Abstract
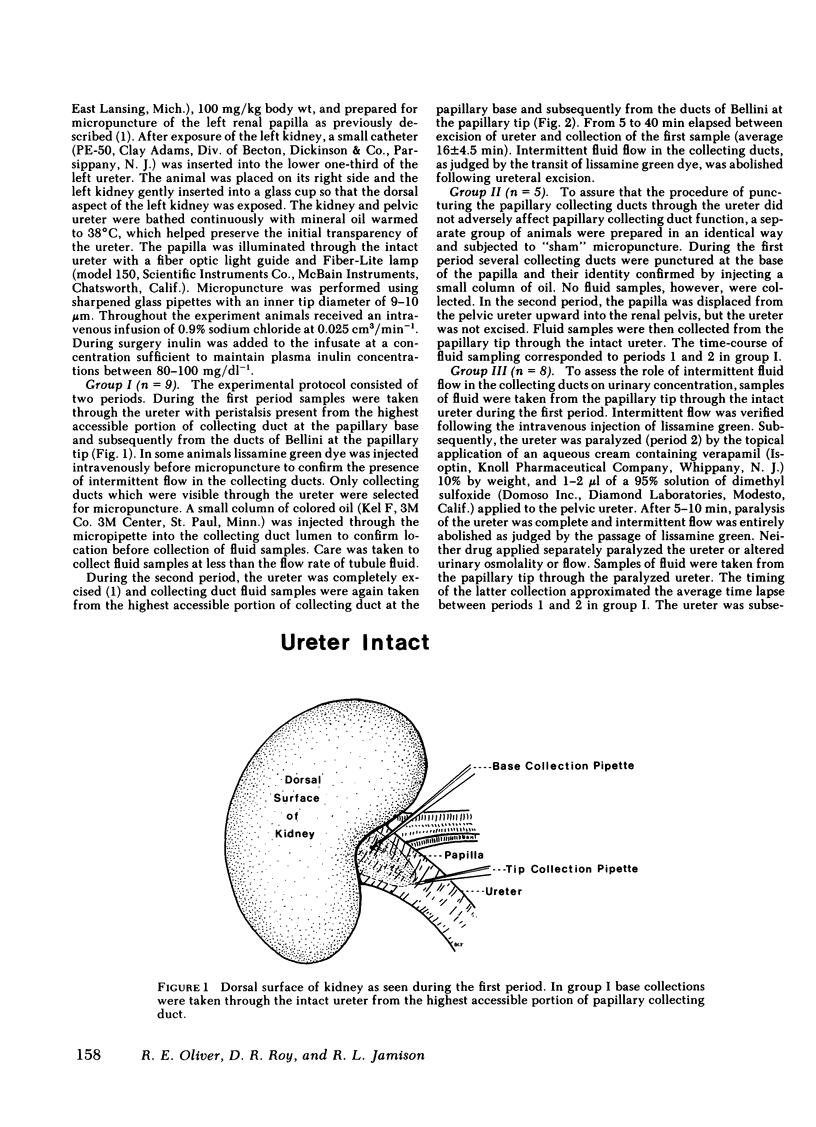
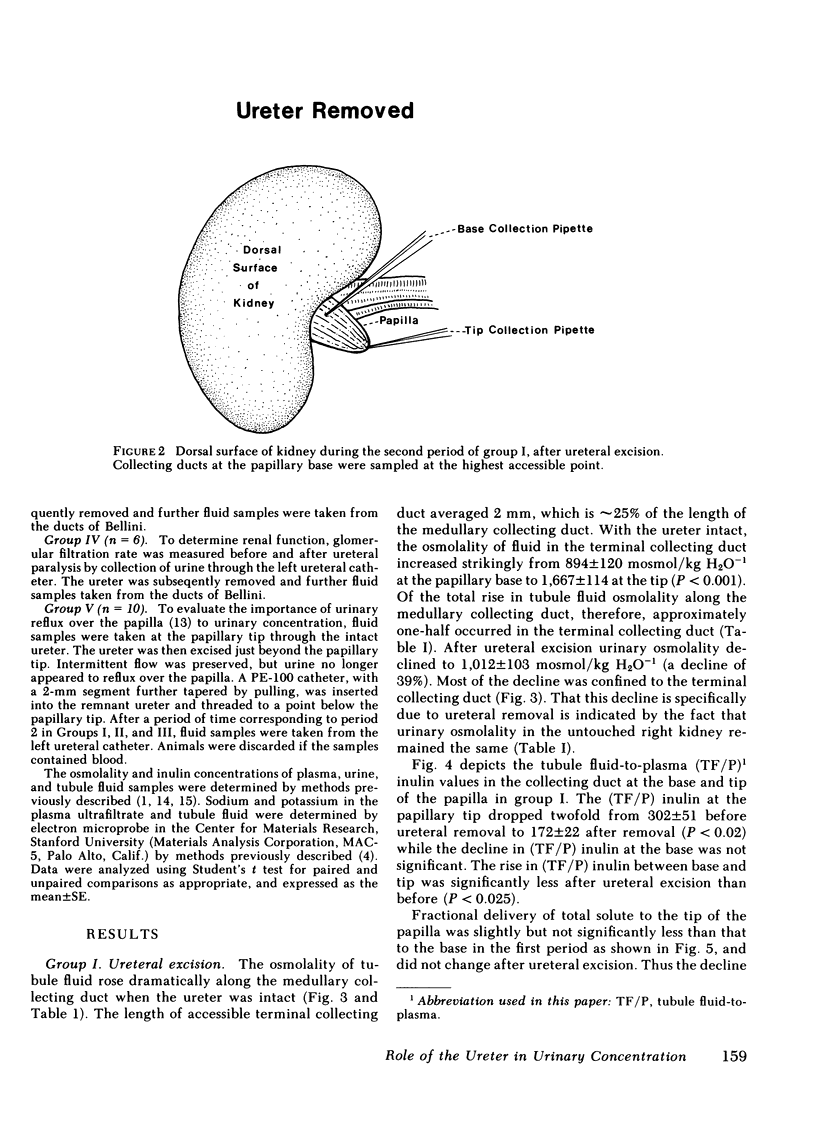
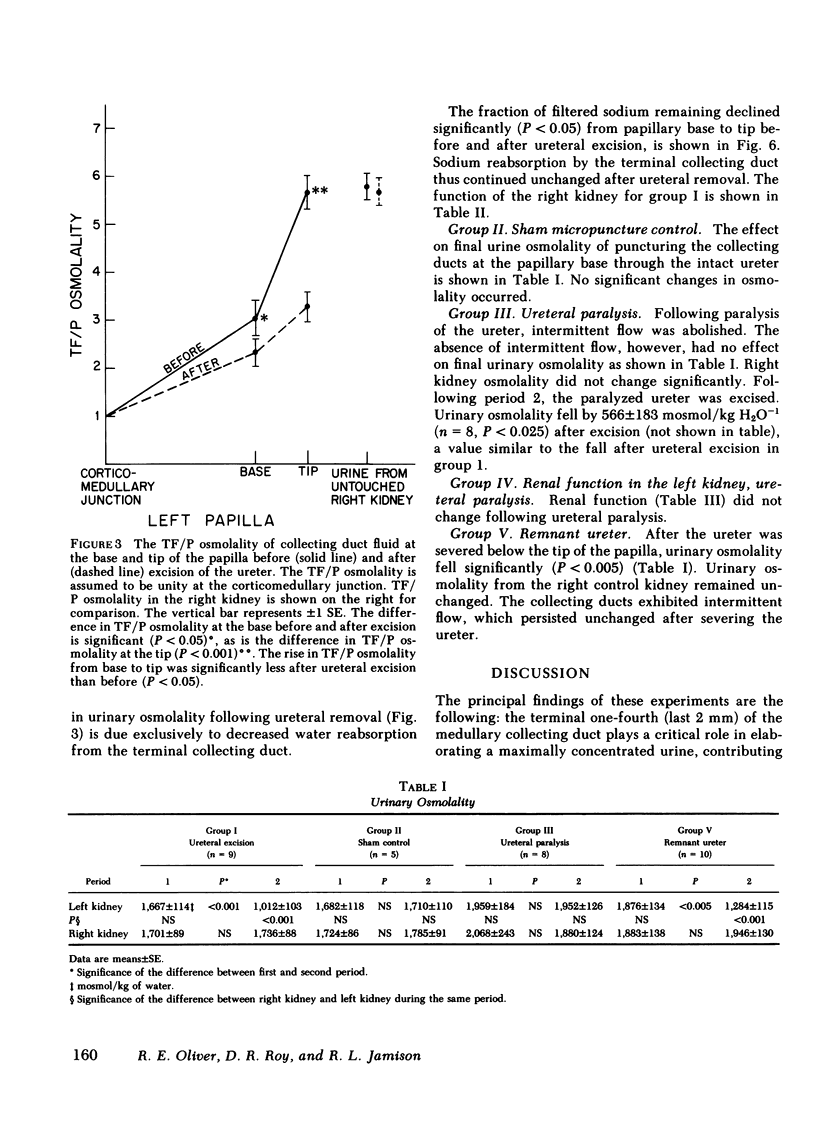
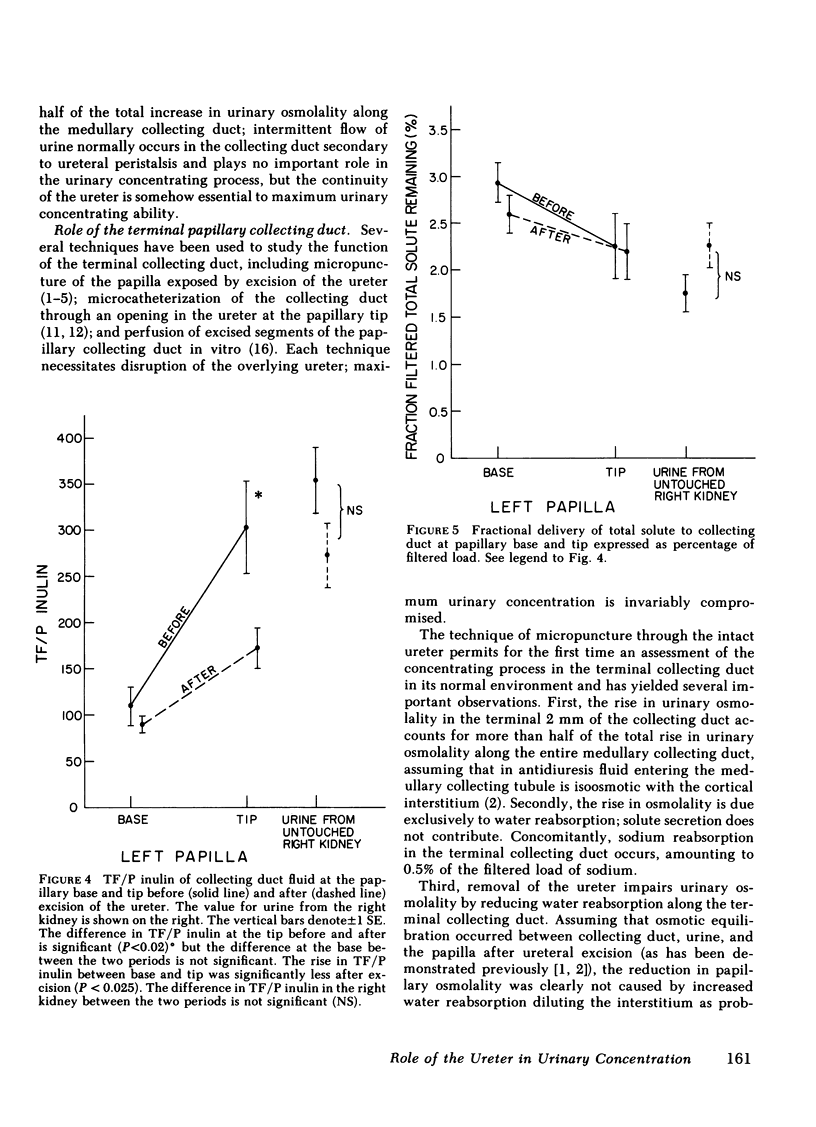
Urine was observed to flow intermittently in the collecting ducts of the extrarenal papilla of antidiuretic rats. The purpose of this investigation was to test Reinking and Schmidt-Nielsen's hypothesis that intermittent flow plays an important role in the production of maximally concentrated urine. Samples of collecting duct fluid were obtained from the base and tip of the papilla by micropuncture through the intact ureter. Fluid osmolality rose sharply from base, 894±120 mosmol/kg H2O−1 (mean±SE), to tip, 1,667±114 (P<0.001), a distance of only 2 mm, and was due exclusively to reabsorption of water. After excision of the ureter, which abolished intermittent flow, osmolality fell modestly at the base to 723±82 mosmol/kg H2O−1 (P < 0.02), but strikingly at the tip to 1,012±103 (P < 0.001). The pelvic ureter was paralyzed by topical verapamil and dimethylsulfoxide, which abolished intermittent flow. Osmolality of urine at the tip was not changed (1,959±184 mosmol/kg H2O−1 before, vs. 1,957±126 after paralysis). The ureter was severed just beyond the papillary tip, a maneuver which preserved intermittent flow but abolished urinary reflux over the papilla. Urinary osmolality fell from 1,876±134 mosmol/kg H2O−1 to 1,284±115 (P < 0.005). These findings demonstrate that when the ureter is intact, over half of the increase in urinary osmolality above isotonicity occurs in the terminal one-fourth of the medullary collecting duct and is due exclusively to water reabsorption (no net solute addition). It is the continuity of the ureter, rather than intermittent flow due to ureteral peristalsis, which is essential for the formation of a maximally concentrated urine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonventre J. V., Karnovsky M. J., Lechene C. P. Renal papillary epithelial morphology in antidiuresis and water diuresis. Am J Physiol. 1978 Jul;235(1):F69–F76. doi: 10.1152/ajprenal.1978.235.1.F69. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Roman R. J., Lechene C. Effect of urea concentration of pelvic fluid on renal concentrating ability. Am J Physiol. 1980 Dec;239(6):F609–F618. doi: 10.1152/ajprenal.1980.239.6.F609. [DOI] [PubMed] [Google Scholar]
- Chuang E. L., Reineck H. J., Osgood R. W., Kunau R. T., Jr, Stein J. H. Studies on the mechanism of reduced urinary osmolality after exposure of renal papilla. J Clin Invest. 1978 Mar;61(3):633–639. doi: 10.1172/JCI108974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C. E., Neubarth J. L., Mensah-Dwumah M. Frequency gradient in the autorhythmicity of the pyeloureteral pacemaker system. Experientia. 1978 May 15;34(5):614–615. doi: 10.1007/BF01936991. [DOI] [PubMed] [Google Scholar]
- Dobyan D. C., Arrascue J. F., Jamison R. L. Terminal papillary collecting duct reabsorption of water, sodium, and potassium in Psammomys obesus. Am J Physiol. 1980 Dec;239(6):F539–F544. doi: 10.1152/ajprenal.1980.239.6.F539. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Gussis G. L., Jamison R. L., Robertson C. R. Determination of erythrocyte velocities in the mammalian inner renal medulla by a video velocity-tracking system. Microvasc Res. 1979 Nov;18(3):370–383. doi: 10.1016/0026-2862(79)90044-x. [DOI] [PubMed] [Google Scholar]
- JARAUSCH K. H., ULLRICH K. J. Zur Technik der Entnahme von Harnproben aus einzelnen Sammelrohren der Säugetierniere mittels Polyäthylen-Capillaren. Pflugers Arch. 1957;264(1):88–94. doi: 10.1007/BF00412575. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Buerkert J., Lacy F. A micropuncture study of collecting tubule function in rats with hereditary diabetes insipidus. J Clin Invest. 1971 Nov;50(11):2444–2452. doi: 10.1172/JCI106743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R. L. Micropuncture study of segments of thin loop of Henle in the rat. Am J Physiol. 1968 Jul;215(1):236–242. doi: 10.1152/ajplegacy.1968.215.1.236. [DOI] [PubMed] [Google Scholar]
- Jamison R. L. Micropuncture study of superficial and juxtamedullary nephrons in the rat. Am J Physiol. 1970 Jan;218(1):46–55. doi: 10.1152/ajplegacy.1970.218.1.46. [DOI] [PubMed] [Google Scholar]
- Pennell J. P., Sanjana V., Frey N. R., Jamison R. L. The effect of urea infusion on the urinary concentrating mechanism in protein-depleted rats. J Clin Invest. 1975 Feb;55(2):399–409. doi: 10.1172/JCI107944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinking L. N., Schmidt-Nielsen B. Peristaltic flow of urine in the renal capillary collecting ducts of hamsters. Kidney Int. 1981 Jul;20(1):55–60. doi: 10.1038/ki.1981.104. [DOI] [PubMed] [Google Scholar]
- STEINHAUSEN M. IN VIVO-BEOBACHTUNGEN AND DER NIERENPAPILLE VON GOLDHAMSTERN NACH INTRAVENOESER LISSAMINGRUEN-INJEKTION. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Apr 29;279:195–213. [PubMed] [Google Scholar]
- Schütz W., Schnermann J. Pelvic urine composition as a determinant of inner medullary solute concentration and urine osmolarity. Pflugers Arch. 1972;334(2):154–166. doi: 10.1007/BF00586788. [DOI] [PubMed] [Google Scholar]
- Sonnenberg H. Secretion of salt and water into the medullary collecting duct of Ringer-infused rats. Am J Physiol. 1975 Feb;228(2):565–568. doi: 10.1152/ajplegacy.1975.228.2.565. [DOI] [PubMed] [Google Scholar]


