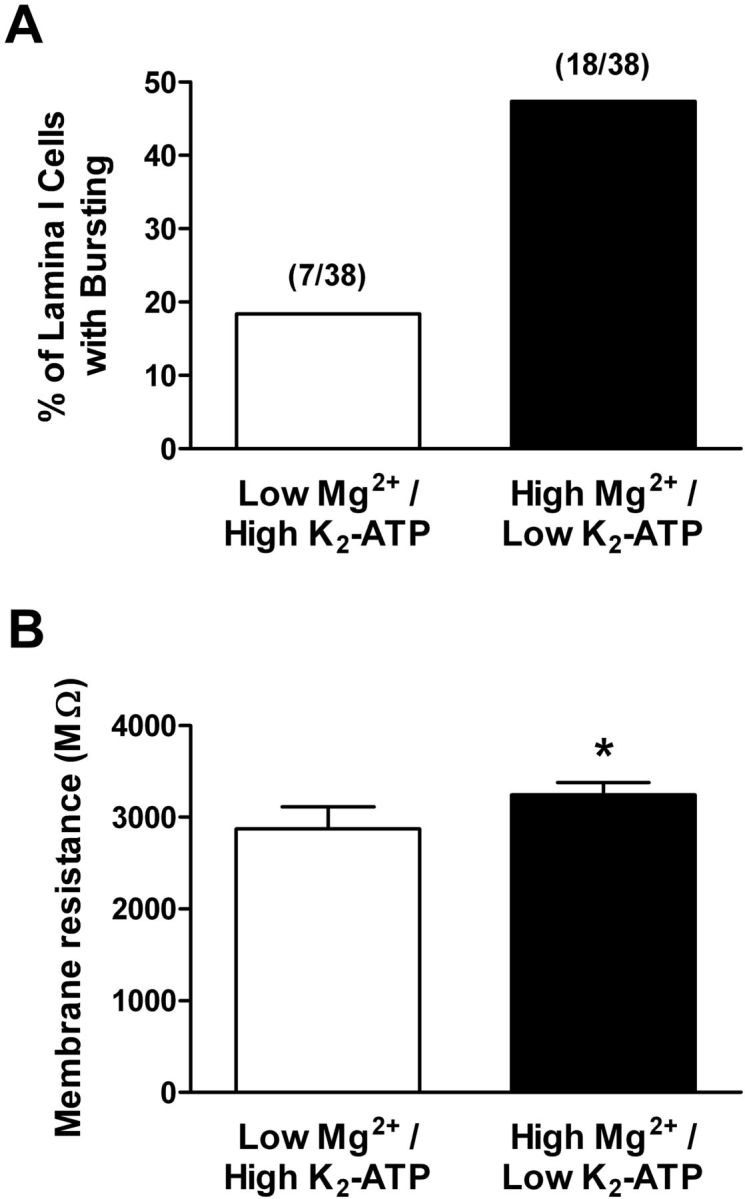
Prevalence of rhythmic burst firing is regulated by intracellular cation levels within neonatal lamina I neurons. A, Elevating the intracellular levels of free Mg2+ (to ∼1 mm) significantly increased the percentage of lamina I neurons demonstrating pacemaker activity during early life (p = 0.014, Fisher's exact test). B, Across the general population of lamina I neurons, a significantly greater membrane resistance was observed with the use of the high-Mg2+ intracellular solution (*p = 0.013, Mann–Whitney test) compared with the use of a patch solution containing low internal Mg2+ within the same slices.
Discussion
These results provide the first evidence that classical Kir channels serve as important regulators of pacemaker activity within the developing spinal cord. Neonatal lamina I neurons exhibiting spontaneous burst firing were characterized by higher membrane resistance (Rm) and more depolarized resting potentials (Vrest) compared with adjacent nonbursting neurons, which was associated with a significantly lower Kir conductance near Vrest within the bursting population. Importantly, block of Kir channels unmasked rhythmic burst firing within newborn spinal pain circuits, suggesting that the ability to generate pacemaker activity may be a latent property of a significant number of SDH neurons during early life.
Kir channels modulate passive membrane properties in neonatal SDH neurons
Neurons with a relatively large Kir conductance are predicted to exhibit more negative resting potentials, lower Rm, and minimal spontaneous activity (Hibino et al., 2010). As in other types of central neurons (Day et al., 2005; Young et al., 2009), Kir channels make a significant contribution to the resting leak conductance within newborn SDH neurons, as the level of Ba2+-sensitive K+ conductance was inversely correlated with Rm (Fig. 2G) and blocking Kir channels significantly elevated Rm in this population (Fig. 7B). As a result, the higher Rm and more depolarized Vrest seen in lamina I pacemaker neurons (Fig. 1B,C) likely reflects, at least in part, a reduced conductance through Ba2+-sensitive Kir channels (Fig. 2E). Given that the prevalence of pacemaker activity significantly decreases after the first postnatal week (Li and Baccei, 2011), it will be interesting to determine whether Kir currents are developmentally upregulated in lamina I neurons.
The available evidence strongly suggests that classical Kir (Kir2.x) channels underlie the Ba2+-sensitive K+ currents observed in the neonatal SDH. First, the strong inward rectification (Fig. 2) and high Ba2+ sensitivity (Fig. 3) of the Kir currents point to the involvement of the Kir2.x family (Preisig-Mülleret al., 2002; Schram et al., 2002) and are inconsistent with the described properties of other rectifying K+ channels (Coetzee et al., 1999). Indeed, our results clearly demonstrate that Kir2.1–2.3 channels are found in immature SDH cells (Fig. 5). In addition, whereas GIRK (Kir3.x) channels are also expressed within lamina I–II (Lüscher et al., 1997; Marker et al., 2005, 2006) and are highly sensitive to Ba2+ (Owen et al., 1999; Lancaster et al., 2000), we found no evidence that these channels were constitutively active in SDH neurons at this age. This implies that differences in GIRK expression between the pacemaker and nonpacemaker populations are unlikely to explain the lower Kir conductance seen in spontaneously bursting neurons. Instead, the data collectively suggest that although rhythmic burst firing is suppressed in the majority of immature lamina I neurons, attributable in part to Kir2.x channel activity, a subset of these cells possesses minimal Kir2.x conductance near Vrest and thus becomes endowed with the ability to generate pacemaker activity. Interestingly, genetic inhibition of Kir2.1 currents unleashes pacemaker activity in ventricular myocytes that were previously quiescent (Miake et al., 2002). However, it should be noted that low Kir2.x conductance by itself is insufficient to generate rhythmic burst firing, as other channels such as persistent voltage-gated Na+ channels and high-threshold voltage-gated Ca2+ channels are critically involved (Li and Baccei, 2011). Lamina I neurons that failed to exhibit pacemaker activity after extracellular Ba2+ application may lack sufficient levels of these other ionic conductances.
Potential mechanisms underlying low Kir conductance in spinal pacemaker neurons
The mechanistic basis for the reduced Kir conductance within the pacemaker population remains to be determined. However, the data suggest that it does not result from an absence of particular Kir2.x isoforms, as Kir2.1, Kir2.2, and Kir2.3 were expressed in lamina I neurons exhibiting spontaneous burst firing (Fig. 5). Although Kir2.4 expression was not examined, the high Ba2+ sensitivity of the isolated Kir currents (Fig. 3) argues against a dominant expression of this isoform within the immature SDH, as Kir2.4 is reported to show significantly lower Ba2+ sensitivity compared with other Kir2.x channels (Töpert et al., 1998; Coetzee et al., 1999). In fact, previous studies in the adult rat SDH suggest Kir2.4 expression is weaker compared with other Kir2.x channels (Prüss et al., 2005).
The low Kir conductance in pacemaker neurons is also unlikely to be explained by a lower number of Kir2.x channels in the somatodendritic membrane compared with other lamina I cells, since the conductance was similar between the two groups at more negative potentials (Fig. 2E, right). Alternatively, since Kir subunits can form heteromultimers (Fink et al., 1996; Preisig-Mülleret al., 2002), it is feasible that bursting neurons express Kir channels with a distinct stoichiometry. Kir2.x isoforms that show similarly high sensitivity to Ba2+ may nonetheless exhibit varying degrees of inward rectification and possess different susceptibilities to block by intracellular polyamines (Dhamoon et al., 2004; Ishihara and Yan, 2007), which are normally present in cells at up to millimolar concentrations (Tabor and Tabor, 1984; Watanabe et al., 1991). Therefore, the higher Rm (Fig. 1B), more depolarized Vrest (Fig. 1C), and greater Kir inward rectification (Fig. 2F) that characterize pacemaker neurons could reflect the preferential expression of Kir isoforms that are more sensitive to internal polyamine block. One potential candidate is Kir2.2, which has been associated with both extremely strong inward rectification and increased polyamine sensitivity compared with other Kir2.x channels (Panama and Lopatin, 2006; Ishihara and Yan, 2007).
Another possibility is that elevated intracellular levels of polyamines predispose a subset of SDH neurons to exhibit the high Rm required for the generation of rhythmic burst firing. The polyamines spermine and spermidine block Kir channels with a much higher (10- to 100-fold) potency than Mg2+ (Lopatin et al., 1994; Fakler et al., 1995) and are highly expressed within lamina I of the spinal cord (Laube et al., 2002), where they are synthesized from the amino acids arginine and methionine via the activation of ornithine decarboxylase (Tabor and Tabor, 1984). Interestingly, ornithine decarboxylase expression in the rat brain is highest at birth and declines rapidly during the first week of life (Pujic et al., 1995). In addition, polyamine levels in the CNS can be modulated by sensory experience during development (Aizenman et al., 2002). Alternatively, it is also feasible that pacemaker neurons are distinguished by differences in Mg2+ transport or buffering, which result in elevated intracellular concentrations of free Mg2+. Large changes in internal Mg2+ levels can occur in response to both hormonal and nonhormonal stimuli (Romani and Scarpa, 2000; Shindo et al., 2011), including alterations in cAMP levels or PKC activity (for review, see Romani, 2007). This raises the possibility that the enhanced rectification seen in pacemakers is explained by a greater block of Kir channels by intracellular Mg2+ at membrane potentials positive to EK.
Nonetheless, the pronounced difference in K+ conductance between pacemakers and nonpacemakers at physiological potentials (Fig. 2) may also reflect the contribution of additional ion channels that act preferentially at potentials positive to EK. For example, some subtypes of voltage-gated K+ channels, including members of the Kv3.x and Kv7.x (KCNQ) families, can be open near Vrest (Schroeder et al., 2000; Abbott et al., 2001). Therefore, the higher Rm within the pacemaker population (Fig. 1) could be partially explained by a lower resting conductance through constitutively active voltage-gated K+ channels.
Kir channels as putative targets of neuromodulators within newborn spinal nociceptive circuits
Although we failed to observe constitutive activation of GIRK (Kir3.x) channels in the neonatal SDH in vitro, previous work suggests that GIRK channels can be opened by metabotropic receptors such as the GABABR from birth (Baccei and Fitzgerald, 2004). Thus, the level of endogenous burst firing within the immature SDH may, under certain conditions, be under tight metabotropic control as previously reported in the deep dorsal horn of the adult (Derjean et al., 2003). One prominent candidate is the NK1 receptor that is expressed in the dorsal horn from the first days of life (Kar and Quirion, 1995; Cheunsuang et al., 2002), as substance P is known to regulate pacemaker activity in other regions of the CNS (Peña and Ramirez, 2004).
It should be noted that classical Kir2.x channels are also influenced by intracellular signaling cascades. For example, PKC and src kinase significantly inhibit Kir2.2 channels (Zitron et al., 2004, 2008). Notably, both PKC and src activity are elevated in the dorsal horn under pathological conditions (Guo et al., 2004; Kawasaki et al., 2004; Liu et al., 2008), suggesting that the prevalence of pacemaker activity within developing spinal nociceptive circuits might be modulated by nerve or tissue injury. Indeed, peripheral nerve damage during adulthood significantly increases the percentage of deep dorsal horn neurons that exhibit plateau potentials (Reali et al., 2011). An excessive number of pacemakers after injury could result in abnormal synchronization of firing within the SDH network, thereby significantly facilitating the output of the spinal pain pathway.
Further elucidation of the molecular properties that distinguish intrinsically bursting neurons within the newborn SDH will yield insight into potential strategies to selectively manipulate their excitability in vivo, which may then reveal the precise role of spinal pacemaker activity in the maturation of central nociceptive circuits.
Footnotes
This work was supported by National Institutes of Health Grants NS060858 and NS072202 (M.L.Ba.). We thank Drs. Peter Szucs and Boris Safronov for technical advice regarding the infrared LED illumination of the intact spinal cord and Elizabeth Kritzer for assistance with confocal image analysis. We also thank Drs. Judith Strong and Jun-Ming Zhang for helpful feedback regarding this manuscript.
References
- Abbott GW, Butler MH, Bendahhou S, Dalakas MC, Ptacek LJ, Goldstein SA. MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell. 2001;104:217–231. doi: 10.1016/s0092-8674(01)00207-0. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Muñoz-Elías G, Cline HT. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron. 2002;34:623–634. doi: 10.1016/s0896-6273(02)00674-8. [DOI] [PubMed] [Google Scholar]
- Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci. 2004;24:4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Brocard F, Tazerart S, Vinay L. Do pacemakers drive the central pattern generator for locomotion in mammals? Neuroscientist. 2010;16:139–155. doi: 10.1177/1073858409346339. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:3787–3792. doi: 10.1523/JNEUROSCI.5312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheunsuang O, Maxwell D, Morris R. Spinal lamina I neurones that express neurokinin 1 receptors: II. Electrophysiological characteristics, responses to primary afferent stimulation and effects of a selective mu-opioid receptor agonist. Neuroscience. 2002;111:423–434. doi: 10.1016/s0306-4522(02)00035-0. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de ME, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-botzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Le Masson G, Landry M, Morisset V, Nagy F. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat Neurosci. 2003;6:274–281. doi: 10.1038/nn1016. [DOI] [PubMed] [Google Scholar]
- Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JM. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94:1332–1339. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- Fakler B, Brändle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Fink M, Duprat F, Heurteaux C, Lesage F, Romey G, Barhanin J, Lazdunski M. Dominant negative chimeras provide evidence for homo and heteromultimeric assembly of inward rectifier K+ channel proteins via their N-terminal end. FEBS Lett. 1996;378:64–68. doi: 10.1016/0014-5793(95)01388-1. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Libman L, Katz E, Gutnick MJ. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 2008;105:18994–18999. doi: 10.1073/pnas.0803464105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Wenner P. Spontaneous network activity in the embryonic spinal cord regulates AMPAergic and GABAergic synaptic strength. Neuron. 2006;49:563–575. doi: 10.1016/j.neuron.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Graham BA, Brichta AM, Callister RJ. Recording temperature affects the excitability of mouse superficial dorsal horn neurons, in vitro. J Neurophysiol. 2008;99:2048–2059. doi: 10.1152/jn.01176.2007. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Milner LD, Landmesser LT. Spontaneous rhythmic activity in early chick spinal cord influences distinct motor axon pathfinding decisions. Brain Res Rev. 2008;57:77–85. doi: 10.1016/j.brainresrev.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Yan DH. Low-affinity spermine block mediating outward currents through Kir2.1 and Kir2.2 inward rectifier potassium channels. J Physiol. 2007;583:891–908. doi: 10.1113/jphysiol.2007.136028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Lu Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- Kar S, Quirion R. Neuropeptide receptors in developing and adult rat spinal cord: an in vitro quantitative autoradiography study of calcitonin gene-related peptide, neurokinins, mu-opioid, galanin, somatostatin, neurotensin and vasoactive intestinal polypeptide receptors. J Comp Neurol. 1995;354:253–281. doi: 10.1002/cne.903540208. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MK, Dibb KM, Quinn CC, Leach R, Lee JK, Findlay JB, Boyett MR. Residues and mechanisms for slow activation and Ba2+ block of the cardiac muscarinic K+ channel, Kir3.1/Kir3.4. J Biol Chem. 2000;275:35831–35839. doi: 10.1074/jbc.M006565200. [DOI] [PubMed] [Google Scholar]
- Laube G, Bernstein HG, Wolf G, Veh RW. Differential distribution of spermidine/spermine-like immunoreactivity in neurons of the adult rat brain. J Comp Neurol. 2002;444:369–386. doi: 10.1002/cne.10157. [DOI] [PubMed] [Google Scholar]
- Leao RM, Li S, Doiron B, Tzounopoulos T. Diverse levels of an inwardly rectifying potassium conductance generate heterogeneous neuronal behavior in a population of dorsal cochlear nucleus pyramidal neurons. J Neurophysiol. 2012;107:3008–3019. doi: 10.1152/jn.00660.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Baccei ML. Pacemaker neurons within newborn spinal pain circuits. J Neurosci. 2011;31:9010–9022. doi: 10.1523/JNEUROSCI.6555-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Liu GX, Derst C, Schlichthörl G, Heinen S, Seebohm G, Brüggemann A, Kummer W, Veh RW, Daut J, Preisig-Müller R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325–1332. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Lorenzo LE, Ramien M, St Louis M, De Koninck Y, Ribeiro-da-Silva A. Postnatal changes in the Rexed lamination and markers of nociceptive afferents in the superficial dorsal horn of the rat. J Comp Neurol. 2008;508:592–604. doi: 10.1002/cne.21691. [DOI] [PubMed] [Google Scholar]
- Lu Z, MacKinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 1994;371:243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Marker CL, Luján R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids. J Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker CL, Luján R, Colon J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci. 2006;26:12251–12259. doi: 10.1523/JNEUROSCI.3693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+ Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Miake J, Marban E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002;419:132–133. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- Owen JM, Quinn CC, Leach R, Findlay JB, Boyett MR. Effect of extracellular cations on the inward rectifying K+ channels Kir2.1 and Kir3.1/Kir3.4. Exp Physiol. 1999;84:471–488. [PubMed] [Google Scholar]
- Panama BK, Lopatin AN. Differential polyamine sensitivity in inwardly rectifying Kir2 potassium channels. J Physiol. 2006;571:287–302. doi: 10.1113/jphysiol.2005.097741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HA, Dolphin AC. Inhibition of omega-conotoxin-sensitive Ca2+ channel currents by internal Mg2+ in cultured rat cerebellar granule neurones. Pflugers Arch. 1993;425:518–527. doi: 10.1007/BF00374880. [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig-Müller R, Schlichthörl G, Goerge T, Heinen S, Brüggemann A, Rajan S, Derst C, Veh RW, Daut J. Heteromerization of Kir2.x potassium channels contributes to the phenotype of Andersen's syndrome. Proc Natl Acad Sci U S A. 2002;99:7774–7779. doi: 10.1073/pnas.102609499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss H, Derst C, Lommel R, Veh RW. Differential distribution of individual subunits of strongly inwardly rectifying potassium channels (Kir2 family) in rat brain. Brain Res Mol Brain Res. 2005;139:63–79. doi: 10.1016/j.molbrainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Pujic Z, Matsumoto I, Wilce PA. Expression of the genes coding for ornithine decarboxylase and its regulatory protein antizyme in the developing rat brain. Dev Neurosci. 1995;17:286–291. doi: 10.1159/000111298. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Peña F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Reali C, Fossat P, Landry M, Russo RE, Nagy F. Intrinsic membrane properties of spinal dorsal horn neurones modulate nociceptive information processing in vivo. J Physiol. 2011;589:2733–2743. doi: 10.1113/jphysiol.2011.207712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Romani A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch Biochem Biophys. 2007;458:90–102. doi: 10.1016/j.abb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Romani AM, Scarpa A. Regulation of cellular magnesium. Front Biosci. 2000;5:D720–D734. doi: 10.2741/romani. [DOI] [PubMed] [Google Scholar]
- Safronov BV, Pinto V, Derkach VA. High-resolution single-cell imaging for functional studies in the whole brain and spinal cord and thick tissue blocks using light-emitting diode illumination. J Neurosci Methods. 2007;164:292–298. doi: 10.1016/j.jneumeth.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–874. 876–879. [PubMed] [Google Scholar]
- Schram G, Melnyk P, Pourrier M, Wang Z, Nattel S. Kir2.4 and Kir2.1 K(+) channel subunits co-assemble: a potential new contributor to inward rectifier current heterogeneity. J Physiol. 2002;544:337–349. doi: 10.1113/jphysiol.2002.026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- Shin J, Shen F, Huguenard JR. Polyamines modulate AMPA receptor-dependent synaptic responses in immature layer v pyramidal neurons. J Neurophysiol. 2005;93:2634–2643. doi: 10.1152/jn.01054.2004. [DOI] [PubMed] [Google Scholar]
- Shindo Y, Fujii T, Komatsu H, Citterio D, Hotta K, Suzuki K, Oka K. Newly developed Mg2+-selective fluorescent probe enables visualization of Mg2+ dynamics in mitochondria. PLoS One. 2011;6:e23684. doi: 10.1371/journal.pone.0023684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Szucs P, Pinto V, Safronov BV. Advanced technique of infrared LED imaging of unstained cells and intracellular structures in isolated spinal cord, brainstem, ganglia and cerebellum. J Neurosci Methods. 2009;177:369–380. doi: 10.1016/j.jneumeth.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells. J Physiol. 2002;539:67–75. doi: 10.1113/jphysiol.2001.012883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazerart S, Viemari JC, Darbon P, Vinay L, Brocard F. Contribution of persistent sodium current to locomotor pattern generation in neonatal rats. J Neurophysiol. 2007;98:613–628. doi: 10.1152/jn.00316.2007. [DOI] [PubMed] [Google Scholar]
- Tazerart S, Vinay L, Brocard F. The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J Neurosci. 2008;28:8577–8589. doi: 10.1523/JNEUROSCI.1437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpert C, Döring F, Wischmeyer E, Karschin C, Brockhaus J, Ballanyi K, Derst C, Karschin A. Kir2.4: a novel K+ inward rectifier channel associated with motoneurons of cranial nerve nuclei. J Neurosci. 1998;18:4096–4105. doi: 10.1523/JNEUROSCI.18-11-04096.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem. 1991;266:20803–20809. [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]
- Young CC, Stegen M, Bernard R, Müller M, Bischofberger J, Veh RW, Haas CA, Wolfart J. Upregulation of inward rectifier K+ (Kir2) channels in dentate gyrus granule cells in temporal lobe epilepsy. J Physiol. 2009;587:4213–4233. doi: 10.1113/jphysiol.2009.170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitron E, Kiesecker C, Lück S, Kathöfer S, Thomas D, Kreye VA, Kiehn J, Katus HA, Schoels W, Karle CA. Human cardiac inwardly rectifying current IKir2.2 is upregulated by activation of protein kinase A. Cardiovasc Res. 2004;63:520–527. doi: 10.1016/j.cardiores.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Zitron E, Günth M, Scherer D, Kiesecker C, Kulzer M, Bloehs R, Scholz EP, Thomas D, Weidenhammer C, Kathöfer S, Bauer A, Katus HA, Karle CA. Kir2.x inward rectifier potassium channels are differentially regulated by adrenergic alpha1A receptors. J Mol Cell Cardiol. 2008;44:84–94. doi: 10.1016/j.yjmcc.2007.10.008. [DOI] [PubMed] [Google Scholar]