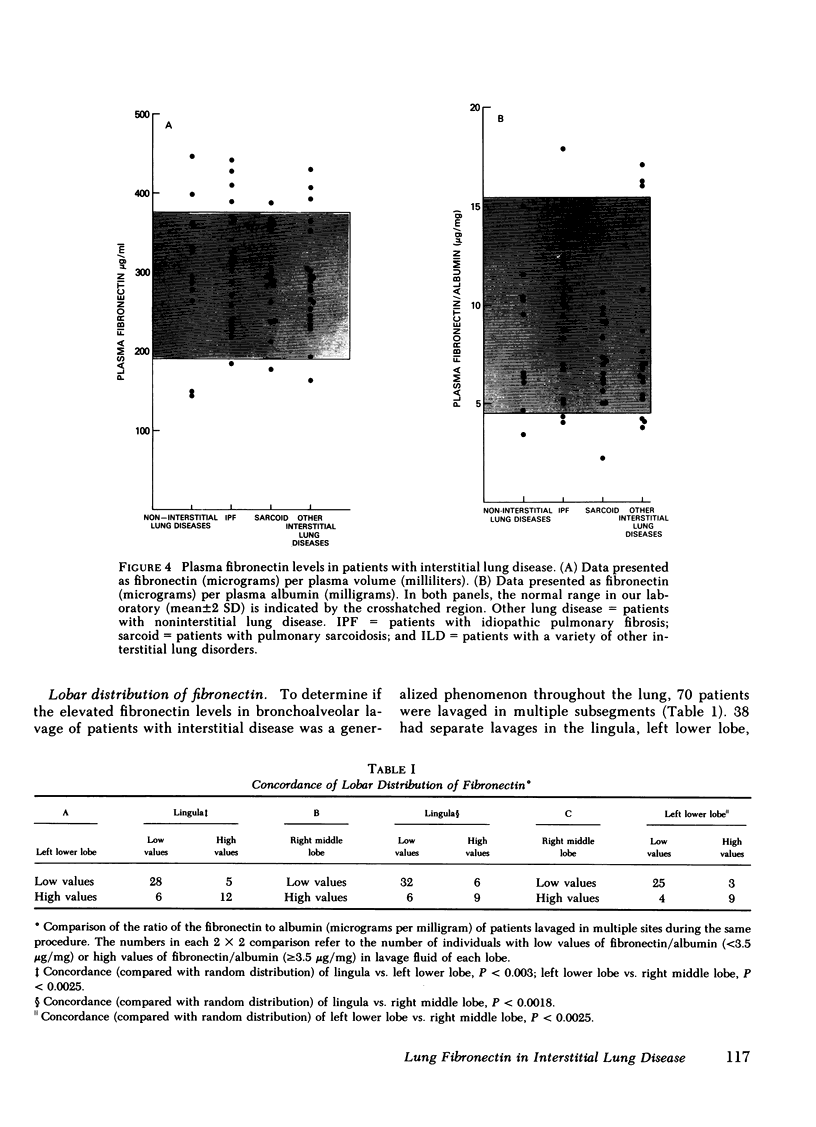
Abstract
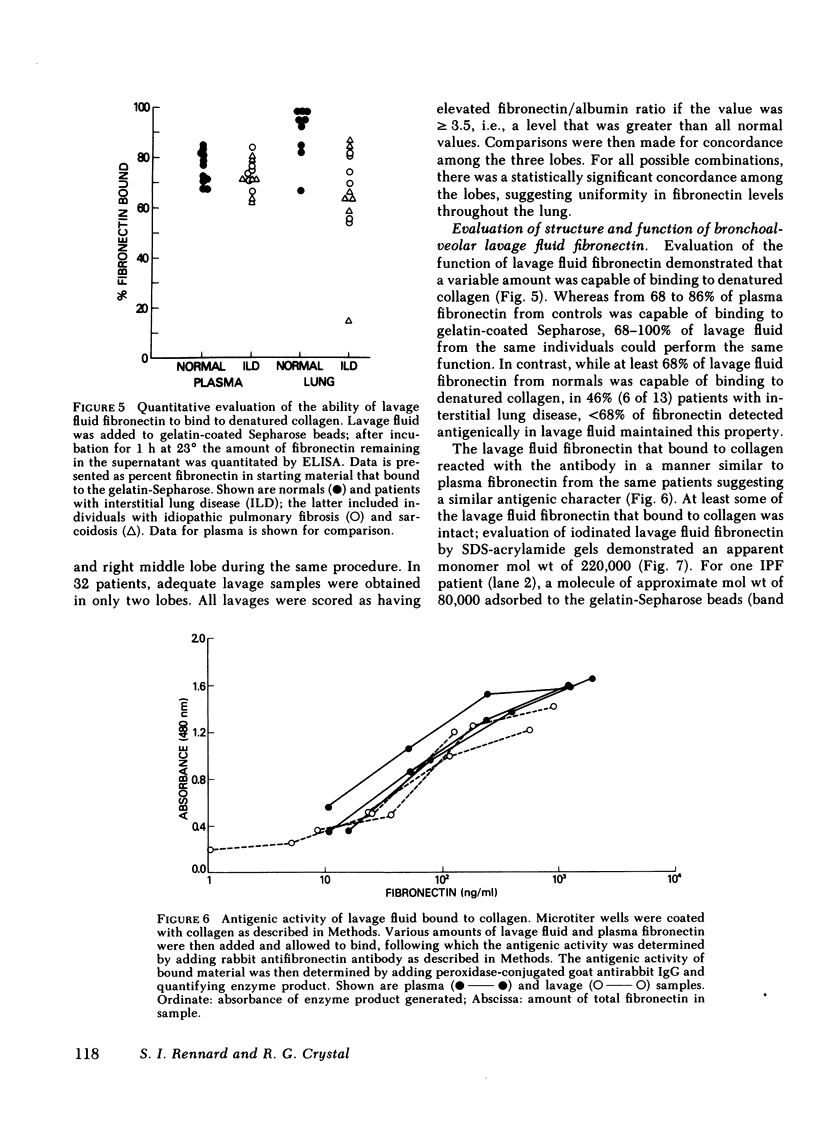
Fibronectin is a major adhesive and opsonic glycoprotein found in plasma and tissues. Because this molecule appears to mediate a number of interactions between cells and extracellular matrix, and because the interstitial lung disease are characterized by marked derangements of the pulmonary extracellular matrix, we evaluated fibronectin in the lower respiratory tract in patients with these disorders. Fibronectin could be detected in the bronchoalveolar lavage fluid of normals (11/11), as well as those with noninterstitial lung diseases (18/18), idiopathic pulmonary fibrosis (21/21), sarcoidosis (20/20), and other interstitial lung diseases (22/22). Compared with normal and those with noninterstitial lung disease, the levels in bronchoalveolar lavage of patients with interstitial disease were significantly higher (P less than 0.01), all comparisons). This was true only for bronchoalveolar lavage fibronectin; plasma levels were similar in all study groups (P greater than 0.2, all comparisons). The lavage fluid fibronectin was intact antigenically and retained collagen binding capability, although in some cases of interstitial disease, the presence of lower molecular weight fragments suggested some degradation. Thus, fibronectin is a normal constituent of the epithelial fluid of the lower respiratory tract and is present in increased amounts in a significant number of individuals with interstitial lung disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Hovi T., Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980 Mar 1;151(3):602–613. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B. J., McDonald J. A., Crystal R. G. Metabolic fate of the major cell surface protein of normal human fibroblasts. Biochem Biophys Res Commun. 1977 Nov 7;79(1):8–15. doi: 10.1016/0006-291x(77)90053-5. [DOI] [PubMed] [Google Scholar]
- Blumenstock F., Weber P., Saba T. M. Isolation and biochemical characterization of alpha-2-opsonic glycoprotein from rat serum. J Biol Chem. 1977 Oct 25;252(20):7156–7162. [PubMed] [Google Scholar]
- Bornstein P., Duksin D., Balian G., Davidson J. M., Crouch E. Organization of extracellular proteins on the connective tissue cell surface: relevance to cell-matrix interactions in vitro and in vivo. Ann N Y Acad Sci. 1978 Jun 20;312:93–105. doi: 10.1111/j.1749-6632.1978.tb16795.x. [DOI] [PubMed] [Google Scholar]
- Bray B. A. Cold-insoluble globulin (fibronectin) in connective tissues of adult human lung and in trophoblast basement membrane. J Clin Invest. 1978 Oct;62(4):745–752. doi: 10.1172/JCI109185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIEN S., SINCLAIR D. G., CHANG C., PERIC B., DELLENBACK R. J. SIMULTANEOUS STUDY OF CAPILLARY PERMEABILITY TO SEVERAL MACROMOLECULES. Am J Physiol. 1964 Sep;207:513–517. doi: 10.1152/ajplegacy.1964.207.3.513. [DOI] [PubMed] [Google Scholar]
- Chen A. B., Mosesson M. W., Solish G. I. Identification of the cold-insoluble globulin of plasma in amniotic fluid. Am J Obstet Gynecol. 1976 Aug 1;125(7):958–961. doi: 10.1016/0002-9378(76)90495-6. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Murray A., Segal R. A., Bushnell A., Walsh M. L. Studies on intercellular LETS glycoprotein matrices. Cell. 1978 Jun;14(2):377–391. doi: 10.1016/0092-8674(78)90123-x. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Dessau W., Sasse J., Timpl R., von der Mark K. Role of fibronectin and collagen types I and II in chondrocytic differentiation in vitro. Ann N Y Acad Sci. 1978 Jun 20;312:404–405. doi: 10.1111/j.1749-6632.1978.tb16819.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Forkman B., Ganrot P. O., Gennser G., Rannevik G. Plasma protein pattern in recurrent cholestasis of pregnancy. Scand J Clin Lab Invest Suppl. 1972;124:89–96. doi: 10.3109/00365517209102756. [DOI] [PubMed] [Google Scholar]
- Fulmer J. D., Roberts W. C., von Gal E. R., Grystal R. G. Small airways in idiopathic pulmonary fibrosis. Comparison of morphologic and physiologic observations. J Clin Invest. 1977 Sep;60(3):595–610. doi: 10.1172/JCI108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furcht L. T., Mosher D. F., Wendelschafer-Crabb G. Immunocytochemical localization of fibronectin (LETS proteins) on the surface of L6 myoblasts: light and electron microscopic studies. Cell. 1978 Feb;13(2):263–271. doi: 10.1016/0092-8674(78)90195-2. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Kelman J. A., Fells G., Weinberger S. E., Horwitz A. L., Reynolds H. Y., Fulmer J. D., Crystal R. G. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979 Oct 4;301(14):737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- Gauss-Müller V., Kleinman H. K., Martin G. R., Schiffmann E. Role of attachment factors and attractants in fibroblast chemotaxis. J Lab Clin Med. 1980 Dec;96(6):1071–1080. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hörmann H., Jelinić V. Fibronectin, VII. Binding of cold-insoluble globulin and of denatured collagen by macrophages. Hoppe Seylers Z Physiol Chem. 1980;361(3):379–387. doi: 10.1515/bchm2.1980.361.1.379. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978 Jun 1;147(6):1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S., Rubin K., Hök M., Ahlgren T., Seljelid R. In vitro biosynthesis of cold insoluble globulin (fibronectin) by mouse peritoneal macrophages. FEBS Lett. 1979 Sep 15;105(2):313–316. doi: 10.1016/0014-5793(79)80637-7. [DOI] [PubMed] [Google Scholar]
- Keogh B. A., Crystal R. G. Clinical significance of pulmonary function tests. Pulmonary function testing in interstitial pulmonary disease. What does it tell us? Chest. 1980 Dec;78(6):856–865. doi: 10.1378/chest.78.6.856. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Martin G. R., Fishman P. H. Ganglioside inhibition of fibronectin-mediated cell adhesion to collagen. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3367–3371. doi: 10.1073/pnas.76.7.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B. Localization of the cell attachment region in types I and II collagens. Biochem Biophys Res Commun. 1976 Sep 20;72(2):426–432. doi: 10.1016/s0006-291x(76)80060-5. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Vaheri A., Palo J., Ruoslahti E. Demonstration of fibronectin in human cerebrospinal fluid. J Lab Clin Med. 1978 Oct;92(4):595–601. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoshida N., Aoki N., Wakabayashi K. Distribution of cold-insoluble globulin in plasma and tissues. Ann N Y Acad Sci. 1978 Jun 20;312:74–92. doi: 10.1111/j.1749-6632.1978.tb16794.x. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Baum B. J., Rosenberg D. M., Kelman J. A., Brin S. C., Crystal R. G. Destruction of a major extracellular adhesive glycoprotein (fibronectin) of human fibroblasts by neutral proteases from polymorphonuclear leukocyte granules. Lab Invest. 1979 Mar;40(3):350–357. [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- Mosher D. F., Williams E. M. Fibronectin concentration is decreased in plasma of severely ill patients with disseminated intravascular coagulation. J Lab Clin Med. 1978 May;91(5):729–735. [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Church R. L., Rohrbach D. H., Shupp D. E., Abe S., Hewitt A. T., Murray J. C., Martin G. R. Localization of the human fibronectin (FN) gene on chromosome 8 by a specific enzyme immunoassay. Biochem Genet. 1981 Jun;19(5-6):551–566. doi: 10.1007/BF00484626. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Saba T. M., Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980 Apr;68(4):577–594. doi: 10.1016/0002-9343(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore J., Robin E. D., Gaudio R., Acevedo J. Transalveolar transport of large polar solutes (sucrose, inulin, and dextran). Am J Physiol. 1975 Oct;229(4):989–996. doi: 10.1152/ajplegacy.1975.229.4.989. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Fibroblast cellular and plasma fibronectins are similar but not identical. J Cell Biol. 1979 Feb;80(2):492–498. doi: 10.1083/jcb.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Kimata K., Pratt R. M. Characterization of fibronectin interactions with glycosaminoglycans and identification of active proteolytic fragments. J Biol Chem. 1980 Jul 10;255(13):6055–6063. [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Quantitation of a transformation-sensitive, adhesive cell surface glycoprotein. Decrease of several untransformed permanent cell lines. J Cell Biol. 1977 Aug;74(2):649–654. doi: 10.1083/jcb.74.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]