Abstract
Decades of research have correlated increased levels of amyloid-β peptide (Aβ) with neuropathological progression in Alzheimer's disease (AD) patients and transgenic models. Aβ precipitates synaptic and neuronal anomalies by perturbing intracellular signaling, which, in turn, may underlie cognitive impairment. Aβ also alters lipid metabolism, notably causing a deficiency of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], a phospholipid that regulates critical neuronal functions. Haploinsufficiency of the gene encoding synaptojanin 1 (Synj1), a major PI(4,5)P2 phosphatase in the brain, provided protection against PI(4,5)P2 breakdown and electrophysiological deficits attributable to Aβ. Based on these data, we tested whether reduction of Synj1 could rescue cognitive deficits and Aβ-induced morphological alterations of synapses. We found that hemizygous deletion of Synj1 in the context of a mouse model expressing the Swedish mutant of amyloid precursor protein rescues deficits in learning and memory without affecting amyloid load. Synj1 heterozygosity also rescued PI(4,5)P2 deficiency in a synaptosome-enriched fraction from the brain of Tg2576 mice. Genetic disruption of Synj1 attenuated Aβ oligomer-induced changes in dendritic spines of cultured hippocampal neurons, sparing mature spine classes, which corroborates the protective role for Synj1 reduction against Aβ insult at the synapse. These results indicate that Synj1 reduction ameliorates AD-associated behavioral and synaptic deficits, providing evidence that Synj1 and, more generally, phosphoinositide metabolism may be promising therapeutic targets. Our work expands on recent studies identifying lipid metabolism and lipid-modifying enzymes as targets of AD-associated synaptic and behavioral impairment.
Introduction
A pathogenic hallmark of Alzheimer's disease (AD) is the cleavage of amyloid precursor protein (APP) leading to accumulation of amyloid-β peptide (Aβ) and subsequent deposition in plaques (Bertram and Tanzi, 2012). Accumulation of soluble Aβ oligomers closely correlates with cognitive decline and disease progression in animal models and AD patients primarily through disruption of synaptic plasticity (Benilova et al., 2012). Soluble Aβ oligomers also profoundly alter dendritic spine morphology in dissociated cultures, hippocampal slices, and in animal models of AD (Pozueta et al., 2012). While these synaptic defects can be modulated by altering glutamatergic receptor trafficking and actin cytoskeleton dynamics (Pozueta et al., 2012), the molecular mechanisms orchestrating downstream effects of Aβ oligomers remain poorly understood.
Recent work has indicated that perturbation of phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] may be relevant to the synaptotoxic actions of Aβ oligomers and more generally to AD (Landman et al., 2006; Berman et al., 2008; Di Paolo and Kim, 2011). The catabolism of PI(4,5)P2, a signaling lipid critical for ion channel regulation, exocytosis/endocytosis, actin cytoskeleton rearrangement, and signal transduction, is tightly controlled by the polyphosphoinositide phosphatase, synaptojanin 1 (Synj1) (Di Paolo and De Camilli, 2006). Synj1 plays a critical role in synaptic vesicle trafficking, actin dynamics, and AMPA receptor (AMPAR) internalization (Di Paolo and De Camilli, 2006; Gong and De Camilli, 2008). Neurons derived from mice deficient in PI(4,5)P2 dephosphorylation due to haploinsufficiency of the gene encoding Synj1 (Synj1) display resistance to Aβ42 oligomer effects on PI(4,5)P2 destabilization and electrophysiological synaptic impairment (Berman et al., 2008). Recent work has further connected phosphoinositide metabolism to AD by identification of the Synj1 ortholog INP52 in an unbiased genome-wide screen for Aβ toxicity modifiers in yeast (Treusch et al., 2011). Further, Synj1 displayed transcriptional changes in a mouse model of AD and in AD patients (Miller et al., 2008; Alldred et al., 2012). Altogether, these studies suggest that PI(4,5)P2 imbalance may play a critical role in AD pathogenesis.
In this study, we show that Synj1 haploinsufficiency protects against memory impairment in an AD mouse model, Tg2576 (Hsiao et al., 1996). To address the underlying cause of this behavioral rescue, we examined morphological changes in dendritic spines triggered by Aβ oligomers in cultured neurons and found that spine alterations were attenuated by reduction of Synj1.
Materials and Methods
Mouse models.
Synj1+/− mice (Cremona et al., 1999) were bred to Tg2576 (Hsiao et al., 1996) (Taconic) to create Tg2576/Synj1 F1 progeny. Since Synj1−/− mice do not survive to adulthood, Synj1+/− mice were used, giving rise to the following four genotypes: Synj1+/+, Synj1+/−, Tg2576/Synj1+/+, and Tg2576/Synj1+/−. Both sexes were used for experiments, but no gender-specific defects were found.
Fear conditioning.
Contextual fear conditioning (FC) is a hippocampus/amygdala-dependent task in which Tg2576 mice show deficits (Barnes and Good, 2005). The test was performed on 5- to 6-month-old mice as previously described, and cued FC, a hippocampus-independent task, was used as a control (Oliveira et al., 2010).
Two-day radial arm water maze.
Since Tg2576 mice show deficits in reference memory in the 2 d radial arm water maze (2 d RAWM), 7- to 8-month-old mice were tested as previously described (Alamed et al., 2006).
Novel object recognition task.
Tg2576 mice show deficits in the novel object recognition (NOR) memory task (Hernandez et al., 2010). Littermates at 5–6 months of age were tested in NOR, and onset of the exploration time was defined as the moment the head of the animal approached the object within a 2.5 cm radius.
Biochemistry.
Western analysis was accomplished using antibodies to Synj1 (Haffner et al., 1997), neuronal β-tubulin (TUJ1, Covance), and human APP (6E10, Covance). Synj1 was quantified using Fujifilm LAS3000 and MultiGauge version 3.0 imaging software, and APP was quantified using LI-COR Biosciences Odyssey infrared detection. For quantification of Aβ40 and Aβ42 (ELISA, Invitrogen) brain tissue was processed as previously described (Schmidt et al., 2005).
Lipid analysis.
To quantify phosphoinositide levels in brain tissue, HPLC combined with suppressed conductivity detection was used (Berman et al., 2008). The crude synaptosome fraction (P2) was isolated from brains before lipid extraction (Wu et al., 1986).
Dendritic spine analysis in cultured primary neurons.
Hippocampal primary cultures were prepared from neonatal pups (Berman et al., 2008) and cultured for 21 d. Cultures were exposed to 200 nm Aβ oligomer for 24 h, fixed with 4% paraformaldehyde, and DiOlistically labeled with DiI (Invitrogen) (Smith et al., 2009). Images were collected with a 100× objective and 3.14× zoom using a z-stack of 0.3 μm sections on a Nikon C1 digital confocal system attached to an Olympus IX71 inverted scope. Neuron Studio (Rodriguez et al., 2008) was used to analyze spine density and spine class.
Statistical methods.
For analysis of behavioral tests, one-way ANOVA was used with Tukey's multiple comparison test except for FC data, which were not normally distributed. The nonparametric Kruskal–Wallis test was used to analyze contextual FC data (p = 0.056). There were no significant differences between the groups at baseline, precue, or postcue freezing using the Kruskal–Wallis test. To better fit the normality assumptions of ANOVA, the contextual FC data were transformed using Log10 and reanalyzed using one-way ANOVA (p = 0.045). One-way ANOVA and Tukey's multiple-comparison test were used for the phospholipid analysis. Student's t test was used for all other biochemical experiments and spine analysis using two-tailed distribution with equal variance (p < 0.05). All data are shown as geometric mean with error bars representing ± SEM. Significance is indicated as *p < 0.05, **p < 0.01.
Results
Cognitive rescue in Tg2576/Synj1+/− mice
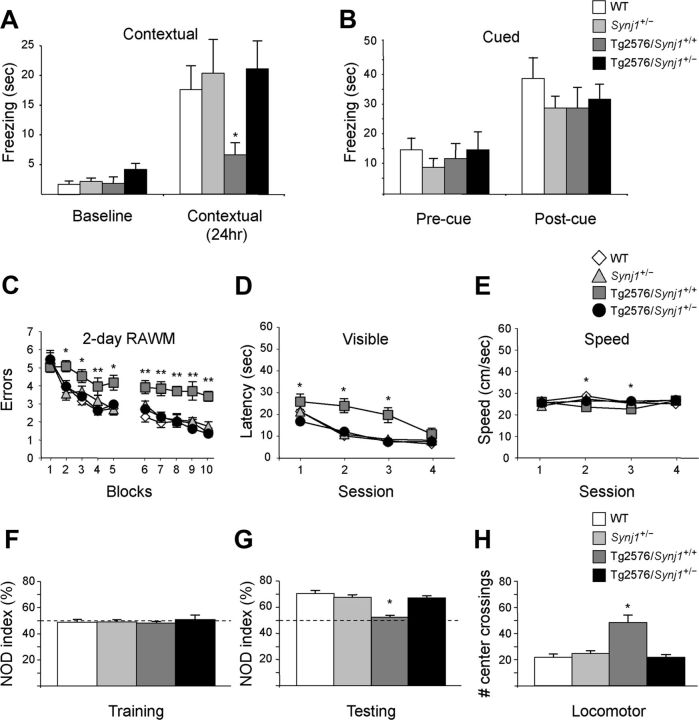
To investigate the effects of hemizygous deletion of Synj1 on learning and memory impairment, we used a battery of three behavioral tests, contextual FC, 2 d RAWM, and NOR. In contextual FC, a hippocampus- and amygdala-dependent learning task (Maren, 2008), Tg2576 mice exhibit deficits at 5–6 months of age, which precedes amyloid plaque deposition (Hsiao et al., 1996; Oliveira et al., 2010). While Tg2576/Synj1+/+ showed decreased freezing and thus impaired contextual fear memory when exposed to the conditioning context 24 h after training, Tg2576/Synj1+/− mice showed normal freezing as their wild-type (WT) and Synj1+/− littermates (Fig. 1A). In contrast to contextual FC, auditory cued FC, a hippocampus-independent task, showed no differences among genotypes (Fig. 1B).
Figure 1.
Hemizygous deletion of Synj1 ameliorates memory and behavioral deficits in Tg2576 mice. A, Freezing response in the FC paradigm in 5- to 6-month-old WT (n = 12), Synj1+/− (n = 16), Tg2576/Synj+/+ (n = 8), and Tg2576/Synj1+/− (n = 12) mice. B, Freezing responses in the auditory cued FC with the mice used in A. C, Two day RAWM. WT (n = 9), Synj1+/− (n = 9), Tg2576/Synj1+/+ (n = 8), and Tg2576/Synj1+/− (n = 9). D, Visible platform test; WT (n = 9), Synj1+/− (n = 9), Tg2576/Synj1+/+ (n = 7), and Tg2576/Synj1+/− (n = 9). E, Swim speed; WT (n = 9), Synj1+/− (n = 9), Tg2576/Synj1+/+ (n = 7), and Tg2576/Synj1+/− (n = 9). F, NOR training; WT (n = 9), Synj1+/− (n = 9), Tg2576/Synj1+/+(n = 7), and Tg2576/Synj1+/− (n = 9). Exploration time is expressed by novel object discrimination (NOD) index = amount of time spent exploring novel object × 100/time exploring novel object + time exploring familiar object. G, NOR testing. H, The hyperlocomotor activity displayed by mice tested in NOR.
The 2 d RAWM task evaluates reference memory by scoring entry into a maze arm without the escape platform as an error. Tg2576 mice typically show impaired ability to learn which arm of the maze has the escape platform (Alamed et al., 2006). Accordingly, Tg2576/Synj1+/+ mice did not effectively learn to locate the escape platform. In contrast, WT, Synj1+/−, and, importantly, Tg2576/Synj1+/− mice showed a progressive decrease in errors over the course of the experiment, indicating that they had learned the task (Fig. 1C). The visible platform task was used to evaluate potential baseline differences in sensory or motor performance or motivation between genotypes. Some Tg2576/Synj1+/+ mice avoided the visible platform due to neophobia (session 2–3), but the difference in latency was abolished by trial 4 (Fig. 1D) (Alamed et al., 2006). Additionally, there were no consistent differences in the mouse swim speed (Fig. 1E).
Published studies have shown that Tg2576 mice exhibit NOR deficits (Hernandez et al., 2010). All mice explored both objects equally on the training day (Fig. 1F). As expected, on the testing day (Fig. 1G), Tg2576/Synj1+/+ mice spent the same amount of time with each object, indicating they were unable to discriminate between the novel and original objects. Tg2576/Synj1+/− animals, however, displayed the same exploratory behavior as WT and Synj1+/− mice, spending more time with the novel object. Consistent with published results (Gil-Bea et al., 2007), we observed hyperlocomotor activity of Tg2576 mice in the open field test of NOR testing, and this phenotype was rescued in the Tg2576/Synj1+/− mice (Fig. 1H). Thus, three separate behavioral tests revealed that reduction of Synj1 in Tg2576 mice was sufficient to ameliorate deficits in learning and memory.
Effect of hemizygous deletion of Synj1 on Aβ and phosphoinositide levels in a mouse model of AD
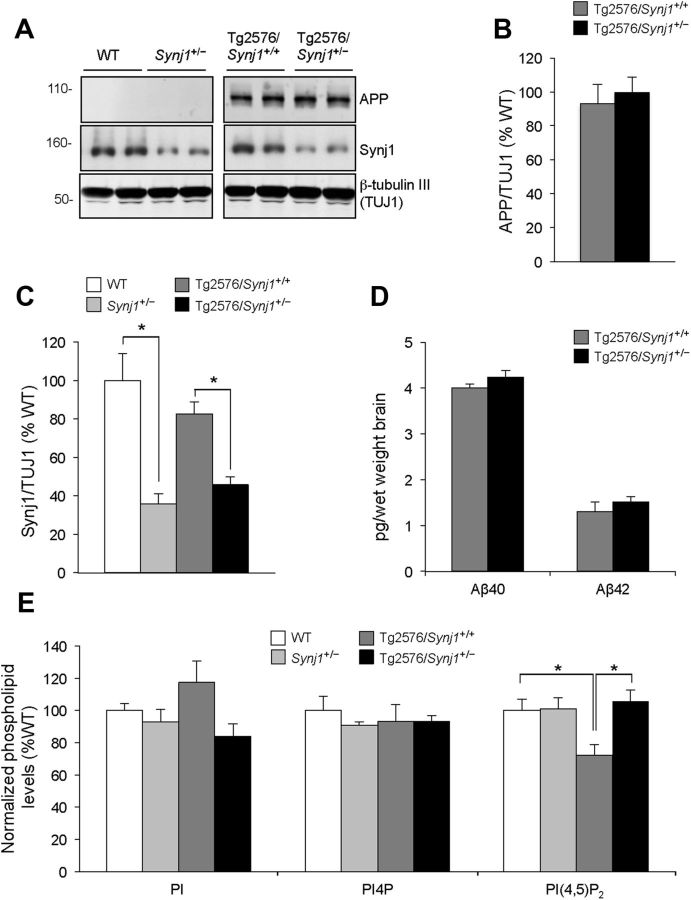
We investigated the possibility that haploinsufficiency of Synj1 may affect Aβ and phosphoinositide levels in the AD model. Western blot analysis showed no differences in the levels of the transgene human APPsw in Tg2576/Synj1+/+ and Tg2576/Synj1+/− mice (Fig. 2A,B). In both the haploinsufficient genotypes, Synj1+/− and Tg2576/Synj1+/−, Synj1 protein levels were reduced compared with Synj1+/+ and Tg2576/Synj1+/+ genotypes (Fig. 2A,C). Neither Aβ42 nor Aβ40 levels were affected by haploinsufficiency of Synj1 (Fig. 2D). Importantly, the levels of PI(4,5)P2, but not control inositol lipids PI and PI4P, were significantly reduced in the synaptosome-enriched (P2) fraction from forebrain tissue of Tg2576 mice and were restored to wild-type levels in Tg2576/Synj1+/− mice (Fig. 2E). Interestingly, these changes were observed in synaptosome-enriched fractions but not in whole forebrain extracts (data not shown), suggesting that alterations in PI(4,5)P2 in the Tg2576 model may be synapse specific.
Figure 2.
APP and Aβ levels are not significantly altered by reduction of Synj1 in Tg2576 mice. A, Western blot detection of human APP (6E10), Synj1, and anti-neuronal β-tubulin (TUJ1) from brain of WT (n = 6), Synj1+/− (n = 7), Tg2576/Synj1+/+ (n = 5) and Tg2576/Synj1+/− (n = 7) mice. B, APP levels as normalized to TUJ1. C, Synj1 levels as normalized to TUJ1. D, Detection of Aβ42 and Aβ40 from brain of Tg2576/Synj1+/+ (n = 5) and Tg2576/Synj1+/− (n = 7) mice. E, Inositol lipid levels from synaptosome-enriched (P2) fractions derived from forebrain tissue of WT (n = 9), Synj1+/− (n = 6), Tg2576/Synj1+/+ (n = 6), and Tg2576/Synj1+/− (n = 6) mice, as determined by HPLC combined with suppressed conductivity.
Effect of Synj1 reduction on Aβ-induced changes in dendritic spine morphology
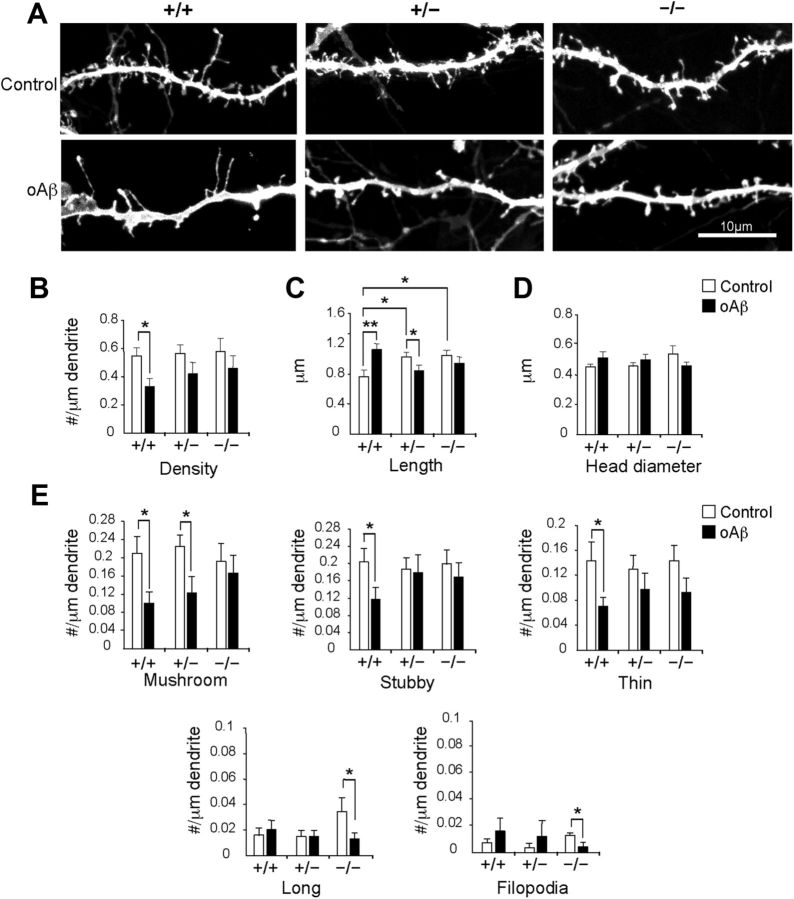
To investigate the underlying cause of the observed behavioral rescue due to reduced Synj1 and PI(4,5)P2 maintenance, we investigated the effects of Synj1 reduction on structural spine modifications caused by soluble Aβ oligomers. Dendritic spines, which are protrusions on dendritic processes of excitatory neurons, undergo dynamic structural changes that are closely associated with learning and memory (Bosch and Hayashi, 2012) and are profoundly affected by Aβ oligomers (Pozueta et al., 2012). We analyzed spine morphology in dissociated hippocampal primary cultures of Synj1+/+, Synj1+/−, or Synj1−/− mice. Under basal conditions, density and head diameter were not altered among the genotypes, though in Synj1+/− and Synj1−/− neurons, dendritic spines were significantly longer (Fig. 3A–D). Prior studies have reported decreased spine density and increased spine length after treatment of cultured neurons with Aβ oligomers (Calabrese et al., 2007; Lacor et al., 2007), both of which were recapitulated in wild-type neurons treated with Aβ (Fig. 3A–C). However, in Synj1+/− and Synj1−/− neurons, density was preserved and spine length did not increase following treatment with Aβ oligomers (Fig. 3A–C). When spines were analyzed by class, we observed that stubby and thin spines in Synj1+/− and Synj1−/− neurons were selectively spared from Aβ oligomer-induced decrease in density (Fig. 3A,E). Furthermore, in Synj1−/− neurons, mushroom spines were spared in addition to thin and stubby spines (Fig. 3A,E). These results support the hypothesis that neurons with reduced levels of Synj1 are fortified against synaptic defects induced by Aβ oligomers.
Figure 3.
Alterations of spine morphology attributed to Aβ are ameliorated with reduction of Synj1. A, Dendritic spines in hippocampal neuronal neurons exposed to 200 nm Aβ oligomer (oAβ) for 24 h and DiOlistically labeled from WT (+/+) cultures (n = 4): DMSO treated (n = 22) and oAβ treated (n = 21) neurons; Synj1+/− (+/−) cultures (n = 4): DMSO treated (n = 22) and oAβ treated (n = 20) neurons; or Synj1−/− (−/−) cultures (n = 3): DMSO treated (n = 19) and oAβ treated (n = 19) neurons. Scale bar, 10 μm. B, Spine density. C, Spine length. D, Spine head diameter. E, Spine class analysis using Neuron Studio, which distinguishes spine classes on the basis of length and presence of a spine head. Mushroom, stubby, and thin spines are <2 μm, while long spines and filopodia are >2 μm long. Mushroom and long spines have a head, while the other classes do not. The total number of spines analyzed was 3745 for 7415 μm length of dendrite as follows: Synj1+/+ (DMSO: 758; oAβ: 414); Synj1+/− (DMSO: 722; oAβ: 408); Synj1−/− (DMSO: 686; Aβ: 757).
Discussion
We previously identified PI(4,5)P2 metabolism as a target of familial AD-linked presenilin mutations and Aβ oligomers (Landman et al., 2006; Berman et al., 2008; Di Paolo and Kim, 2011). Haploinsufficiency of Synj1 was found to cause a decrease in the dephosphorylation of brain PI(4,5)P2 (Voronov et al., 2008) and conferred protection against Aβ-induced defects in long-term potentiation (Berman et al., 2008). In this study, we investigated the role Synj1 reduction plays in behavioral deficits in a mouse model of AD as well as morphological alterations in dendritic spines triggered by Aβ oligomers. We found that Synj1 haploinsufficiency was sufficient to ameliorate learning and memory deficits in the Tg2576 mouse model of AD in three independent behavioral paradigms: contextual FC, RAWM, and NOR. Because Tg2576 animals do not develop extensive plaque pathology in the age group we studied (5–9 months), their learning deficits are likely due to the accumulation of soluble pools of Aβ (Hsiao et al., 1996). However, Aβ levels were unaltered by the reduction of Synj1, suggesting that the protective effects on behavior are independent of Aβ levels in Tg2576/Synj1+/− mice, likely reflecting interference with Aβ-induced synaptotoxic signaling. Because our previous work showed Aβ42 oligomers cause a decrease in PI(4,5)P2, the major substrate for Synj1 (Berman et al., 2008), we investigated PI(4,5)P2 metabolism in our in vivo models. Though we found no global reduction of PI(4,5)P2 levels in whole forebrain extracts, synaptosome-enriched (P2) fractions displayed reduced PI(4,5)P2 levels in Tg2576 mice but not in Tg2576/Synj1+/− mice. This supports the hypothesis that synaptic pools of PI(4,5)P2 are affected by Aβ and preserved by the absence of one copy of Synj1 in this mouse model. Interestingly, despite reports indicating Aβ biogenesis and APP processing are modulated by PI(4,5)P2 (Landman et al., 2006; Osawa et al., 2008; Osenkowski et al., 2008), we found no alterations in full-length APP, Aβ40, or Aβ42 levels in the Tg2576/Synj1+/− cross, suggesting that specific pools of PI(4,5)P2 involved in APP processing may not be affected by the partial reduction of Synj1 in the context of APPsw overexpression.
To understand the cellular mechanism underlying the protective role of Synj1 deficiency, we investigated changes in spine morphology after Aβ insult. Cultured hippocampal neurons with reduced Synj1 maintained normal spine density, length, and mature classes of spines, namely, mushroom, thin, and stubby, in the presence of Aβ oligomer. One possible mechanism underlying maintenance of spine morphology in the presence of Aβ challenge, as well as amelioration of the learning and memory deficits, is regulation of synaptic glutamate receptor trafficking, which has been shown to affect spine morphology under nonpathological conditions (Kopec et al., 2006) and to be sensitive to Aβ (Pozueta et al., 2012). Importantly, a recent study has demonstrated that ablation of Synj1 delays AMPAR internalization (Gong and De Camilli, 2008). Reduction of Synj1 and resulting PI(4,5)P2 maintenance may thus delay the loss of AMPAR from the synaptic membrane due to Aβ challenge, thereby preserving spine morphology and synaptic function. It has also been reported that the NMDA receptor (NMDAR) interacts with PI(4,5)P2 through α-actinin, an actin cross-linking protein (Michailidis et al., 2007), and PI(4,5)P2-mediated regulation of NMDAR trafficking is impaired by Aβ challenge (Mandal and Yan, 2009). Since Aβ has been reported to decrease NMDAR surface expression (Pozueta et al., 2012), reduction of Synj1 could prevent this phenomenon through maintenance of PI(4,5)P2, resulting in more persistent NMDAR signaling. Synj1 may also have a role downstream of Aβ signaling as a substrate of calcineurin, a Ca2+-activated phosphatase that controls synaptic plasticity and has been shown to stimulate Synj1 via dephosphorylation (Lee et al., 2004). Indeed, calcineurin signaling has been shown to mediate Aβ-induced spine loss (Shankar et al., 2007), raising the possibility that a relevant target of this phosphatase downstream of Aβ may be Synj1. Finally, reduced Synj1 levels may maintain synaptic pools of PI(4,5)P2, preventing actin destabilization, which has been previously reported to occur in response to Aβ oligomer treatment (Shankar et al., 2007).
Overall, our studies validate Synj1 at the genetic level as a candidate therapeutic target for AD. Since Aβ-lowering strategies targeting late-stage AD have had only modest success in recent clinical trials (Huang and Mucke, 2012), identification of alternate targets that are independent of amyloid load, such as Synj1, is critical for progress in the development of AD therapeutics. Our data revealed that heterozygous deletion of the Synj1 gene ameliorates AD-associated cognitive deficits and protects synapses against Aβ, without interfering with normal behavior or synaptic function. Thus, reducing Synj1 levels may give rise to the desired protective phenotype without interfering with normal brain function in AD patients. Reducing Synj1 activity may also be beneficial in ameliorating the cognitive deficits of Down syndrome, characterized by overexpression of genes on chromosome 21, including Synj1 and App, and inevitably leading to AD pathology in adulthood (Voronov et al., 2008 and Cossec et al., 2012). Confluent with a validated therapeutic target for diabetes, phosphoinositide phosphatases represent a new and promising class of therapeutic targets in human diseases (McCrea and De Camilli, 2009). In addition to phosphatases, a phosphoinositide kinase has also been implicated in AD pathology. Inhibition of PI3 kinase has been demonstrated to decrease Aβ biogenesis (Petanceska and Gandy, 1999; Haugabook et al., 2001) and ameliorate AD-associated cognitive defects (Chiang et al., 2010). Finally, recent work has shown that genetic disruption of phospholipases, such as phospholipase D2 and cytosolic phospholipase A2, improves pathological phenotypes in AD mouse models (Oliveira et al., 2010; Sanchez-Mejia and Mucke, 2010; and Chan et al., 2012). Collectively, these studies substantiate that modulation of neuronal lipid networks can ameliorate AD-associated pathologies, and therefore targeting lipid-modifying enzymes represents an engaging strategy for future development of AD therapeutics.
Footnotes
This work has been supported by the Alzheimer's Association and NIH Grant MN015174 to L.B.J.M.; NIH Grant AG08702 to L.B.J.M. and D.E.B.; NIH Grant NS049442 to O.A.; NIH Grants HD055457 and AG033212 to G.D.P.; and NIH Grant NS074536 and Cure Alzheimer's Fund support to T.-W.K. We thank Michael Shelanski and Julio Pozueta for help with DiOlistic labeling of neurons. We thank Pietro De Camilli for the gift of the Synj1 knock-out mice and the anti-Synj1 antibody. We thank Dara Dickstein for help with Neuron Studio. We thank the Irving Institute for Clinical and Translational Research Design, Biostatistics and Database Support for help with the statistical analysis (Grant UL1 RR 024156).
The authors declare no conflicting financial interests.
References
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- Alldred MJ, Duff KE, Ginsberg SD. Microarray analysis of CA1 pyramidal neurons in a mouse model of tauopathy reveals progressive synaptic dysfunction. Neurobiol Dis. 2012;45:751–762. doi: 10.1016/j.nbd.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P, Good M. Impaired Pavlovian cued fear conditioning in Tg2576 mice expressing a human mutant amyloid precursor protein gene. Behav Brain Res. 2005;157:107–117. doi: 10.1016/j.bbr.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Berman DE, Dall'Armi C, Voronov SV, McIntire LB, Zhang H, Moore AZ, Staniszewski A, Arancio O, Kim TW, Di Paolo G. Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nat Neurosci. 2008;11:547–554. doi: 10.1038/nn.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. The genetics of Alzheimer's disease. Prog Mol Biol Transl Sci. 2012;107:79–100. doi: 10.1016/B978-0-12-385883-2.00008-4. [DOI] [PubMed] [Google Scholar]
- Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22:383–388. doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese B, Shaked GM, Tabarean IV, Braga J, Koo EH, Halpain S. Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-beta protein. Mol Cell Neurosci. 2007;35:183–193. doi: 10.1016/j.mcn.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, Wenk MR, Shui G, Di Paolo G. Comparative lipidomic analysis of mouse and human brain with Alzheimer's disease. J Biol Chem. 2012;287:2678–2688. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang HC, Wang L, Xie Z, Yau A, Zhong Y. PI3 kinase signaling is involved in Abeta-induced memory loss in Drosophila. Proc Natl Acad Sci U S A. 2010;107:7060–7065. doi: 10.1073/pnas.0909314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossec JC, Lavaur J, Berman DE, Rivals I, Hoischen A, Stora S, Ripoll C, Mircher C, Grattau Y, Olivomarin JC, de Chaumont F, Lecourtois M, Antonarakis SE, Veltman JA, Delabar JM, Duyckaerts C, Di Paolo G, Potier MC. Trisomy for Synaptojanin1 in Down syndrome is functionally linked to the enlargement 1 of early endosomes. Hum Mol Genet. 2012;21:3156–3172. doi: 10.1093/hmg/dds142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O, Di Paolo G, Wenk MR, Lüthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Kim TW. Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Bea FJ, Aisa B, Schliebs R, Ramírez MJ. Increase of locomotor activity underlying the behavioral disinhibition in tg2576 mice. Behav Neurosci. 2007;121:340–344. doi: 10.1037/0735-7044.121.2.340. [DOI] [PubMed] [Google Scholar]
- Gong LW, De Camilli P. Regulation of postsynaptic AMPA responses by synaptojanin 1. Proc Natl Acad Sci U S A. 2008;105:17561–17566. doi: 10.1073/pnas.0809221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner C, Takei K, Chen H, Ringstad N, Hudson A, Butler MH, Salcini AE, Di Fiore PP, De Camilli P. Synaptojanin 1: localization on coated endocytic intermediates in nerve terminals and interaction of its 170 kDa isoform with Eps15. FEBS Lett. 1997;419:175–180. doi: 10.1016/s0014-5793(97)01451-8. [DOI] [PubMed] [Google Scholar]
- Haugabook SJ, Le T, Yager D, Zenk B, Healy BM, Eckman EA, Prada C, Younkin L, Murphy P, Pinnix I, Onstead L, Sambamurti K, Golde TE, Dickson D, Younkin SG, Eckman CB. Reduction of Aβ accumulation in the Tg2576 animal model of Alzheimer's disease after oral administration of the phosphatidylinositol kinase inhibitor wortmannin. FASEB J. 2001;15:16–18. doi: 10.1096/fj.00-0528fje. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. Loss of α7 nicotinic receptors enhances β-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer's disease. J Neurosci. 2010;30:2442–2453. doi: 10.1523/JNEUROSCI.5038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman N, Jeong SY, Shin SY, Voronov SV, Serban G, Kang MS, Park MK, Di Paolo G, Chung S, Kim TW. Presenilin mutations linked to familial Alzheimer's disease cause an imbalance in phosphatidylinositol 4,5-bisphosphate metabolism. Proc Natl Acad Sci U S A. 2006;103:19524–19529. doi: 10.1073/pnas.0604954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci U S A. 2004;101:546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Yan Z. Phosphatidylinositol (4,5)-bisphosphate regulation of N-methyl-D-aspartate receptor channels in cortical neurons. Mol Pharmacol. 2009;76:1349–1359. doi: 10.1124/mol.109.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- McCrea HJ, De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology (Bethesda) 2009;24:8–16. doi: 10.1152/physiol.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidis IE, Helton TD, Petrou VI, Mirshahi T, Ehlers MD, Logothetis DE. Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through α-actinin. J Neurosci. 2007;27:5523–5532. doi: 10.1523/JNEUROSCI.4378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Oldham MC, Geschwind DH. A systems level analysis of transcriptional changes in Alzheimer's disease and normal aging. J Neurosci. 2008;28:1410–1420. doi: 10.1523/JNEUROSCI.4098-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira TG, Chan RB, Tian H, Laredo M, Shui G, Staniszewski A, Zhang H, Wang L, Kim TW, Duff KE, Wenk MR, Arancio O, Di Paolo G. Phospholipase d2 ablation ameliorates Alzheimer's disease-linked synaptic dysfunction and cognitive deficits. J Neurosci. 2010;30:16419–16428. doi: 10.1523/JNEUROSCI.3317-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S, Funamoto S, Nobuhara M, Wada-Kakuda S, Shimojo M, Yagishita S, Ihara Y. Phosphoinositides suppress gamma-secretase in both the detergent-soluble and -insoluble states. J Biol Chem. 2008;283:19283–19292. doi: 10.1074/jbc.M705954200. [DOI] [PubMed] [Google Scholar]
- Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanceska SS, Gandy S. The phosphatidylinositol 3-kinase inhibitor wortmannin alters the metabolism of the Alzheimer's amyloid precursor protein. J Neurochem. 1999;73:2316–2320. doi: 10.1046/j.1471-4159.1999.0732316.x. [DOI] [PubMed] [Google Scholar]
- Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer's disease and its models. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.05.050. Advance online publication. Retrieved September 15, 2012. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mejia RO, Mucke L. Phospholipase A2 and arachidonic acid in Alzheimer's disease. Biochim Biophys Acta. 2010;1801:784–790. doi: 10.1016/j.bbalip.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SD, Jiang Y, Nixon RA, Mathews PM. Tissue processing prior to protein analysis and amyloid-beta quantitation. Methods Mol Biol. 2005;299:267–278. doi: 10.1385/1-59259-874-9:267. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci U S A. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treusch S, Hamamichi S, Goodman JL, Matlack KE, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS, Han H, Lindhagen-Persson M, Reiman EM, Evans DA, Bennett DA, Olofsson A, DeJager PL, Tanzi RE, Caldwell KA, Caldwell GA, et al. Functional links between Aβ toxicity, endocytic trafficking, and Alzheimer's disease risk factors in yeast. Science. 2011;334:1241–1245. doi: 10.1126/science.1213210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov SV, Frere SG, Giovedi S, Pollina EA, Borel C, Zhang H, Schmidt C, Akeson EC, Wenk MR, Cimasoni L, Arancio O, Davisson MT, Antonarakis SE, Gardiner K, De Camilli P, Di Paolo G. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down's syndrome. Proc Natl Acad Sci U S A. 2008;105:9415–9420. doi: 10.1073/pnas.0803756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Sachs L, Carlin RK, Siekevitz P. Characteristics of a Ca2+/calmodulin-dependent binding of the Ca2+ channel antagonist, nitrendipine, to a postsynaptic density fraction isolated from canine cerebral cortex. Brain Res. 1986;387:167–184. doi: 10.1016/0169-328x(86)90008-2. [DOI] [PubMed] [Google Scholar]