Abstract
Background/Aims
HO-1 and EETs are functionally linked and their interactions influence body weight, insulin sensitivity, and serum levels of inflammatory cytokines in metabolic syndrome phenotype of HO-2 null mice. The HO-2 isozyme is essential for regulating physiological levels of ROS. Recent studies have suggested a potential role of EET in modifying adipocyte differentiation through up-regulation of HO-1-adiponectin-AkT signaling in human mesenchymal stem cells (MSCs). Our aim was to examine the consequences of HO deficiency on MSC-derived adipogenesis in vitro using MSC derived from HO-2 null and WT mice in vivo.
Methods
Four-month-old HO-2 null (HO-2−/−) and B6/129SF2/J (WT) mice were divided into three groups (four mice/group): WT, HO-2−/−, and HO-2−/− +CoPP. Adipogenesis was performed on purified MSC-derived adipocytes cultured in adipogenic differentiation media and an EET-agonist was added every 3 days.
Results
HO-2 depletion of MSC adipocytes resulted in increased adipogenesis (p<0.01) and increased levels of inflammatory cytokines including (TNF)-alpha (p<0.05), (MCP)-1 (p<0.05), and (IL-1)-beta (p<0.05). These results were accompanied by decreases in HO-1 (p<0.05) and subsequently EET and HO activity (p<0.05). Up-regulation of HO-1 resulted in decreased MSC-derived adipocyte differentiation, decreased production of TNF-alpha and MCP-1 and increased levels of adiponectin (p<0.05). Cyp2J5 (p<0.05), HO-1 (p<0.05), and adiponectin mRNA levels (p<0.05) were also decreased in visceral adipose tissue isolated from HO-2 null compared to WT mice. EET agonist stimulation of MSC adipocytes derived from HO-2 null mice yielded similar results.
Conclusion
Increased levels of EET and HO-1 are essential for protection against the adverse effects of adipocyte hypertrophy and the ensuing metabolic syndrome. These results offer a portal into therapeutic approaches for the prevention of the metabolic syndrome.
Key Words: HO, Stress response genes, Antioxidants, Cardiovascular, Diabetes, Carbon monoxide, Bilirubin
Introduction
Metabolic syndrome is a multifactorial disease consisting of visceral adiposity, hypertension, dyslipidemia and insulin resistance. Obesity is a central and causal component of the metabolic syndrome [1], therefore much focus has been placed on impaired adipocyte function and inflammation as primary defects linking obesity to its associated pathological disorders [2]. Impaired adipocyte function is caused by a plethora of environmental and genetic factors; characterized by visceral accumulation of adipose tissue, changes in cellular composition adipose tissue, adipocyte hypertrophy, increases in secretion of pro-inflammatory cytokines (IL-6, TNF-α) [3] with concurrent decreases in cytoprotective adipokine-adiponectin levels, and alterations in adipocyte and adipose tissue mRNA and protein expression patterns [2]. Adipokines have important autocrine/paracrine roles in regulating adipocyte differentiation and metabolism and local inflammatory processes [4]. These changes contribute to insulin resistance and the eventual pathogenesis of obesity-associated metabolic syndrome.
Mesenchymal stem cells (MSCs) have emerged as an important model for the study of adipogenesis and adipocyte development [5]. Several studies have shown mesenchymal stem cells to be a reservoir for recruitment of new preadipocytes [4] and generation of adipocytes through the process of adipogenesis [6]. In addition to resident stem cells, bone marrow MSCs can be recruited to adipose tissue and generate new healthy adipocytes in response to treatment such as thiazolidinediones (TZDs).
TZDs and other PPARy agonists, have been shown to upregulate plasma adiponectin levels, increase adipose mass via production of small adipocytes, and to cause a reduction in oxidative stress. In addition, TZDs have been proposed to ameliorate insulin resistance by increasing the number of small adipocytes that are more sensitive to insulin. An increase in the number of small adipocytes has been associated with increased adiponectin [7, 8, 9]. We and others have proposed methods to enhance the production of adiponectin, for instance through the generation of small adipocytes which abundantly express and secrete adiponectin and/or through directly activating adiponectin gene transcription [10, 11, 12].
Oxidative stress is an additional component preceding metabolic syndrome. Oxidative stress is an imbalance in reactive oxygen species (ROS) with a reduction in protective antioxidant systems. This imbalance in ROS leads not only to an impairment in mesenchymal function but also to adipocyte derangements and an elevation of inflammatory cytokines secreted from adipose tissue [8, 13].
Heme oxygenase, which consists of inducible and constitutive forms (HO-1, HO-2 respectively) catalyzes the breakdown of heme into equimolar of bilirubin, carbon monoxide and iron [14, 15]. HO-2 is responsible for maintaining normal metabolic cellular functions, i.e. vascular tone, renal channel function and activity [15] and, maintenance of body weight, insulin sensitivity, and normal blood pressure [16]. In the absence of HO-2, there is a marked increase in oxidative stress, chronic inflammation, and a loss of protection against diabetes-induced renal injury [17, 18]. HO-1 is a stress responsive enzyme which, through its breakdown of heme, causes the production of potent antioxidant and anti-inflammatory molecules [19, 20] and affords protection against disease progression [15]. Due to the ubiquitous expression, HO is able to affect many pathways including COX-1, COX-2 and act as a major regulator of several CYP450 enzymes [15].
Sacerdoti et al, described the interaction of HO-1 expression and HO activity with P450 epoxygenase metabolites epoxyeicosatrienoic acids (EETs) and showed in endothelial cells that an increase in EET levels was associated with an increase in HO activity [21, 22]. Recently, an EET agonist has been reported to affect adipocyte formation and adipogenesis in human MSCs [23]. The present study was designed to elucidate the interaction(s) between the HO system and EETs in the regulation of MSC adipocyte differentiation, and to determine if this approach offered therapeutic insights into the amelioration of the metabolic syndrome in the HO-2 null mice animal model.
Materials and Methods
Animal Experimentation
All animal experiments followed an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. HO-2−/− mice are derived as described by Poss et al. [24]. HO-2−/− mice have a C57BL/6 × 129/Sv genetic background that was used on age- and gender-matched controls. Four-month-old HO-2−/− and B6/129SF2/J (WT) mice were used for these studies. Mice were divided into three groups (four mice/ group): WT, HO-2−/−, and HO-2−/− + cobalt protophoryrin (CoPP). CoPP was injected intraperitoneally (i.p.) weekly at a dose of 3 mg/kg for four weeks. Food intake did not change, and the animals had access to water ad libitum. Tin mesoporphyrin (SnMP) was injected i.p. at a dose of 3 mg/kg twice a week for 4 weeks. At the time of sacrifice, abdominal visceral fat and bone marrow were isolated, from each mouse, and weighed. Specimens were maintained at −80°C until needed.
Chemicals
CoPP and SnMP dichloride were purchased from Frontier Science, Logan, UT. The EET agonist 13-(2-(butylamino)-2-oxoacetamido)tridec-8(Z)-enoic acid was synthesized by Dr. Falck. The compound was used at final concentrations in cell culture of 1 µM and 10 µM.
Isolation of bone marrow cells
Bone marrow cells were obtained from 3-4 month old HO-2−/− mice through marrow flushing. Cells were plated at a density of 1-2×106 cells per 100 cm2 dish. The cultures were maintained at 37 °C in a 5% CO2 incubator and the medium was changed after 48h and every 3~4 days thereafter. Cells were used after passage 2-3.
Adipogenesis
When the MSCs were confluent, the cells were recovered by the addition of TrypLE Express (Invitrogen, Carlsbad, CA). MSCs were plated in a 75-cm2 flask at a density of 1-2×104 cells and cultured in alpha-MEM with 10% FBS for 7 days. The medium was replaced with adipogenic medium, and the cells were cultured for an additional 15 days. The adipogenic media consisted of complete culture medium supplemented with DMEM-high glucose, 10% (v/v) FBS, 10 ug/ml insulin, 0.5 dexamethasone (Sigma-Aldrich, St.Louis, MO), 0.5 mM isobutylmethylxanthine (Sigma-Aldrich) and 0.1 mM indomethacin (Sigma-Aldrich). MSC-derived adipocytes were cultured in adipogenic differentiation media and an EET-agonist was added every 3 days at a dose of 1 µM, or 10 µM.
Oil Red O Detection of Adipocytes and Lipid Droplet Size and Number
For Oil Red O staining, 0.21% Oil Red O in 100% isopropanol (Sigma-Aldrich) was used. Briefly, adipocytes were fixed in 10% formaldehyde, washed in Oil Red O for 10 min, rinsed with 60% isopropanol (Sigma-Aldrich), and the Oil Red O eluted by adding 100% isopropanol for 10 min, where after OD was measured at 490nm, for an 0.5 sec reading. MSC-derived adipocytes were measured by Oil Red O staining (OD=490nm) after day 14. The Oil Red O staining was normalized to a specific number (Values at OD490nm/104 cells ratio).
Lipid Droplet Size Analysis
After induction of adipogenesis, lipid droplets were stained with Oil Red O. Cell size was measured using ImagePro Analyzer Software (MediaCybernetics, Inc., MD). The classification of the size of lipid droplets was based on the size of the two-dimensional area and expressed as pixels.
Western Blotting
Adipocytes were incubated with stimulants in T75 flasks for 24 hours. They were then washed with PBS and trypsinized (0.05% trypsin w/v with 0.02% EDTA) and then pelleted by centrifugation. The pellets were lysed in buffer (Tris-HCl 50 mM, EDTA 10 mM, Triton X-100 1% v/v, PMSF 1%, pepstatin A 0.05 mM and leupeptin 0.2 mM) and, after mixing with sample loading buffer (Tris-HCl 50 mM, SDS 10% w/v, glycerol 10% v/v, 2-mercaptoethanol 10% v/v and bromophenol blue 0.04%) at a ratio of 4:1, were boiled for 5 min. Samples (10 µg protein) were loaded onto 12% gels and subjected to electrophoresis (150 V, 80 min). The separated proteins were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA; 1 h, 200 mA per gel). After transfer, the blots were incubated overnight with 5% nonfat milk in TBS followed by incubation with an 1:1000 dilution of the primary antibody for 3 h. Polyclonal rabbit anti HO-1 antibody was purchased from Stressgen Biotechnologies (Victoria, BC). After washing with TTBS, the blots were incubated for 2 h with secondary antibody (1:5000) and conjugated with horse radish peroxidase. Finally, the blots were developed using a premixed solution containing 0.56 mM 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and 0.48 mM nitro blue tetrazolium (NBT) in buffer (Tris-HCl 10 mM, NaCl 100 mM, MgCl2 59,3 µM, pH 9.5). The blots were scanned and the optical densities of the bands were measured using Scion (New York, NY) Image Software [25].
Cytokine Analysis
Levels of IL-6, IL-1β, IL-α, MCP-1 and TNF-α were determined using an enzyme-linked immunoabsorbent assay mouse obesity strip (Signosis, Sunnyvale, CA). The high-molecular-weight-form of adiponectin was determined by enzyme-linked immunosorbent assay (Assaygate, Ijamsville, MD).
EET measurements
Mononuclear cells were homogenized in 66% methanol containing a 500-pg mixture of internal standards (prostaglandin E2-d4, 8(9)-EET-d11, 11(12)-EET-d8, 12-hydroxyeicosatrenoic acid-d8, 20-hodroxyeicosatrenoic acid-d6, and 11, 12- DHET-d11). EETs and DHETs were extracted using solid phase C18-ODS Accubond II 500-mg cartridges (Agilent Technologies, Santa Clara, CA). In brief, each sample was centrifuged for 15 min at 4°C. The supernatant was then collected, diluted with 15 ml of water, and acidified to pH 4.0 with HCl. Cartridges were primed with 20 ml of methanol followed by 20 ml of water. Samples were loaded, washed with 20 ml water and 8 ml hexane, and eluted with 8 ml of methanol. The collected methanol fraction was dried under nitrogen and resuspended in 200 µl of methanol and stored at −80°C until analysis. Identification and quantification of EETs and DHETs were performed using a Q-trap 3200 linear trap quadruple liquid chromatography-tandem mass spectrometry system equipped with a Turbo V ion source operated in negative electrospray mode (Applied Biosystems, Foster City, CA). Extracted samples were suspended in 10 µl of methanol and injected into a high-performance liquid chromatography via an Agilent 1200 standard series autosampler equipped with a thermostat set at 4 °C (Agilent Technologies). The highperformance liquid chromatographic component comprised of an Agilent 1100 series binary gradient pump equipped with an Eclipse plus C18 column (50 × 4.6 mm; 1.8 mm) (Agilent Technologies). The column was eluted at a flow rate of 0.5 ml/min with 100% mobile phase A; methanol/water/acetic acid (60:40:0.01, v/v/v) from 0 to 2 min and a gradient increasing to 100% B (100% methanol) at 13 min. Multiple reaction monitoring was used with a dwell time of either 25 or 50 ms for each compound, with the following source parameters: ion spray voltage, −4500 V; curtain gas, 40 U; ion source gas flow rate 1, 65 U; ion source gas flow 2, 50 U; and temperature, 600°C. Synthetic standards were used to obtain standard curves (5-500pg) for each compound. These standard curves were used to calculate the final EET and DHET concentrations, which are expressed as picograms per 106 cells.
RNA purification
The RNA was extracted from frozen adipose tissue by RNeasy® Lipid Tissue (Qiagen, Valencia, CA), as described by the manufacturer. The amount of RNA was determined by measuring the absorbance at 260 nm (A260) with Nanodrop (Thermo Scientific, Wilmington, DE), and quality was assessed by the A260/A280 ratio with values of 1.8-2.0 and A260/A230 ratio with values greater than 1.7, regarded as satisfactory purity. The Ribosomal RNA band integrity was checked by denaturing agarose/formaldehyde gel electrophoresis and ethidium bromide staining.
Reverse transcription
Total RNA from different samples was reverse transcribed using Super Script III First-Strand Super Mix (Invitrogen), with random hexamers as primers. Briefly, the mix with RNA, random Hexamer primer, and annealing buffer were incubated at 65°C for 5 min, followed by a short incubation on ice. Then 400 U of superscript III reverse transcriptase and 2X first-Strand Reaction Mix were added and the mixture incubated at 25°C for 5 min, followed by incubation at 50°C for 50 min. The reaction was terminated at 85°C for 5 min and chilled on ice. All cDNA samples were stored at −20°C.
Real Time-PCR reaction
The PCR reactions were performed in duplicate using 96-well optical reaction plates on an ABI Prism® 7500 Fast Real Time PCR System (Applied Biosystems). For each reaction, 25 ng of cDNA was placed in a well with 10 µl of Power SYBR Green Master Mix (Applied Biosystems) and 1 µl of each pair of primers (SABiosciences, Frederick, MD). The specificity of PCR amplification was checked with the heat dissociation protocol following the final cycle of PCR.
To correct for differences in the amount of starting first-strand cDNAs, the mouse GADPH gene was amplified in parallel as a reference gene. The quantification of the HO-1, Adiponectin, and CYP2J5 genes was performed according to the comparative method (2-ΔΔCt; [26]). Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays was carried out with the ΔCt for HO-2−/− as calibrator. 2-(ΔCtsample-ΔCtcalibrator), where ΔCt sample is Ct sample - Ct reference gene, and Ct is the threshold cycle, the PCR cycle number at which emitted fluorescence exceeds 10 times the SD of baseline emissions [26].
Statistical Analysis
Statistical significance (p<0.05) between experimental groups were determined by the Fisher method of analysis of multiple comparisons. For comparison between treatment groups, the null hypothesis was tested by either a single-factor ANOVA for multiple groups or unpaired t tests for two groups and the data presented as the mean ± SE.
Results
Increased adipocyte hypertrophy in HO-2−/− mice compared to WT-MSC derived adipocyte during adipogenesis
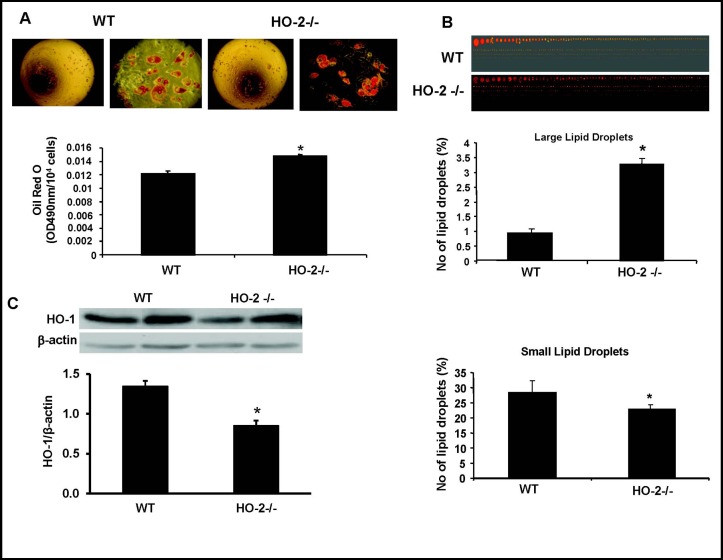
We measured the size and number of lipid droplets formed after induction of adipogenesis in MSCs derived from WT and HO-2−/− mice. There was an 18% increase in adipogenesis in HO-2−/− MSC-derived adipocytes compared to WT MSC-derived adipocytes (p< 0.01, Fig. 1A). In addition to an increase in adipogenesis, there was a 3.5 fold increase in the percentage of large adipocytes compared to WT MSC-derived adipocytes (p<0.05, Fig. 1B). There was a small but significant decrease in the percentage of small MSC derived adipocytes from HO-2−/− compared to WT MSC-derived adipocytes (p<0.05, Fig. 1B).
Fig. 1.
A) Adipocyte hypertrophy in HO-2−/− and WT mice. Representative photographs showing WT and HO-2−/− MSCs during adipogenesis. These pictures are representative of six experiments at 14 days, *p<0.01 compared to WT. B) Lipid droplets were stained with Oil Red O and size of droplets were measure using Image Pro Analyzer (ver. 6.2, Media Cybernetics, Inc., MD, *p<0.05 vs. WT). Percentage of small and large lipid droplets were calculated as described in the Methods section. C) Western blot and densitometry analysis of HO-1 expression in HO-2−/− and WT MSC-derived measured after 14 days of adipogenic differentiation. We performed quantitative densitometry evaluation of HO-1 in the cells. *p<0.05 vs control. Each bar represents mean ± SE of 4 experiments.
HO-1 protein expression is decreased in HO-2−/−derived adipocytes
We measured the protein expression of HO-1 in both WT and HO-2−/− MSC-derived adipocytes. Western blot analysis showed a significant decrease (p<0.05) in the ratio of HO-1 to actin in MSC-derived adipocytes after Day 14 in HO-2−/− MSC-derived adipocytes compared to WT cells (Fig. 1C).
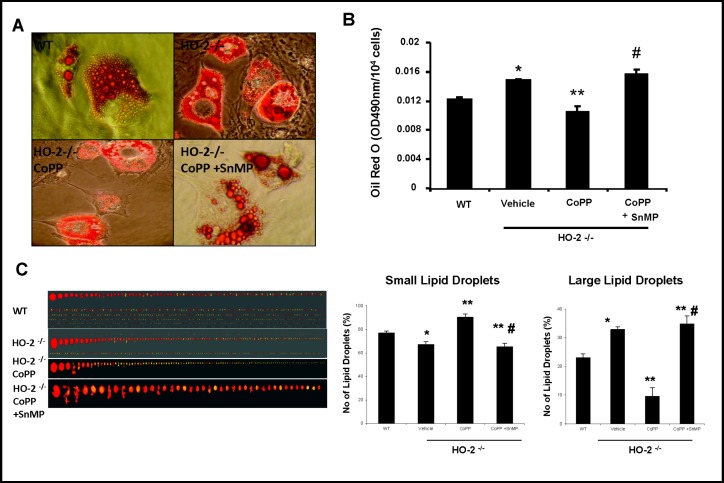
Effect of HO-1 induction and inhibition in HO-2−/− MSC derived adipocytes
We examined the effect of CoPP on lipid size and number by counting cells with lipid droplets in the cytoplasm and measuring lipid size by area of pixels using ImagePro analysis (Fig. 2). The percentage of large lipid droplets defined by areas larger than 100 pixels was increased in HO-2−/− MSC-derived adipocytes. There was an increase in the percentage of small lipid droplets (90.3% ± 2.7 vs. 61.7% ± 2.3%) (Fig. 2C) accompanied by a decrease in the percentage of large lipid droplets (14.3% ± 3% vs. 38.4 ± .98 %) (Fig. 2C) in cells with up-regulated HO-1 after CoPP treatment as compared to untreated cells. A reversal of these changes was observed with the addition of SnMP. These results indicate that upregulation of HO-1 and increased HO activity not only decreases adipogenesis, but also directly shifts the adipogenic profile of HO-2 derived cells into increasing production and/or limiting growth of small adipocytes while also decreasing the presence of larger lipid droplets.
Fig. 2.
Pharmacological effect of and HO-1 inducer (CoPP) and an inhibitor (SnMP) of HO activity for 4 weeks on HO-2−/− MSCs-derived adipocyte cell differentiation. A, B) Representative photographs of size by size comparison of the effect of CoPP and SnMP on Oil Red O staining of lipid droplets. C) The percent of small and large adipocytes as determined by Image Pro Analyzer. Values are means ± S.E., n=4-6; *p<0.05 versus untreated WT; **p<0.05 versus vehicle HO-2−/−; # p<0.05 versus HO-2−/− + CoPP.
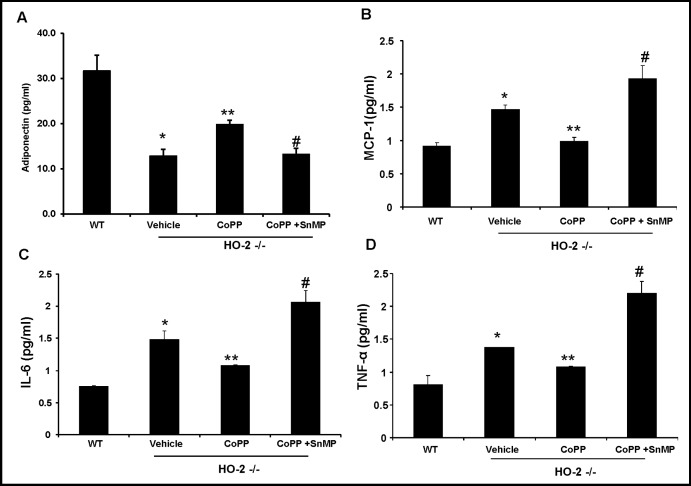
Effect of HO-1 induction on adiponectin and inflammatory cytokines: MCP-1, TNF-α, and IL-1β levels
As shown in Fig. 3A, adiponectin levels were 27.2 ± 9.8 % lower (p<0.05) in HO-2−/− MSC-derived adipocytes compared to WT MSC-derived adipocytes.
Fig. 3.
Effect of CoPP and SnMP treatment on serum cytokine levels in MSC derived adipocytes. A) Supernatant adiponectin levels. B) Supernatant MCP-1 levels. *p< 0.05 versus WT; **p<0.05 versus HO-2−/−; #p<0.05 versus HO-2−/− +CoPP. C) Supernatant IL-1β levels. *p< 0.05 versus WT; **p<0.05 versus HO-2−/−; #p<0.05 versus HO-2−/− +CoPP. Values are means ± S.E., n=4. D) Supernatant TNF-α levels. *p< 0.01 versus WT; **p<0.05 versus HO-2−/−; #p<0.05 versus vehicle HO-2−/− +CoPP.
HO-1 induction resulted in a 1.8 fold increase in adiponectin levels compared to levels of adipocytokine in untreated HO-2−/− cells (p<0.05, Fig. 3A). This change was reversed by HO-1 inhibition. A reciprocal effect was seen in inflammatory cytokine levels: MCP-1, IL-1β, and TNF-α. The HO-2−/− MSC-derived adipocytes displayed increased levels of inflammatory molecules compared with WT MSC-derived adipocytes. As seen in Figure 3B, culture media levels of MCP-1 from HO-2−/− derived cells were 32.1 ± 1.1% higher (p<0.05) than in media collected from WT derived cells. Likewise, IL-1β and TNF-α levels were 41.5 ± 9.9% and 42.5 ± 4.7% repectively higher (p<0.05) than WT derived cells (Fig. 3C and 3D). Treatment of HO-2−/− derived cells with CoPP decreased levels of MCP-1, IL-1β, and TNF-α to levels not significantly different from those in WT derived cells. These changes with CoPP treatment were reversed by the addition of SnMP, levels of IL-1β and TNF-α were significantly (p<0.05) elevated compared to HO-2−/−derived cells.
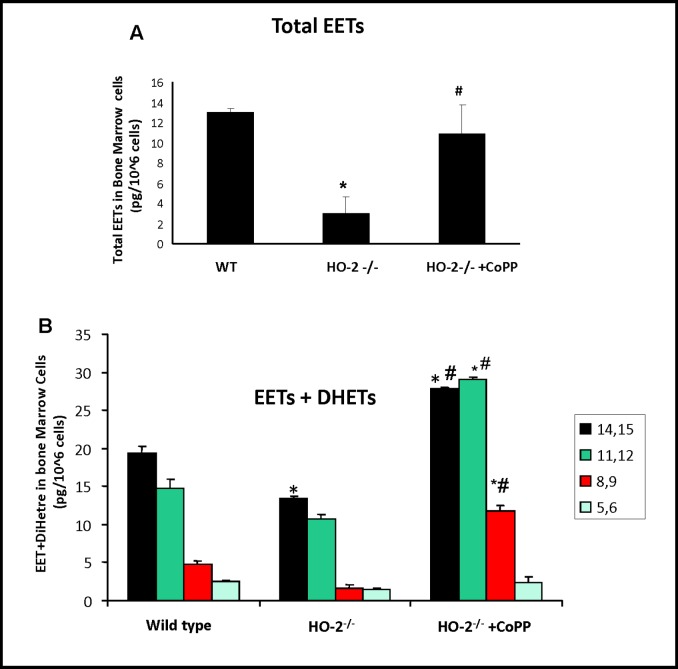
Effect of HO-2 deletion on EET levels and the estimated epoxygenase activity
Epoxyeicosatrienoic acids (EETs) share many common biological activities with HO in addition to vasodilatory properties, both HO and EETs act as anti-inflammatory agents and stimulate insulin sensitivity. We therefore examined whether basal levels of EETs were lowered in HO-2−/− derived bone marrow cells and whether stimulation via EET agonists would induce changes in adipogenesis and shift the cytokine profile from inflammatory to a healthier immune profile. In WT mice, mononuclear EETs were composed of 14, 15-, 11,12-, and 8,9-EETs at a ratio of 3:1:1. As seen in Fig. 4, mononuclear EETs levels derived from HO-2−/− as well as total epoxygenase activity (EETS+DHETS) were significantly lower than that in the mononuclear cells derived from WT. The reduction was comparable for all four EET regioisomers (Fig. 4B). Treatment with CoPP increased EET levels by 8.2-fold and epoxygenase activity, as determined by the sum of EETs+DiHET, was increased by 2.6 fold.
Fig. 4.
The levels of EETs/DHETS in HO-2−/− derived bone marrow cells. A) EET levels in bone marrow cells. Values are means ± S.E., n=8; *p<0.05 versus WT mice; #p<0.05 versus vehicle HO-2−/− mice. B) Levels of EETs + DHETs in bone marrow cells. Values are means ± S.E., n=9; *p<0.05 versus WT mice; **p<0.05 versus HO-2−/− mice; #p<0.05 versus HO-2−/− mice.
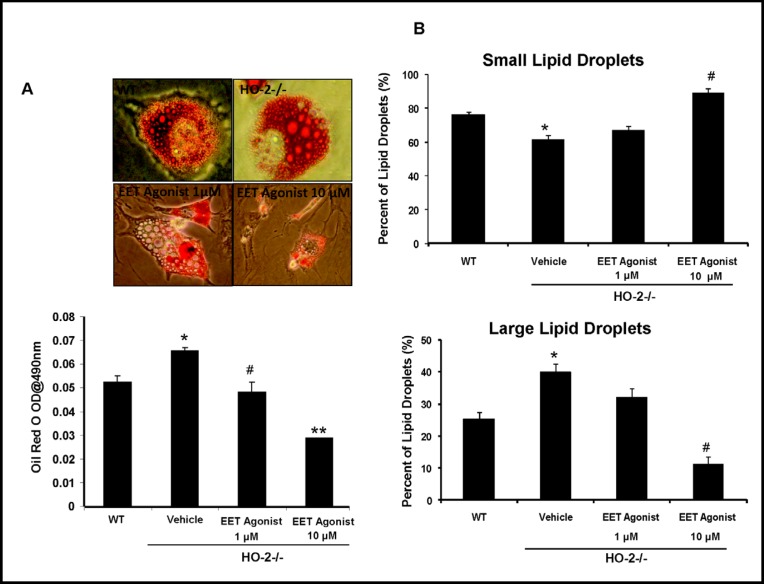
Effect of EET-agonist 13-(2-(butylamino)-2-oxoacetamido)tridec-8(Z)-enoic acid on adipogenesis
We investigated whether the EET-agonist 13-(2-(butylamino)-2- oxoacetamido)tridec-8(Z)-enoic acid would reverse the effect of increased adipogenesis in HO-2−/− compared to WT derived cells. The percentage of cells with morphological large lipid droplets was decreased as EET concentration increased. As shown in Fig. 5, EET-agonist increased the percentage of smaller lipid droplets while decreasing the percentage of larger lipid droplets in a concentration dependent manner. These results indicate that up-regulation of EETs has a similar effect to CoPP in reducing adipogenesis and increasing the number of small lipid droplets in HO-2−/−; thus; resembling the lipid droplet composition seen in WT MSC-derived adipocytes.
Fig. 5.
Pharmacological effect of an EET-agonist on HO-2−/− MSCs-derived adipocyte cell differentiation. The final concentrations of the EET agonist were 1 µM and 10 µM. A) Dose-response effect of EET-agonist on Oil red O stained lipid droplets. Values are means ± S.E., n=4; *p< 0.05 versus WT, #p<0.05 versus HO-2−/−, **p<0.05 versus HO-2−/− +EET 1 µM. B) The percent of small and large adipocytes determined by computerized image analysis. Values are means ± S.E., n=4; *p< 0.05 versus vehicle HO-2−/−. **p<0.05 versus vehicle HO-2−/− mice; #p<0.05 versus vehicle HO-2−/−.
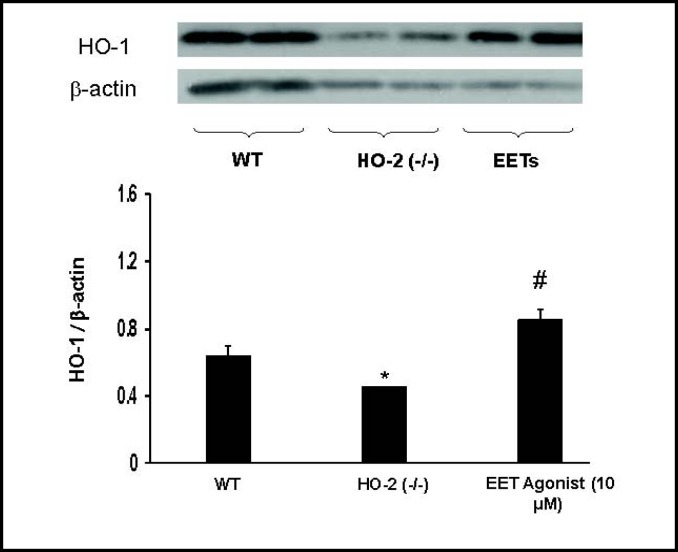
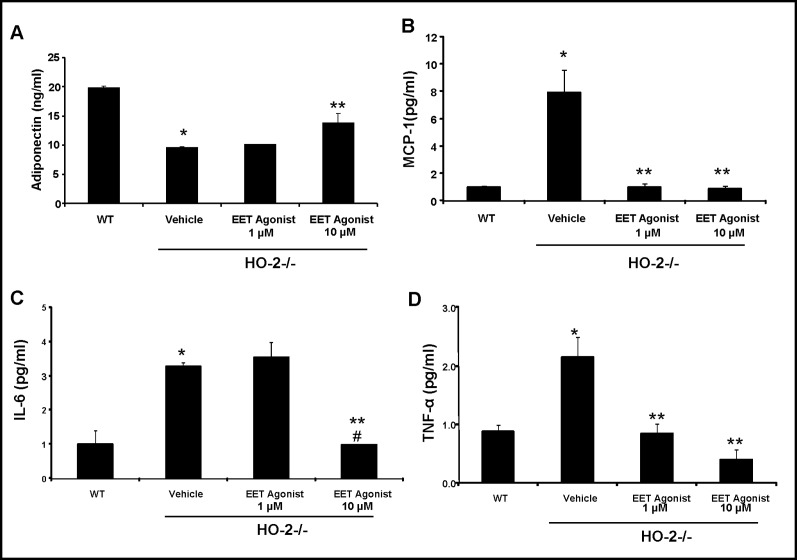
Effect of EET-agonist on HO-1 protein expression, adiponectin, MCP-1, TNF-α, and IL-1β levels
Western blot analysis clearly demonstrated that EET up-regulation via 13-(2-(butylamino)-2- oxoaceta-mido)tridec-8(Z)-enoic acid increased HO-1 expression, restoring previously low levels of HO-1 in HO-2−/− derived cells (Fig. 6). Since EET up-regulation rescued both the adipocyte profile and HO-1 expression in HO-2−/− derived cells, we examined whether EET induction would also produce increases in adiponectin and decreases in inflammatory cytokines. Adiponectin levels were significantly lower (p<0.05) in HO-2−/− MSC-derived adipocyte compared to WT MSC-derived adipocytes. Treatment of HO-2−/− MSC-derived adipocytes with the EET agonist led to a partial restoration of adiponectin levels (p<0.05, Fig. 7A). This effect was dose dependent. The HO-2−/− derived MSC adipocytes displayed high levels of MCP-1, TNF-α and IL-1α culture media, treatment with EET agonist reduced MCP-1, TNF-α and IL-1α levels in a dose dependent manner (Fig. 7).
Fig. 6.
The effect of EET agonist on the levels of expression of HO-1 in MSC-derived adipocytes. The MSC derived adipocytes were obtained from EET treated HO-2−/− mice. EET agonist (10 µM final concentration) was added to MSC adipocytes obtained from HO-1−/− mice. Western blot and densitometry analysis analysis of HO-1 in MSC derived adipocytes after Day 14. Values are means ± S.E., n=3;#p<0.05 vs. WT,*p<0.05 vs vehicle HO-2−/−.
Fig. 7.
The effect of EET agonist (1 µM and 10 µM concentration) on serum cytokines in MSC adipocytes derived from HO-2 mice. A) Supernatant adiponectin levels. *p<0.01 vs WT, **p<0.05 vs vehicle HO-2−/−. B) Supernatant MCP-1 levels. *p< 0.05 versus WT; **p<0.05 versus vehicle HO-2−/− C) Supernatant IL-1α levels. *p< 0.05 versus WT; **p<0.05 versus vehicle HO-2−/−; #p<0.01 versus HO-2−/− +EET 1 µM. Values are means ± S.E., n=6. D) Supernatant TNF-α levels. *p< 0.05 versus WT; **p<0.05 versus vehicle HO-2−/−.
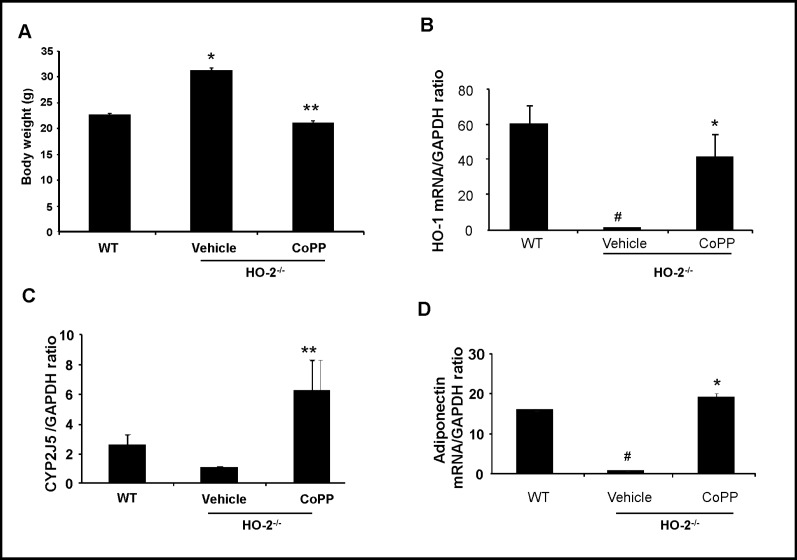
Effect of CoPP treatments on body weight and mRNA expression levels of HO-1, adiponectin, and CYP2J5 levels from visceral adipose tissue of HO-2−/− null mice
Given the effects of CoPP on EET production, adipocyte size, adiponectin and inflammatory cytokine levels in our in vitro studies, we wanted to confirm whether similar changes take place in vivo. CoPP treatment significantly decreased total body weight of HO-2−/− mice (p<0.05) Fig. 8A. The decrease in body weight was accompanied by changes in mRNA levels of HO-1, adiponectin, and CYP2J5, a gene responsible for production of primarily 14,15 and 11,12 EETs Figure 8. HO-1 and adiponectin mRNA levels were significantly increased to levels similar to those of WT (p<0.05 and p<0.05 respectively), while mRNA of CYP2J5 was increased and surpassed that of basal levels of CYP2J5 in WT visceral adipose tissue (p< 0.05) (Fig. 8B-D).
Fig. 8.
The effect of CoPP on body weight and mRNA expression HO-1, CYP2J5, and Adiponectin within visceral adipose tissue in HO-2−/− mice. A) Body weight. Values are means ± S.E., n=6; *p<0.05 versus WT mice; **p< 0.05 versus HO-2−/−mice. B-D) Quantitative analysis of HO-1, CYP2J5, and adiponectin mRNA levels measured after 4 week treatment with CoPP. Values are mean ± S.E., n=4-5; B) HO-1 mRNA levels; #p<0.01 versus WT mice; *p<0.05 versus vehicle HO-2−/− mice. C) CYP2J5 mRNA levels; *p<0.05 versus WT mice: **p<0.05 versus vehicle HO-2−/− mice. D) Adiponectin mRNA levels; #p<0.05 versus WT mice; *p<0.05 versus vehicle HO-2−/− mice.
Discussion
We clearly demonstrate that HO-2 plays a vital role in adipogenesis and visceral adipose function. We have defined a role for HO-1, in the regulation of body weight, adipocyte differentiation, and secretion of inflammatory cytokines. We examined the consequences of HO deficiency on adipocyte function during adipogenesis. We show that HO-2 deletion can act directly on adipocytes and their progenitors to increase adipogenesis and adipocyte hypertrophy. HO-1 rescue of HO-2 deficiency resulted in restoration of adipocyte function via cellular composition change of lipid droplets, decrease in secretion of inflammatory cytokines, and up-regulation of cytoprotective-adiponectin.
Our findings indicate that HO-2 not only plays a vital role in the regulation of metabolic functions of differentiated adipocytes, but also in the regulation of adipogenesis. We showed an increase in adipogenesis and accumulation of lipid droplets resulting in adipocyte hypertrophy in HO-2 null mice compared to WT mice. Previously, in vivo studies in our lab have demonstrated that HO-2 deficiency leads to manifestations of the metabolic syndrome, including obesity, hypertension, and insulin resistance [16]. HO-2 deletion has also been shown to result in enhanced diabetes-induced renal dysfunction and morphological injury, and with induction of HO-1 these morphological changes are prevented [17]. Our results also showed that accompanied with HO-2 deletion, there was a decrease in HO-1 protein expression within MSC-derived adipocytes compared to cells derived from WT mice. However, this is not surprising because HO-2 is critical for HO-1 expression and failure to up-regulate the HO system has dire consequences i.e. oxidative stress [27, 28], insulin resistance [29], and attenuation of hypertension [30, 31]. HO levels have been shown to be lower in several models of obesity [32], diabetes [17], atherosclerosis [33] and metabolic syndrome [34]. Hence, it is likely diminished activity of the HO system may contribute to increased oxidative stress and inflammatory conditions and subsequent derangements in adipocyte and metabolic function.
Our findings indicated that the induction of HO-1 via CoPP treatment caused a decrease in droplet formation and adipogenesis in HO-2−/− bone marrow-derived adipocyte stem cells and increased the secretion of adiponectin in the culture media. This novel observation was accompanied by the demonstration that induction of HO-1 resulted in a reciprocal decrease in secretion of TNF-α, IL-1α, and MCP-1. These results were reversed with SnMP, a known inhibitor of human HO activity [35]. The decrease in lipid droplet size indicative of smaller adipocytes, decreased cytokine levels accompanied by increases in adiponectin levels in vitro are suggestive of changes in the pre-adipocyte environment similar to changes seen in treatment of metabolic syndrome. Several studies have examined the inextricable link between adipocyte hypertrophy and inflammation; followed by a reduction in adipocyte size leading to amelioration of metabolic syndrome [7, 36]. This phenomenon has been extensively studied in both mice and humans treated with thiazolidinedione PPAR-γ agonists. Mammals treated with thiazolidinediones show a reduction in adipocyte size and an increased number of small adipocytes that are more insulin sensitive [7]. Similar results were obtained by HO-1 pre-condtioning using CoPP in diabetic mice or diabetic rats [12, 13, 32]. Up-regulation of the HO system in adipose tissue of obese mice led to a reduction of adipocyte size and abrogation of the underlying chronic inflammatory environment [13].
We show here for the first time that EET levels are decreased in bone marrow derived cells from HO-2 null mice prior to adipocyte generation; and that EET levels are restored with HO-1 induction via CoPP. These results also show a decrease in activity of the arachidonic acid metabolic pathway that yield EETs. We also demonstrate that treatment with the EET agonist 13-(2-(butylamino)-2-oxoacetamido)tridec-8(Z)-enoic acid is very effective in suppressing adipogenesis. EETs also showed similar results as CoPP in reducing the levels of inflammatory cytokines with an accompanying increase in adiponectin secretion in vitro.
The specific mechanisms by which HO-1 and EETs regulate adipogenesis and adipocyte metabolism will require further study, but a number of mechanisms are thought to participate. Several studies have shown, in addition to the vasodilatory effects of EETs, that EETs also cause an anti-inflammatory effect on the adipocyte and endothelium via inhibition of cytokine and activation of PPARs [9, 37, 38, 39, 40]. In addition to their beneficial effects in the vasculature and their anti-inflammatory actions, EETs also affect lipid metabolism and insulin sensitivity. Furthermore, CYP expression is decreased and sEH expression is increased in both obese Zucker rats [41] and human MSC derived adipocytes [23]; indicating that a deficiency in EET production and increased EET degradation occurs early in developing adipocyte hypertrophy and dysfunction [9]. Sacerdoti et al showed EETs to be potent inducers of HO-1 and HO activity, suggesting one of the possible mechanisms involved in its actions on inflammation may be through up-regulation of the HO system [21]. Our results also confirm a positive feedback loop in which HO-1 induction increases EET levels, and vice versa [9, 23]. Another possible mechanism by which HO increases EETs may be related to the well known antioxidant actions of the HO system. Oxidative stress is an integral component of metabolic syndrome that plays a role both in endothelial dysfunction and insulin resistance becoming a causal factor in disease progression and future risk outcomes [42, 43]. Additionally, several reports show that oxidative stress and O2− cause a reduction of EET levels [39, 44]. A lack of HO-2 creates an environment that promotes oxidative stress-related disturbances reflected in increased levels of O2− and decreases in EC-SOD [45]. Therefore, suggesting that the highly pro-oxidant environment in HO-2−/− mice allows for reduced production of EET levels and thereby possible activation of the ER stress response initiating the inflammatory signaling cascade [23, 46]. Our results strongly suggest that this cascade can be prevented via HO-1 induction. Induction of HO-1 in obese and diabetic models, increases HO activity resulting in decreases in oxidative stress and ROS production and a reestablishment of the antioxidant and ROS balance [12], restoration of EET levels, attenuation of adipogenesis, and prevention of inflammatory cytokine recruitment.
Our in vitro studies suggest that HO-2 and EET deficiency results in increased adipogenesis and production of circulating inflammatory cytokines that can be corrected by induction of HO-1 and/or EET agonist treatment. We wanted to confirm whether this phenomenon also occurs in vivo. HO-2 deficient mice have increased body weight that is preventable via weekly treatments with a low dose of CoPP. HO-1, CYP2J5, and adiponectin mRNA expression were lowered in visceral adipose tissue of HO-2 deficient mice compared to WT mice. Induction of HO-1 via CoPP resulted in restoration of HO-1, CYP2J5, and adiponectin levels similar to that of WT mice, (Fig. 9) indicating EET and adiponectin production are reduced within the visceral adipose tissue of HO-2 deficient mice but can be rescued (Fig. 9). Together with our in vitro studies, our results suggest that HO-1 and EETs are primary influences of the regulation of adipogenesis and adipocyte function before the development of obesity. Additionally, deficiencies within these two systems have a negative effect of resulting in reduction of adiponectin levels and a systemic inflammatory response. HO-2 deficient mice may serve as a useful model for the study of adipogenesis and adipocyte development within a chronic inflammation and oxidative stress environment. Additionally, the HO-EET-adiponectin relationship may be a novel useful target for future drug therapies of the leading disease pathologies facing Westernized countries: obesity and metabolic syndrome.
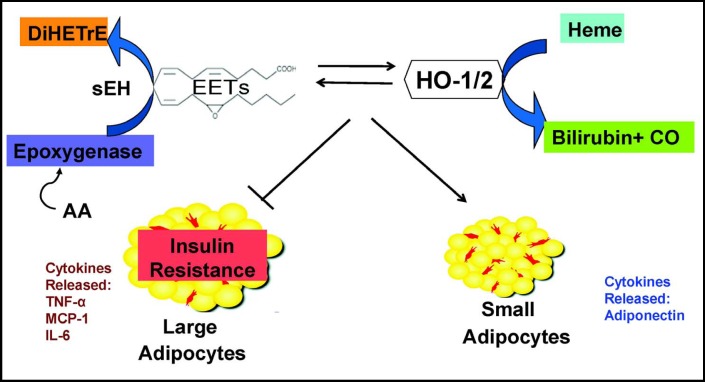
Fig. 9.
Proposed mechanism for the EET agonist-mediated suppression of MSCs-derived adipocyte differentiation and lipid accumulation. HO-2 deletion and EET deficiency act directly on adipocytes and their progenitors to increase adipogenesis and adipocyte hypertrophy. EET agonist-induced HO-1 resulted in restoration of adipocyte function via cellular composition change of lipid droplets, decrease in secretion of inflammatory cytokines, and up-regulation of cytoprotective-adiponectin.
Acknowledgements
This work was supported by NIH grants DK068134, HL55601 (NGA), HL34300 (MLS), and The Beatrice Renfield Foundation (AK).
Abbreviations
- HO-1
(Heme oxygenase-1)
- HO-2
(Heme oxygenase-2)
- EET
(epoxyeicosatrienoic acid)
- ROS
(reactive oxygen species)
- MSC
(mesenchymal stem cells)
- TNFα
(tumor necrosis factor α)
- MCP-1
(Macrophage chemoattractant protein-1)
- Cyp2J5
(Cytochrome P4502J5)
- IL-1
(interleukin-1)
- IL-6
(interleukin-6)
- TZD
(thiazolidinedione)
- i.p.
(intraperitoneally)
- SnMP
(tin (Sn4+) mesoporphyrin dichloride)
- EET
agonist (13-(2-(butylamino)-2-oxoacetamido)tridec-8(Z)-enoic acid)
References
- 1.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117:241–250. doi: 10.1055/s-0029-1192044. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 4.Gustafson B. Adipose Tissue, Inflammation and Atherosclerosis. J Atheroscler Thromb. 2010;17:332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 5.Armani A, Mammi C, Marzolla V, Calanchini M, Antelmi A, Rosano GM, Fabbri A, Caprio M. Cellular models for understanding adipogenesis, adipose dysfunction, and obesity. J Cell Biochem. 2010;110:564–572. doi: 10.1002/jcb.22598. [DOI] [PubMed] [Google Scholar]
- 6.Crossno JT, Jr., Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JY, van de WE, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev. 2010;19:1863–1873. doi: 10.1089/scd.2010.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanella L, Kim DH, Sodhi K, Barbagallo I, Burgess AP, Falck JR, Schwartzman ML, Abraham NG. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat. 2011;96:54–62. doi: 10.1016/j.prostaglandins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 11.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Burgess AP, Li M, Tsenovoy PL, Addabbo F, McClung JA, Puri N, Abraham NG. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther. 2008;325:833–840. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- 13.Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, Sodhi K, Canestraro M, Martasek P, Peterson SJ, Kappas A, Abraham NG. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension. 2010;56:1124–1130. doi: 10.1161/HYPERTENSIONAHA.110.151423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 16.Sodhi K, Inoue K, Gotlinger K, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman AI, Chander PN, Rezzani R, Schwartzman ML, Regan RF, Rodella L, Turkseven S, Lianos EA, Dennery PA, Abraham NG. Heme oxygenase-2 deficiency contributes to diabetesmediated increase in superoxide anion and renal dysfunction. J Am Soc Nephrol. 2006;17:1073–1081. doi: 10.1681/ASN.2004121082. [DOI] [PubMed] [Google Scholar]
- 18.Spitz PADR, Yang G, Tatarov A, Lee CS, Shegog ML, Poss KD. Oxygen toxicity and iron accumulation in the lungs of mice lacking heme oxygenase-2. J Clin Invest. 1998;101:1001–1011. doi: 10.1172/JCI448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abraham NG, Drummond G. CD163-Mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circ Res. 2006;99:911–914. doi: 10.1161/01.RES.0000249616.10603.d6. [DOI] [PubMed] [Google Scholar]
- 20.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 21.Sacerdoti D, Colombrita C, Di PM, Schwartzman ML, Bolognesi M, Falck JR, Gatta A, Abraham NG. 11,12-epoxyeicosatrienoic acid stimulates heme-oxygenase-1 in endothelial cells. Prostaglandins Other Lipid Mediat. 2007;82:155–161. doi: 10.1016/j.prostaglandins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Sacerdoti D, Bolognesi M, Di PM, Gatta A, McGiff JC, Schwartzman ML, Abraham NG. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H1999–H2002. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- 23.Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, Abraham NG. EET-Agonist Regulates Human Mesenchymal Stem Cells-Derived Adipocytes Through Activation of HO-1-pAKT Signaling and a decrease in PPARgamma. Stem Cells Dev. 2010;19:1863–1873. doi: 10.1089/scd.2010.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poss KD, Thomas MJ, Ebralidze AK, O'Dell TJ, Tonegawa S. Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron. 1995;15:867–873. doi: 10.1016/0896-6273(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 25.Vanella L, Kim DH, Asprinio D, Peterson SJ, Barbagallo I, Vanella A, Goldstein D, Ikehara S, Kappas A, Abraham NG. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236–243. doi: 10.1016/j.bone.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 27.Farhangkhoee H, Khan ZA, Mukherjee S, Cukiernik M, Barbin YP, Karmazyn M, Chakrabarti S. Heme oxygenase in diabetes-induced oxidative stress in the heart. J Mol Cell Cardiol. 2003;35:1439–1448. doi: 10.1016/j.yjmcc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Ghattas MH, Chuang LT, Kappas A, Abraham NG. Protective effect of HO-1 against oxidative stress in human hepatoma cell line (HepG2) is independent of telomerase enzyme activity. Int J Biochem Cell Biol. 2002;34:1619–1628. doi: 10.1016/s1357-2725(02)00097-3. [DOI] [PubMed] [Google Scholar]
- 29.Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, Piccolomini F, Puri N, Gastaldelli A, Kusmic C, L'Abbate A, Abraham NG. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vera T, Kelsen S, Stec DE. Kidney-specific induction of heme oxygenase-1 prevents angiotensin II hypertension. Hypertension. 2008;52:660–665. doi: 10.1161/HYPERTENSIONAHA.108.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension. 2011;57:941–948. doi: 10.1161/HYPERTENSIONAHA.111.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 33.Brydun A, Watari Y, Yamamoto Y, Okuhara K, Teragawa H, Kono F, Chayama K, Oshima T, Ozono R. Reduced expression of heme oxygenase-1 in patients with coronary atherosclerosis. Hypertens Res. 2007;30:341–348. doi: 10.1291/hypres.30.341. [DOI] [PubMed] [Google Scholar]
- 34.Deshane J, Wright M, Agarwal A. Heme oxygenase-1 expression in disease states. Acta Biochim Pol. 2005;52:273–284. [PubMed] [Google Scholar]
- 35.Kappas A, Drummond GS, Henschke C, Valaes T. Direct comparison of Snmesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns. Pediatrics. 1995;95:468–474. [PubMed] [Google Scholar]
- 36.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 38.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 40.Norwood S, Liao J, Hammock BD, Yang GY. Epoxyeicosatrienoic acids and soluble epoxide hydrolase: potential therapeutic targets for inflammation and its induced carcinogenesis. Am J Transl Res. 2010;2:447–457. [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao X, Dey A, Romanko OP, Stepp DW, Wang MH, Zhou Y, Jin L, Pollock JS, Webb RC, Imig JD. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R188–R196. doi: 10.1152/ajpregu.00018.2004. [DOI] [PubMed] [Google Scholar]
- 42.Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 43.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 44.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turkseven S, Drummond G, Rezzani R, Rodella L, Quan S, Ikehara S, Abraham NG. Impact of silencing HO-2 on ECSOD and the mitochondrial signaling pathway. J Cell Biochem. 2007;100:815–823. doi: 10.1002/jcb.21138. [DOI] [PubMed] [Google Scholar]
- 46.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]