Abstract
The objective of this study was to determine the effect of benzo[a]pyrene (BaP), an abundant environmental polycyclic aromatic hydrocarbon compound, on the pathogenesis of abdominal aortic aneurysms (AAA). Earlier studies have shown that BaP promotes vasculopathy, including atherosclerosis, a predisposing factor for AAA development. In two experimental arms, 203 apolipoprotein E knockout (ApoE-/-) mice were evaluated in 4 groups: BaP, angiotensin II (AngII), BaP+AngII and control. Mice in the first arm were exposed to 5mg/kg/week of BaP for 42 days, and in the second arm to 0.71mg/kg daily for 60 days. In arm one, AAA incidence was higher in the BaP+AngII (14/28) versus AngII (8/27) group (p < 0.05), rupture (n=3) was observed only in BaP+AngII treated mice (p < 0.05). In the second arm, AAA incidence did not differ between AngII (17/30) and BaP+AngII (16/29) groups. However, intact AAA diameter was larger in the BaP+AngII (2.3 ± 0.1mm) versus AngII (1.9 ± 0.1mm) group (p < 0.05), but AAA rupture did not differ (p=NS). In both experimental arms, BaP+AngII mice showed increased expression of tumor necrosis factor alpha (TNF-α), cyclophilin A (Cyp A), and matrix metalloproteinase-9 (MMP9) (p < 0.05). No AAA occurred in control or BaP groups. These findings suggest the role of BaP exposure in potentiating AAA pathogenesis, which may have potential public health significance.
Key Words: Abdominal aortic aneurysm, Benzo[a]pyrene, Inflammation, Proteolysis, ApoE deficient mice, Environmental toxicants
Introduction
Abdominal aortic aneurysm (AAA) is the thirteenth leading cause of death in the United States and poses a substantial health care burden due the consequences of progression and rupture [1, 2, 3]. The disorder of AAA is conventionally diagnosed if there is ≥ 3cm dilatation of the cross-sectional diameter of the abdominal aorta. Risk factors for AAA development include advanced age, male gender, hypertension, atherosclerosis, connective tissue disease, and familial predisposition [1, 2, 3, 4]. Despite recognition of some of the risk factors for AAA, its pathogenesis has not been clearly elucidated. Accumulating evidence suggests that inflammation, enzymatic degradation of vascular wall, and apoptosis [5, 6, 7, 8], all possibly triggered by oxidative stress [9, 10], are central to the pathobiology of AAA. Benzo(a)pyrene (BaP) is a polycyclic aromatic hydrocarbon (PAH) compound known to promote atherosclerosis via multiple mechanisms, notably oxidative stress [11, 12, 13, 14, 15]. This toxicant is found in barbequed foods, cigarette smoke, exhaust fumes, and smoke from fires. The ubiquitous environmental presence of BaP makes it a potential factor in AAA development, either indirectly via the promotion of atherosclerosis, an AAA predisposing factor, or directly via oxidative stress. This concern is currently heightened by the known abundance of BaP in red meat, fatty food, and fish samples from crude oil contaminated waters, a worsening fear given the recent oil spill in the Gulf of Mexico [16, 17, 18] Therefore, in this study we sought to evaluate the potential effects of BaP in AAA pathogenesis using the well- established angiotensin II (AngII) induced model of AAA in apolipoprotein E knockout (ApoE-/-) mice [19, 20]. We tested the hypothesis that daily or weekly exposure of mice to BaP increases oxidant stress and inflammation burden, and exacerbates AngII-induced AAA in ApoE-/- mice. Our findings indicate that the combined exposure to BaP and AngII increases the incidence of AAA, and precipitates AAA of larger sizes, with evidence of increased aortic expression of TNF-α, Cyp A, and MMP9. We conclude that exposure to BaP potentiates vascular inflammation and proteolysis, resulting in increased incidence of AAA and development of AAA of larger sizes.
Material and Methods
Animals and treatment
Male ApoE-/- mice, backcrossed in C57BL/6 for over 10 generations, were obtained from Jackson Laboratory (Bar Harbor, ME). The mice were housed in groups of four per cage, maintained on a twelve-hour light/dark cycle (lights on at 6:00 a.m. and off at 6:00 p.m.), and allowed free access to rodent chow (2016 Teklad Global 16% protein rodent diet [3.5% fat]; Harlan Laboratories, Indianapolis, IN, USA) and water. All mice involved in this study were housed in polycarbonate cages (Lab Products, Inc., Seaford, DE, USA) with laboratory grade 7089 Teklad Diamond Soft Cellulose (Harlan Laboratories) material as bedding. Mice were housed in an Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited animal care facility. All mice were allowed a seven-day acclimation period prior to being randomly assigned to a control or treatment group.
In a study composed of two experimental arms, a total of 203 ApoE-/- mice (7 weeks old at the start of the experiment) were evaluated in 4 group assignments (BaP, AngII, BaP+AngII and control). The experimental arms were distinguished by the dose of BaP administered. In the first arm, mice were exposed to 5mg/kg/week of BaP for 42 days, and in the second arm to 0.71mg/kg daily for 60 days. The rationale for this design centers on the well-documented dose- and time-related effects of BaP on atherogenesis, which indicate that higher doses and/ or longer duration of treatment precipitates larger atherosclerotic lesions [21, 22]. Consequently, we sought to determine whether exposure to small daily doses of BaP over a longer duration of time (60 days) would exert similar vascular degenerative effects as large weekly doses over a shorter period of exposure (42 days). Benzo(a)pyrene exposure was via oral gavage, and AngII exposure (1000ng/kg/min × 2 wks) occurred via subcutaneous osmotic mini pumps (Alzet Model 2004, Durect Corporation, CA) as previously described [23]. In the BaP+AngII group, Angll was infused over the last 2 weeks of BaP exposure. Mice in the control group were treated with tricaprylin (vehicle for BaP) via oral gavage and underwent saline infusion via subcutaneous osmotic mini pumps.
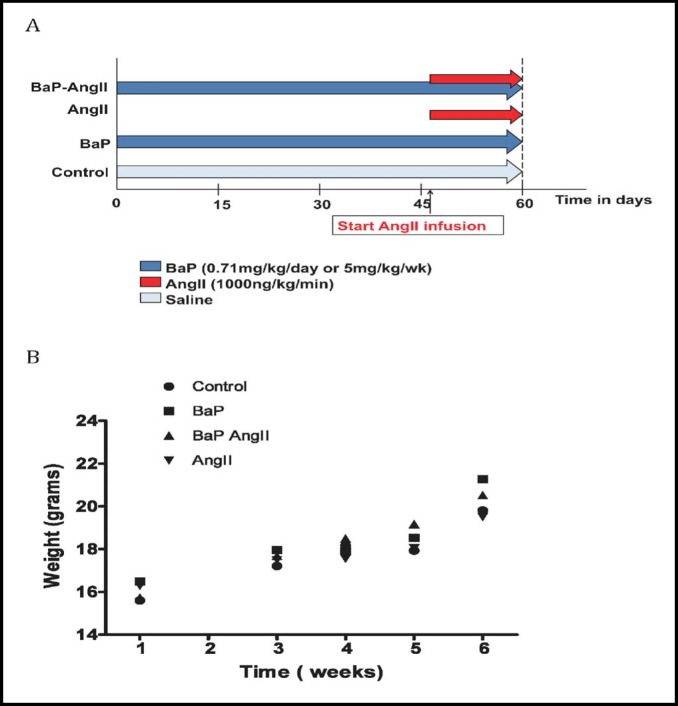
The experimental protocol is delineated in Fig. 1a, and the allocation of mice per group in each experimental arm is detailed in the Table 1. Mice were evaluated for signs of toxicity and failure to thrive by observation of behavior and monitoring of weight gain. At the end of the experiment mice were euthanized to harvest the aorta for gross morphological evaluation and for immuno-histochemical and molecular studies as previously described [23]. Necropsy was conducted on mice that died prior to the end of study to determine whether a ruptured AAA was the cause of death. Animal care and experimental procedures were approved and carried out according to the regulations of the Meharry Medical College IACUC.
Fig. 1.
Study group assignment and assessment of normal development. A) Mice were evaluated in 4 groups in 2 study arms: BaP, AngII, BaP+AngII, and control. Exposure to BaP was via oral gavages and AngII exposure via subcutaneous osmotic mini pumps. In the BaP+AngII group, AngII infusion overlapped the last two weeks of BaP treatment. In the first study arm, mice were exposed to 5mg/kg/week of BaP for 42 days, and in the second arm 0.71mg/kg daily for 60 days (Figure only illustrates 60-day marker for the first study arm). B) Assessment of normal development. During the duration of the study, body weight was used as an indirect measure of exposure toxicity (failure to thrive). Incremental weight gain during the study period suggests normal growth. No statistically significant differences were observed between groups. Graph was derived from the weekly-exposure study arm (5 mg/kg/week of BaP); similar findings were noted in the daily-exposure arm (0.71 mg/kg/day) (data not shown).
Table 1.
Distribution of mice per study group
| Experimental arm | Group | Number (n) | Aneurysm (AAA) | Rupture |
|---|---|---|---|---|
| Weekly BaP exposure | Control | 8 | 0 | 0 |
| BaP | 28 | 0 | 0 | |
| AngII | 27 | 8 | 0 | |
| BaP+AngII | 28 | 14 | 3 | |
| Daily BaP exposure | Control | 24 | 0 | 0 |
| BaP | 29 | 0 | 0 | |
| AngII | 30 | 17 | 7 | |
| BaP+AngII | 29 | 16 | 8 |
Gross examination and immunohistochemistry
Measurement of the maximal aortic diameter was performed with a digital caliper (Tresna) under microscope guidance as previously described [23]. For the purpose of this study, an increase in AAA diameter of 50% compared to the aortic diameter in control mice was the threshold for AAA definition. All measurements were performed by the same operator to avoid inter-observer variability. Embedded tissue was cut into 3-micron serial sections and stained for H&E and Elastin van Gieson. Sections were deparaffinized prior to being stained with Weigert's Iron Hematoxylin for 15 minutes and, after washing with running water, followed by a van Gieson stain for 5 minutes. Formaldehyde fixed paraffin embedded sections were de-paraffinized by placing sections at 70°C for one hour followed by re-hydration to H2O using several changes of xylenes and ethanol. When required, heat mediated antigen retrieval with 10mM citrate buffer (pH 6) was done for 30 minutes as well as quenching of endogenous peroxidase by submerging sections in 3% H2O2 for 15 minutes. Slides were incubated with primary antibody (1:100) at room temperature for 1 hour [(F4/80 (GeneTex, CA)] or at 4°C overnight [(CypA (Enzo Life Sciences, PA) and Cycloxygenase-2 (Cox-2) (Thermo Scientific, CA)]. Negative controls were prepared using no primary antibody. Anti-Mouse, Anti-Rat and Anti-Rabbit Vectastain Elite ABC kits (Vector Laboratories, CA) were used for the secondary biotinylated antibody and streptavidin-peroxidase complex. A Vector Nova Red substrate kit (Vector Laboratories, CA) was used as substrate for the peroxidase, but if counterstained, Gills Hematoxylin III (EMD, USA) was used. Staining of slides was done simultaneously and quantification was done by at least two investigators who were blinded to knowledge of treatment assignment. A Vanguard 1490-FLP01 (VeeGee Scientific, WA) microscope with mounted camera (Canon Eos Rebel xsi) was used for analysis and image capture of slides. Analysis of elastin staining was based on a semi quantitative scoring system (1+ minor to no stain, 4+ intensive staining). Stains for macrophages, Cox-2 and CypA were analyzed using stereology. For this a microscope with mounted camera (Olympus BX50W1) and stereology software (stereologer 2000) was used.
Protein expression
Aortic tissue protein was extracted by pooling 3 aortas per specific group after which the tissue was homogenized followed by lysis in Ripa buffer supplemented with a protease inhibitor cocktail (Pierce, USA). After centrifugation, the protein concentration of the cell lysates was determined using a DC protein assay (Bio-rad, USA). Total cell lysates were separated on SDS- Page electrophoresis, followed by electro transfer into PVDF membranes. Membranes were blocked for 1h at room temperature in 4% non-fat milk followed by incubation with primary [TNF-α, MMP9 (Abcam), CypA (Enzo Life Sciences, PA)] and secondary antibodies [anti mouse/rabbit IgG-Hrp (Promega)]. Western blots were developed and exposed using chemiluminescence.
Serum analysis
At the time of sacrifice, blood samples were collected via the portal vein and centrifuged at 4°C, and then serum was extracted for analysis. Analysis of the serum employed a BD™ Cytometric Bead Array kit for murine inflammatory cytokines on a BD FACSArray™ Bioanalyzer platform (BD Biosciences, San Jose, CA). The analysis of serum total cholesterol, triglycerides, and free fatty acid levels were done by spectrophotemetry using commercially available kits as previously described [24].
Statistical analysis
Unless otherwise stated results are expressed as mean ± standard error of measurement (SEM). Differences between groups were analyzed for statistical significance using Students t-test, Mann Whitney test, or Fisher's exact test as deemed appropriate. A p value of < 0.05 was considered the threshold for statistical significance. All analyses of study data were performed with GraphPad Prism version 5.0a for Mac OS × (GraphPad Software, San Diego CA, www.graphpad.com).
Results
Incidence and size of AAA
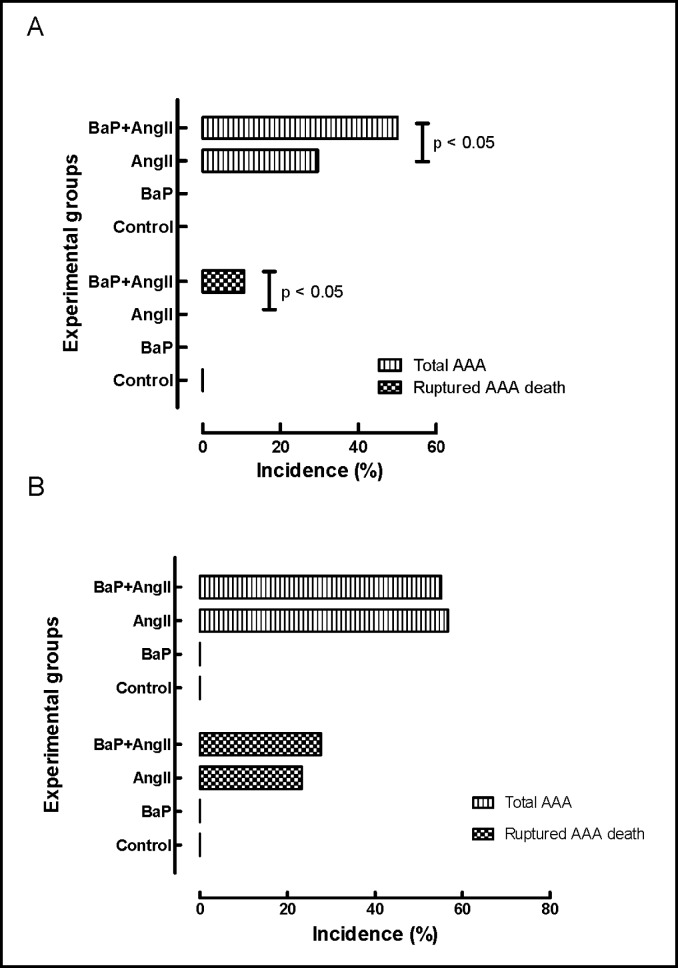
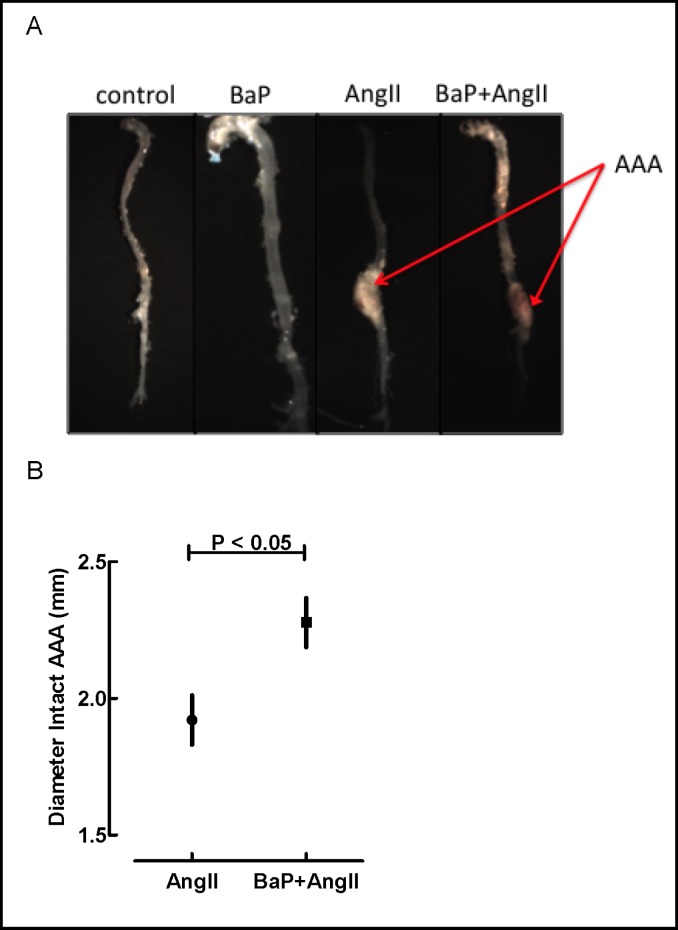
There was no evidence of failure to thrive due to treatment assignment as inferred from concordant pattern of weight gain in all groups of mice (Fig. 1b). In the weekly exposure study arm (Fig. 2a), 8 mice in the AngII treated group (n=27) and 14 in the BaP+AngII (n=28) treatment group developed aneurysms, but none in the control (n=8) or BaP (n=28) groups. The difference in AAA incidence was statistically significant (p < 0.05). Also, 3 of the mice treated with BaP+AngII died from AAA rupture whereas none in the other groups did. The incidence of AAA rupture was statistically significant (p < 0.05). In the daily exposure arm (Fig. 2b), 17 mice in the AngII treated group (n=30) developed AAA, of these, 7 died from ruptured AAA. Sixteen of 29 BaP+AngII treated mice developed AAA, of which 8 ruptured. There was no statistical difference (p = NS) in the overall incidence of AAA and rupture between the AngII and BaP+AngII treated mice. None of the control (n= 24) or BaP (n= 29) treated mice developed AAA. Gross evaluation revealed more extensive atherosclerotic plaques in the aortic arches of both BaP and BaP+AngII groups (Fig. 3a). In the daily exposure arm, the maximum diameter of intact AAA in BaP+AngII treated mice was significantly larger (2.3 ± 0.1 N=8) than in AngII treated mice (1.9 ± 0.1 N=10) (p < 0.05) (Fig. 3b). We did not observe any significant difference in the diameter of intact AAA in the weekly exposure arm.
Fig. 2.
AAA incidence according to experimental groups. A) In the weekly-exposure experiment, 8 mice in the AngII treated group (n=27) and 14 in the combined BaP+AngII (n=28) treatment group developed aneurysms, but none in the control (n=8) or BaP (n=28) groups. The difference in AAA incidence was statistically significant (p < 0.05). Also 3 of the mice treated with BaP+AngII died from AAA rupture whereas none in the other groups did. The incidence of AAA rupture was statistically significant (p < 0.05). B) In the daily-exposure experiment, 17 mice in the AngII treated group (n=30) developed AAA, of these, 7 died from ruptured AAA. Sixteen of 29 BaP+AngII treated mice developed AAA, of which 8 ruptured. There was no statistical difference in the overall incidence of AAA and rupture between the AngII and BaP+AngII treated mice. None of the control (n= 24) or BaP (n= 29) treated mice developed AAA.
Fig. 3.
Anatomical evaluation of the aorta. A) Representative gross images of aortas from control, BaP, AngII and BaP+AngII groups. AAA is indicated (arrow) and increased atherosclerosis is noted in the arch of aortas from BaP and BaP+AngII groups. Images are derived from the weekly-dosing experiment. B) Sizes of AAA. In the daily-exposure arm, the maximum diameter of intact AAA was measured using a digital caliper under microscope guidance. Diameters of AAA in BaP+AngII treated mice were significantly larger (2.3 mm ± 0.1, N=8) than diameters of those in AngII treated mice (1.9 mm ± 0.1, N=10) (p < 0.05). We did not observe any significant differences in the diameter of intact AAA in the weekly-dosing experiment (data no shown).
Inflammation and proteolysis
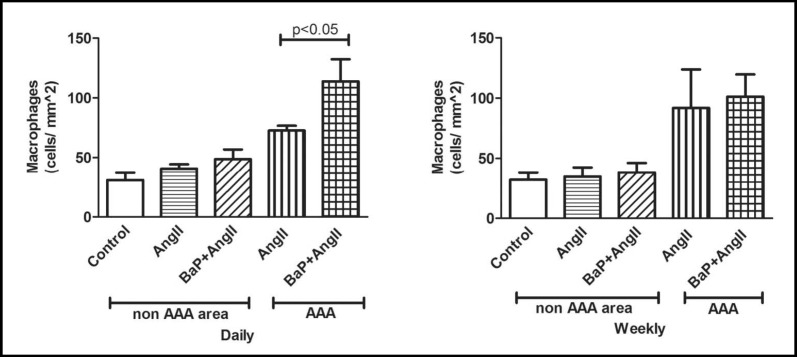
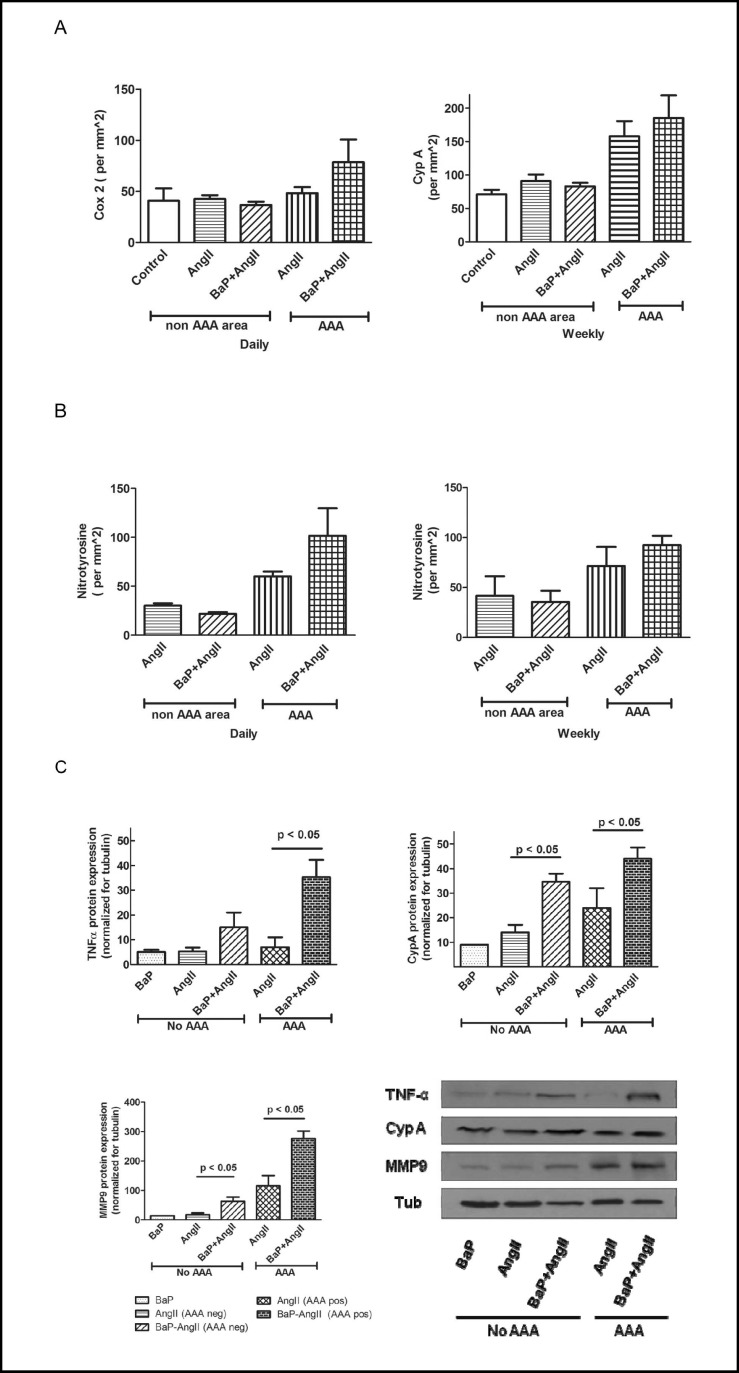
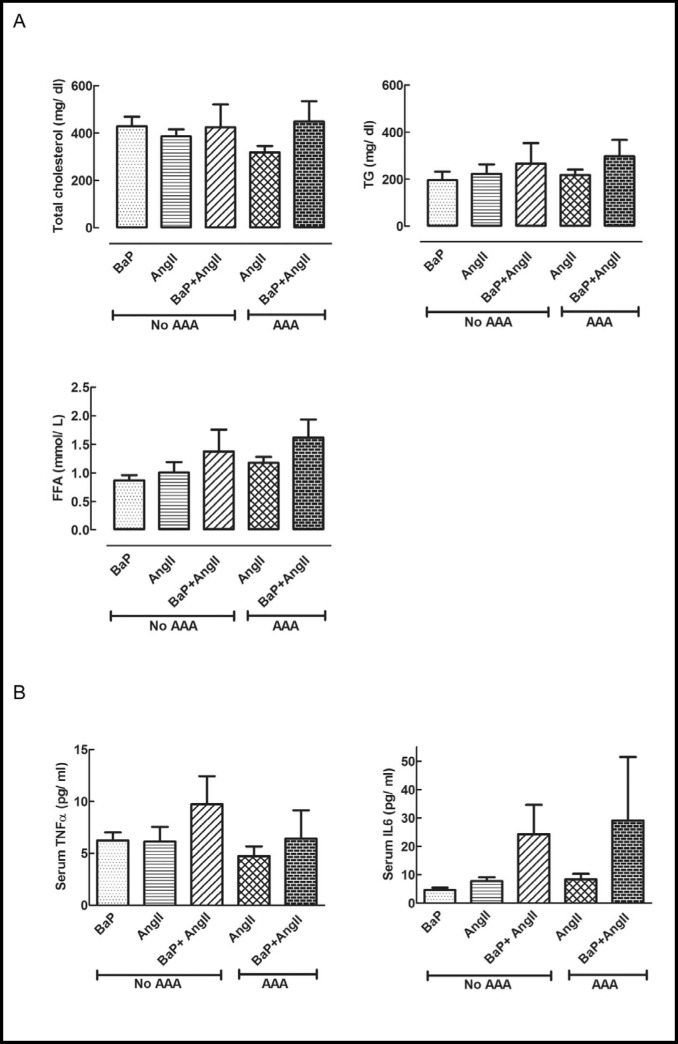
We also observed an increase in accumulation of macrophages in both AAA and non-AAA regions of AngII treated mice (with or without BaP). Increased macrophage accumulation was observed at AAA sites of mice from the AngII and BaP+AngII treated groups for both daily (Fig. 4, left) and weekly exposure (Fig. 4, right) study arms. However, we observed a statistically significant difference in the number of macrophages present in AAA regions of BaP+AngII versus AngII treated mice in the daily exposure arm (p < 0.05). We noted a similar trend, although not statistically significant (p = NS), in the weekly exposure arm. Immuno-histochemical evaluation revealed an increased expression of Cox-2 (daily arm), and CypA (weekly arm), a protein that promotes inflammation and is expressed under oxidant stress by vascular smooth muscle cells in AAA regions with a trend toward an additional effect in BaP+AngII versus AngII only group; however this was not statistically significant (p = NS; Fig. 5a). Similar findings were noted for CypA in the daily BaP exposure experiment arm. Also, an increased level of oxidant stress indicated by nitrotyrosine stain was observed at AAA sites with a trend toward an additional effect in BaP+AngII versus AngII treated mice in the daily but not weekly treatment, however the trend did not reach the threshold for statistical significance (p=NS; Fig. 5b). Independent of the presence of AAA, we observed an increased expression of TNF-α protein in aortas of the BaP + AngII treated mice compared to AngII treated mice (Fig. 5c). Moreover, the expression of TNF-α was significantly higher in BaP + AngII treated mice that developed AAA (p < 0.05). Tissue expression of CypA protein was also significantly elevated in the aortas from BaP + AngII compared to AngII treated mice independent of the presence of AAA (p < 0.05; Fig. 5c). Since metalloproteinases mediate extracellular matrix degeneration, we evaluated the expression of MMP9 protein in aortas from mice that developed AAA versus those that did not. Notably, there was an apparent increase in MMP9 expression in aortas from mice that developed AAA compared to those that did not (Fig. 5c). More importantly, both in AAA and non-AAA aortas, the combined treatment with BaP + AngII led to a significant increase in MMP9 expression compared to AngII treatment alone (p < 0.05; Fig. 5c). Serum analysis of mice from the daily exposure arm revealed there was no difference in the total cholesterol, triglycerides, and free fatty acid levels of mice in the BaP, AngII, or BaP + AngII groups (p = NS; Fig. 6a). However, analysis of serum inflammatory cytokines in mice with or without aneurysm demonstrated a trend toward elevated levels of TNF-α and IL-6 in mice treated with BaP+AngII compared to AngII though it did not reach the threshold of statistical significance (p = NS; Fig. 6b).
Fig. 4.
Effects on tissue macrophage accumulation. Inflammation - macrophage infiltration - in the aortic walls both at AAA and non-AAA sites were quantified by stereology. Non-AAA and AAA are from the same aortas. Increased macrophage accumulation was observed at AAA sites in both the AngII and BaP+AngII treated groups for both daily (left) and weekly treatment (right). We observed a statistically significant difference in the number of macrophages present in AAA regions of BaP+AngII (n=4) versus AngII (n=6) treated mice in the daily exposure arm. We noted a similar relationship, although not statistically significant (p=NS), in the weekly exposure arm.
Fig. 5.
Effects on markers of inflammation, proteolysis, and oxidant stress. A) Cox-2 positive cells are present in the aortic wall with a trend toward increased positive cells in AAA sites of BaP+AngII (n=4) versus AngII (n=5) treated mice. Also, increased levels of CypA which promotes inflammation and is expressed by vascular smooth muscle cells were observed at AAA sites of mice in both AngII (n=5) and BaP+AngII (n=4). There was no statistically significant difference in the expression of Cox2 and CypA in both groups. Similar trends were noted for CypA in the daily exposure experiment (data not shown). B) Increased oxidant stress is suggested (p= NS) by the nitrotyrosine stain of AAA sites in the daily (AngII n= 5, BaP+AngII n= 3) but not the weekly (AngII n= 4, BaP+AngII n= 4) exposure experiments. Cross sections used for AAA and non AAA were from the same aortas. C) The expression of TNF-α, CypA, and MMP9 protein were significantly higher in aorta from mice in BaP+AngII compared to Ang II group independent of the presence of AAA (p < 0.05). Results shown are data from 2 sets of groups, each group representing a triplicate assessment of protein pooled from 3 aortas from AAA negative and AAA positive mice, respectively. Data shown are only for daily exposure experiment.
Fig. 6.
Effects on serum lipids and cytokines. A) Treatment with BaP, AngII, or their combination, did not change total serum cholesterol, free fatty acids, and triglycerides in mice with or without AAA (p=NS; N=3 per group) (Data was derived from daily exposure experiment). B) Serum inflammatory cytokines. Compared to AngII group, mice from BaP+AngII treated group with AAA or without AAA showed a trend toward increased serum levels of TNF-α and IL6 (p= NS; N=3 per group) (Data was derived from daily exposure experiment).
Discussion
We have used the established model of AngII induced AAA in ApoE -/- mice to demonstrate that BaP exposure may play a role in the development of AAA. Our data suggest that daily or weekly exposure to BaP potentiates the inflammatory and proteolytic processes that attend AngII-induced development of AAA in ApoE-/- mice.
Weekly exposure to BaP in conjunction with the pro-aneurysmal stimulus led to an increased incidence of AAA formation and rupture. Although the daily treatment arm of our study did not show an aggravating effect of BaP on AAA incidence, a significant increase in the size of AAA in the BaP+AngII exposure group was nonetheless observed. This suggests that, relative to intermittent exposure to large doses of BaP, prolonged daily exposure to small amounts of BaP also causes vascular degeneration. This notion is substantiated by the observed increase in the expression of TNF-α and MMP9 in the AngII induced AAA when primed by BaP exposure. This increased expression of TNF-α and MMP9 provides mechanistic support for the observed larger AAA sizes in mice subjected to BaP and AngII exposure. Overall, our findings support the notion that environmental contamination of food substances resulting in prolonged dietary exposure to polycyclic aromatic hydrocarbon compounds such as BaP, primes the development of more extensive and progressive degenerative vascular disease when triggered by degenerative or inflammatory stimuli.
Although the cellular responses to exposure to BaP were consistent between study arms, the differences in AAA incidence can be explained by insights from the well-documented dose- and time-related atherogenic role of BaP in different animal models [14, 15, 22, 25]. Similarly, our group reported that BaP increases atherosclerotic lesion area in the mouse aorta in a dose-and time-dependent manner [21]. We also reported that BaP treatment aggravates the severity of the lesions, increased calcification and acellular areas in the plaques, but did not alter serum cholesterol and triglyceride levels of these mice [21]. Furthermore, it has been reported that biliary concentrations of BaP metabolites in fish increase with dosage and decrease between day 3 and day 12 after exposure, with higher single doses yielding greater and more long-lasting concentrations of metabolites 6 days after exposure compared to the concentration of metabolites after 3 days of exposure to a smaller single doses [26, 27, 28, 29, 30]. These reports provide perspective on the differences in AAA incidence between the daily (small dose) and weekly (high dose) exposure arms of our study. However, our findings also demonstrate the harmful vascular effects that exposure to oxidant and/ or inflammatory stress can precipitate, and that these effects are independent of serum cholesterol or triglycerides levels as reported by other investigators in the context of atherosclerosis [31, 32, 33, 34, 35, 36, 37, 38].
The results of this study add significant insight to the understanding of the pathobiology of AAA. It is now understood that the pathogenesis of AAA implicates a complex interplay of key mechanisms that include oxidative stress [39, 40, 41], local production of pro-inflammatory cytokines [42, 43], vascular smooth muscle migration and proliferation [44, 45, 46], and activation of proteases such as matrix metalloproteinases and cysteine/ serine proteases [6, 47, 48, 49, 50, 51]. Substantial experimental evidence implicates early involvement of the monocyte/ macrophage innate immune response, with consequent production of pro-inflammatory cytokines during AAA development [7, 52, 53, 54]. However, the potential impact of BaP exposure in conjunction with proaneurysmal stimulus has not been investigated extensively. We know that BaP induces cellular reactive oxygen species (ROS) production and promotes atherogenesis [11, 14, 15, 54, 55, 56, 57, 58, 59, 60], both essential elements in the pathogenesis of AAA. We previously reported that overexpression of antioxidant enzymes reduce BaP-induced atherosclerosis in ApoE-/- mice, thus implicating oxidant stress in the pathogenesis of atherosclerosis [60]. Despite these reported findings on the effect of BaP on atherosclerosis via ROS mechanisms, its possible involvement in the pathogenesis of AAA needs further evaluation. Zhang and Ramos recently reported that weekly exposure of C57/B6J mice to BaP resulted in increased numbers of AAA as well as increased numbers of aortic ruptures [61]. They also noted that BaP treatment caused thinning of the arterial wall and increased elastin degradation. Immunohistological examination of the aortas from our study revealed similar changes in arterial wall thickness and elastin degradation (data not shown). Not only did BaP treatment by itself show increased damage and structural disorganization, but also BaP combined with AngII visibly potentiated the damage seen compared to that caused by AngII only.
We are cognizant of the fact that exposure of animal models to lower ambient concentrations of toxicants is desirable to understand the pathogenesis of human disease and avoid extrapolation of toxicology data from high experimental doses. However, studies conducted by our group and others have shown that if animal models are exposed to ambient human BaP exposure settings, one would seldom be able to see any disease manifestations. These inherent problems associated with assessing the environmental health effects of toxicants prompted us to employ higher BaP doses. This debate notwithstanding, the BaP doses we used will be of relevance to “unusual BaP exposure situations to humans”. Examples of such situations arise from cumulative human exposure to BaP from a wide gamut of exposures, which include diet, inhalation of tobacco smoke (both mainstream and side stream), contaminated air released from occupational settings (workers from petrochemical, graphite electrode and aluminum manufacturing industries, restaurant cooks, fire fighters, etc.), and people living in the vicinity of hazardous waste sites and in unvented homes using biomass for cooking and home heating. Some of these sources are outlined in a WHO report [17], and Ramesh et al [18]. Therefore, cumulative annual intake of BaP by some susceptible populations may approximate the dose used in this study.
Conclusions
We have demonstrated that the combination of exposure to BaP and AngII increases the incidence of AAA, and precipitates AAA of larger sizes, with increased aortic expression of TNF-α, Cyp A, and MMP9. We conclude that exposure to BaP potentiates vascular inflammation, oxidant stress, and proteolysis, resulting in increased incidence of AAA and development of AAA of larger sizes. In this context, the abundance of BaP in the environment may have potential significance in cardiovascular disease due to chronic exposure, e.g. through diet. Since biotransformation of BaP is linked to toxicity, studies are in progress in our laboratories using samples from the treatment groups employed in this study to examine the generation of BaP reactive metabolites that might promote the development of aneurysms through the modulation of processes of apoptosis and inflammation. However, given that BaP exposure may have major implications in aneurysm formation, future directions should include epidemiological studies to determine the association between BaP and vascular diseases such as AAA in humans particularly those resident in geographical regions marred by significant environmental pollution from crude oil spills, hazardous waste sites (brownfields), and other industrial wastes. Also, occupationally exposed individuals such as jet plane repair personnel, workers in petroleum industry, coke oven workers, fire fighters, and personnel in armed forces serve as excellent cohorts for epidemiology studies in this area.
Acknowledgements
The research embodied in this manuscript was facilitated by The Harold Amos Faculty Development Award of the Robert Wood Johnson Foundation (Princeton, NJ), and the Vanderbilt Clinical and Translational Scholars Award to UKS. Also, funding support through the National Institutes of Health (NIH) grants 5S11ES01415602 from the National Institute of Environmental Health Sciences (NIEHS) to UKS and AR, 1RO1CA142845-01A1 from the National Cancer Institute (NCI) to AR, and 1RO1ES014471-01A1 from the NIEHS to ZG. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIH or Vanderbilt University or Meharry Medical College.
Abbreviations
- AAA
(Abdominal aortic aneurysm)
- BaP
(Benzo[a]pyrene)
- AngII
(Angiotensin II)
- ApoE-/-
(Apolipoprotein E Knockout)
- TNF-α
(Tumor necrosis alpha)
- IL-6
(Interleukin-6)
- CypA
(Cyclophilin A)
- MMP9
(Matrix metalloproteinase-9)
- Cox2
(Cycloxygenase-2)
- ROS
(Reactive oxygen species)
References
- 1.Gillum RF. Epidemiology of aortic aneurysm in the United States. J of Clin Epidemiol. 1995;48:1289–1298. doi: 10.1016/0895-4356(95)00045-3. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard JF, Armenian HK, Friesen PP. Risk Factors for abdominal aortic aneurysm: results of a case-control study. Am J Epidemiol. 2000;151:575–583. doi: 10.1093/oxfordjournals.aje.a010245. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard JF. Epidemiology of abdominal aortic aneurysms. Epidemiol Rev. 1999;21:207–221. doi: 10.1093/oxfordjournals.epirev.a017997. [DOI] [PubMed] [Google Scholar]
- 4.Thompson MM. Controlling the expansion of abdominal aortic aneurysms. Br J Surg. 2003;90:897–898. doi: 10.1002/bjs.4280. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 6.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15:1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 7.Newman KM, Jean-Claude J, Li H, Ramey WG, Tilson MD. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation. 1994;90:II224–227. [PubMed] [Google Scholar]
- 8.Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: basic mechanisms and clinical implications. Current Prob Surg. 2002;39:110–230. doi: 10.1067/msg.2002.121421. [DOI] [PubMed] [Google Scholar]
- 9.Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr, Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin II-Induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Lee E, Song W, Ricciotti E, Rader DJ, Lawson JA, Pure E, FitzGerald GA. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation. 2008;117:1302–1309. doi: 10.1161/CIRCULATIONAHA.107.731398. [DOI] [PubMed] [Google Scholar]
- 11.Albert RE, Vanderlaan M, Burns FJ, Nishizumi M. Effect of carcinogens on chicken atherosclerosis. Cancer Res. 1977;37:2232–2235. [PubMed] [Google Scholar]
- 12.Paigen B, Holmes PA, Morrow A, Mitchell D. Effect of 3- Methylcholanthrene on atherosclerosis in two congenic strains of mice with different susceptibilities to methylcholanthrene-induced tumors. Cancer Res. 1986;46:3321–3324. [PubMed] [Google Scholar]
- 13.Curfs DMJ, Lutgens E, Gijbels MJJ, Kockx MM, Daemen MJAP, van Schooten FJ. Chronic exposure to the carcinogenic compound benzo[a]pyrene induces larger and phenotypically different atherosclerotic plaques in ApoE-knockout mice. Am J Pathol. 2004;164:101–108. doi: 10.1016/S0002-9440(10)63101-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batastini G, Penn A. An ultrastructural comparison of carcinogen-associated and spontaneous aortic lesions in the cockerel. Am J Pathol. 1984;114:403–409. [PMC free article] [PubMed] [Google Scholar]
- 15.Penn A, Snyder C. Arteriosclerotic plaque development is promoted by polynuclear aromatic hydrocarbons. Carcinogenesis. 1988;9:2185–2189. doi: 10.1093/carcin/9.12.2185. [DOI] [PubMed] [Google Scholar]
- 16.Anyakora C, Arbabi M, Coker H. A screen for benzo(a)pyrene in fish samples from crude oil polluted environments. Am JEnviron Sci. 2008;4:145–150. [Google Scholar]
- 17.World Health Organization : Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monographs on the evaluation of the carcinogenic risks to humans. Geneva, Switzerland 2010, vol 92, pp 1–861. [PMC free article] [PubMed]
- 18.Ramesh A, Archibong AE, Hood DB, Guo Z, Loganathan BG. Global Environmental distribution and human health effects of polycyclic aromatic hydrocarbons. In: Loganathan BG, Lam PK-S, editors. Global Contamination Trends of Persistent Organic Chemicals. Boca Raton: Florida, CRC Press; 2011. pp. 95–124. [Google Scholar]
- 19.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler, Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 21.Yang H ZL, Wang Z, Roberts LJ, 2nd, Lin X, Zhao Y, Guo Z. Overexpression of antioxidant enzymes in ApoE-deficient mice suppresses benzo(a)pyrene-accelerated atherosclerosis. Atherosclerosis. 2009;207:51–58. doi: 10.1016/j.atherosclerosis.2009.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revis NW, Bull R, Laurie D, Schiller CA. The effectiveness of chemical carcinogens to induce atherosclerosis in the white Carneau pigeon. Toxicology. 1984;32:215–227. doi: 10.1016/0300-483x(84)90075-1. [DOI] [PubMed] [Google Scholar]
- 23.Sampson UK, Perati PP, Prins PA, Pham W, Liu Z, Harrell Jr FE, Linton MF, Gore JC, Kon V, Fazio S. Quantitative estimates of variability of in vivo ultrasound imaging measurements of mouse aorta important for studies of abdominal aortic aneurysms and related arterial diseases. J Ultrasound Med. 2011;30:773–784. doi: 10.7863/jum.2011.30.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazio S, Babaev VR, Murray AB, Hasty AH, Carter KJ, Gleaves LA, Atkinson JB, Linton MF. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc Nat Acad Sci USA. 1997;94:4647–4652. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bond JA, Gown AM, Yang HL, Benditt EP, Juchau MR. Further investigations of the capacity of polynuclear aromatic hydrocarbons to elicit atherosclerotic lesions. J Toxicol Environ Health. 1981;7:327–335. doi: 10.1080/15287398109529983. [DOI] [PubMed] [Google Scholar]
- 26.van Schanke A, Holtz F, van der Meer J, Boon JP, Ariese F, Stroomberg G, van den Berg M, Everaarts JM. Dose- and time-dependent formation of biliary benzo[a]pyrene metabolites in the marine flatfish Dab (Limanda limanda) Environ Toxicol Chem. 2001;20:1641–1647. [PubMed] [Google Scholar]
- 27.Leadly TA, Arcand-Hoy LD, Haffner GD, Metcalfe CD. Fluorescent aromatic hydrocarbons in bile as a biomarker of exposure of brown bullheads (Ameiurus nebulosus) to contaminated sediments. Environ Toxicol Chem. 1999;18:750–755. [Google Scholar]
- 28.Collier TK, Varanasi U. Hepatic activities of xenobiotic metabolizing enzymes and biliary levels of xenobiotics in English Sole (Parophrys vetulus) exposed to environmental contaminants. Arch Environl Contam Toxicol. 1991;20:462–473. doi: 10.1007/BF01065834. [DOI] [PubMed] [Google Scholar]
- 29.Beyer J, Sandvik M, Skare JU, Egaas E, Hylland K, Waagbo R, Goksoyr A. Time-and dose-dependent biomarker responses in flounder (Platichthys flesus L.) exposed to benzo[a]pyrene, 2,3,3’,4,4’,5- - hexachlorobiphenyl (PCB-156) and cadmium. Biomarkers. 1997;2:35–44. doi: 10.1080/135475097231959. [DOI] [PubMed] [Google Scholar]
- 30.Willett K, Steinberg M, Thomsen J, Narasimhan TR, Safe S, McDonald S, Beatty K, Kennicutt MC. Exposure of killifish to benzo[a]pyrene: comparative metabolism, DNA adduct formation and aryl hydrocarbon (Ah) receptor agonist activities. Comp Biochem Physiol Part B: Biochem Mol Biol. 1995;112:93–103. [Google Scholar]
- 31.Ayabe N, Babaev VR, Tang Y, Tanizawa T, Fogo AB, Linton MF, Ichikawa I, Fazio S, Kon V. Transiently heightened angiotensin II has distinct effects on atherosclerosis and aneurysm formation in hyperlipidemic mice. Atherosclerosis. 2006;184:312–321. doi: 10.1016/j.atherosclerosis.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Babaev VR, Runner RP, Fan D, Ding L, Zhang Y, Tao H, Erbay E, Gorgun CZ, Fazio S, Hotamisligil GS, Linton MF. Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-(gamma) regulated genes. Arterioscler Thromb Vasc Biol. 2001;31:1283–1290. doi: 10.1161/ATVBAHA.111.225839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kon V, Linton MF, Fazio S. Atherosclerosis in chronic kidney disease: the role of macrophages. Nat Rev Nephrol. 2011;7:45–54. doi: 10.1038/nrneph.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babaev VR, Whitesell RR, Li L, Linton MF, Fazio S, May JM. Selective macrophage ascorbate deficiency suppresses early atherosclerosis. Free Radic Biol Med. 2011;50:27–36. doi: 10.1016/j.freeradbiomed.2010.10.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babaev VR, Li L, Shah S, Fazio S, Linton MF, May JM. Combined vitamin C and vitamin E deficiency worsens early atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:1751–1757. doi: 10.1161/ATVBAHA.110.209502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babaev VR, Porro F, Linton MF, Fazio S, Baralle FE, Muro AF. Absence of regulated splicing of fibronectin EDA exon reduces atherosclerosis in mice. Atherosclerosis. 2008;197:534–540. doi: 10.1016/j.atherosclerosis.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Major AS, Dove DE, Ishiguro H, Su YR, Brown AM, Liu L, Carter KJ, Linton MF, Fazio S. Increased cholesterol efflux in Apolipoprotein AI (ApoAI)-producing macrophages as a mechanism for reduced atherosclerosis in ApoAI(-/-) Mice. Arterioscler Thromb Vasc Biol. 2001;21:1790–1795. doi: 10.1161/hq1101.097798. [DOI] [PubMed] [Google Scholar]
- 39.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 40.Griendling KK, FitzGerald GA. Oxidative Stress and cardiovascular injury: Part II: Animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 41.Satoh K, Nigro P, Matoba T, O'Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J-i, Illig KA, Berk BC. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin II accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellenthal FAMVI, Buurman WA, Wodzig WKWH, Schurink GWH. Biomarkers of abdominal aortic aneurysm progression. Part 2: inflammation. Nat Rev Cardiol. 2009;6:543–552. doi: 10.1038/nrcardio.2009.102. [DOI] [PubMed] [Google Scholar]
- 44.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 45.Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, Berk BC. Purification and Identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- 46.Satoh K, Matoba T, Suzuki J, O'Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin ZG, Yan C, Abe JI, Berk BC. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newman KM, Jean-Claude J, Li H, Scholes JV, Ogata Y, Nagase H, Tilson MD. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J Vasc Surg. 1994;20:814–820. doi: 10.1016/s0741-5214(94)70169-5. [DOI] [PubMed] [Google Scholar]
- 48.Newman KM, Ogata Y, Malon AM, Irizarry E, Gandhi RH, Nagase H, Tilson MD. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1994;14:1315–1320. doi: 10.1161/01.atv.14.8.1315. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura K IM, Kanaoka Y, Ohgi S, Ueta E, Nanba E, Ito H. Relationships between matrix metalloproteinases and tissue inhibitor of metalloproteinases in the wall of abdominal aortic aneurysms. Int Angiol. 2003;22:229–238. [PubMed] [Google Scholar]
- 50.Yoshimura K, Aoki H, Ikeda Y, Furutani A, Hamano K, Matsuzaki M. Regression of Abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase in mice. Ann NY Acad Sci. 2006;1085:74–81. doi: 10.1196/annals.1383.031. [DOI] [PubMed] [Google Scholar]
- 51.Hellenthal FAMVI, Buurman WA, Wodzig WKWH, Schurink GWH. Biomarkers of AAA progression. Part 1: extracellular matrix degeneration. Nat Rev Cardiol. 2009;6:464–474. doi: 10.1038/nrcardio.2009.80. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 53.Hellenthal FAMVI, Geenen ILA, Teijink JAW, Heeneman S, Schurink GWH. Histological features of human abdominal aortic aneurysm are not related to clinical characteristics. Cardiovasc Pathol. 2009;18:286–293. doi: 10.1016/j.carpath.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 54.Juvonen J, Surcel H-M, Satta J, Teppo AM, Bloigu A, Syrjala H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 55.Burdick AD, Davis JW, Liu KJ, Hudson LG, Shi H, Monske ML, Burchiel SW. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003;63:7825–7833. [PubMed] [Google Scholar]
- 56.Flowers L, Bleczinski WF, Burczynski ME, Harvey RG, Penning TM. Disposition and biological activity of benzo[a]pyrene-7,8-dione. A genotoxic metabolite generated by dihydrodiol dehydrogenase. Biochemistry. 1996;35:13664–13672. doi: 10.1021/bi961077w. [DOI] [PubMed] [Google Scholar]
- 57.Kerzee JK, Ramos KS. Activation of c-Ha-ras by Benzo(a)pyrene in vascular smooth muscle cells involves redox stress and aryl hydrocarbon receptor. Mol Pharmacol. 2000;58:152–158. doi: 10.1124/mol.58.1.152. [DOI] [PubMed] [Google Scholar]
- 58.Kim HS, Kwack SJ, Lee BM. Lipid peroxidation, antioxidant enzymes, and benzo[a]pyrene-quinones in the blood of rats treated with benzo[a]pyrene. Chem-Biol Interact. 2000;127:139–150. doi: 10.1016/s0009-2797(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 59.Penning TM, Ohnishi ST, Ohnishi T, Harvey RG. Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase. Chem Res Toxicol. 1996;9:84–92. doi: 10.1021/tx950055s. [DOI] [PubMed] [Google Scholar]
- 60.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/ Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Ramos KS. The development of abdominal aortic aneurysms in mice is enhanced by benzo(a)pyrene. Vasc Health Risk Manag. 2008;4:1095–1102. doi: 10.2147/vhrm.s3038. [DOI] [PMC free article] [PubMed] [Google Scholar]