Abstract
Most drug interaction resources suggest that levothyroxine can dramatically potentiate the effect of warfarin. However, the mechanistic basis of the interaction is speculative, and little evidence supports a meaningful drug interaction. We conducted a population-based nested case–control study to examine the risk of hospitalization for hemorrhage following the initiation of levothyroxine in a cohort of 260,076 older patients receiving warfarin. In this group, we identified 10,532 case subjects hospitalized for hemorrhage and 40,595 controls. In the primary analysis, we found no association between hospitalization for hemorrhage during warfarin therapy and initiation of levothyroxine in the preceding 30 days (adjusted odds ratio 1.11, 95% confidence interval 0.67–1.86). Secondary analyses using more remote initiation of levothyroxine also found no association. These findings suggest that concerns about a clinically meaningful levothyroxine–warfarin drug interaction are not justified. Drug interaction resources that presently characterize this interaction as important should reevaluate this classification.
INTRODUCTION
Warfarin has been in clinical use for more than 50 years.1 With more than 31 million outpatient prescriptions in 2010, it is the most commonly prescribed oral anticoagulant in the United States.2 Treatment with warfarin remains a challenge, however, and the drug is one of the top 10 cited in the US Food and Drug Administration’s Adverse Event Reporting System.3 The adverse events reported are largely the result of warfarin’s narrow therapeutic window.4–6 Genetic polymorphisms and dietary factors influence the individual response to warfarin,7–11 but avoidable drug interactions are of particular importance.12–15 Hundreds of drugs can alter the response to warfarin, and in most cases these interactions, although predictable and avoidable, are underappreciated.2,13,14
Several authoritative reference sources suggest that a significant drug interaction exists between warfarin and levothyroxine, predisposing patients to bleeding.16–20 Two pharmacodynamic mechanisms have been advanced to explain this potential interaction. First, thyroid hormone may enhance the affinity of warfarin for its target, the enzyme vitamin K–dependent epoxide reductase.21,22 Second, supplemental thyroid hormone may increase the metabolic clearance of vitamin K–dependent clotting factors, particularly factors II and VII.23–27 Because warfarin interferes with activation of the same factors, these mechanisms are thought to explain the potential drug interaction between the two drugs.
Because levothyroxine and warfarin are used by millions of patients each year,2 it is probable that many patients worldwide will receive these drugs in combination. Several drug interaction textbooks warn that the potential interaction between levothyroxine and warfarin is clinically important16–18 and characterize its severity as major.17 The interaction is also described in popular evidence-based resources such as UpToDate and Micromedex, as well as drug interaction databases used by pharmacists such as First DataBank.
Although this potential interaction was first described in animal studies more than half a century ago,28,29 case reports and small prospective studies have done little to expand our understanding of its frequency and clinical relevance.19,22–26,30–34 Increases in the international normalized ratio have been observed in clinical settings after patients receiving warfarin became thyrotoxic for various reasons, including after being given levothyroxine.30 Similarly, small prospective studies observed increases in prothrombin time of up to 26 s in participants receiving warfarin when they were also given thyroid hormone.24,32 However, only one case report describes a patient presenting with clinical evidence of bleeding thought to result from the concomitant use of levothyroxine and warfarin.30 No controlled studies of this interaction have explored clinically relevant outcomes such as hemorrhage. We examined the clinical consequences of the possible drug interaction between levothyroxine and warfarin.
RESULTS
Over the 16-year study period, we identified 260,076 patients with at least 3 months of continuous warfarin use beginning after their 66th birthday. Within this group, we identified 10,937 patients who were hospitalized for hemorrhage. We excluded 405 cases (3.7%) because they could not be matched with at least one control. The remaining 10,532 cases were matched to 40,693 controls. The median age of cases and controls was 80 years (interquartile range 75–86 years), and slightly more than half were women (50.8%; Table 1). As expected, cases were more likely than controls to have various comorbidities, including stroke, atrial fibrillation, and chronic kidney disease. Other characteristics of the cases and controls are shown in Table 1.
Table 1.
Characteristics of patients receiving warfarin
| Variable | Cases | Controls | Standardized differencea |
|---|---|---|---|
| n = 10,532 | n = 40,693 | ||
| Age | |||
| Median (IQR) | 80 (75–86) | 80 (75–86) | 0 |
| Male | 5,181 (49.2%) | 20,018 (49.2%) | 0 |
| Income quintile | |||
| 1 | 2,374 (22.5%) | 8,294 (20.4%) | 0.05 |
| 2 | 2,293 (21.8%) | 8,744 (21.5%) | 0.01 |
| 3 | 2,099 (19.9%) | 8,080 (19.9%) | 0 |
| 4 | 1,888 (17.9%) | 7,520 (18.5%) | 0.01 |
| 5 | 1,841 (17.5%) | 7,907 (19.4%) | 0.05 |
| Missing | 37 (0.4%) | 148 (0.4%) | 0 |
| Residence in long-term care | 1,800 (17.1%) | 5,386 (13.2%) | 0.11 |
| Number of different drugs used in previous year | 13 (9–17) | 11 (8–15) | 0.3 |
| Comorbid conditions (previous 3 years) | |||
| Hemorrhage | 395 (3.8%) | 1,526 (3.8%) | 0 |
| Atrial fibrillation | 7,864 (74.7%) | 27,796 (68.3%) | 0.14 |
| Stroke | 1,314 (12.5%) | 3,964 (9.7%) | 0.09 |
| Hypertension | 6,277 (59.6%) | 24,072 (59.2%) | 0.01 |
| Alcohol-use disorder | 455 (4.3%) | 1,440 (3.5%) | 0.04 |
| Chronic kidney disease | 3,362 (31.9%) | 8,954 (22.0%) | 0.23 |
| Chronic liver disease | 367 (3.5%) | 948 (2.3%) | 0.07 |
| Other medication use within 60 days before index date | |||
| Aspirin | 320 (3.0%) | 1,327 (3.3%) | 0.01 |
| Other antiplatelet drugs | 228 (2.2%) | 841 (2.1%) | 0.01 |
| Other NSAIDs | 865 (8.2%) | 1,927 (4.7%) | 0.15 |
| Cyclo-oxygenase-2 inhibitor | 281 (2.7%) | 852 (2.1%) | 0.04 |
| Acetaminophen | 2,741 (26.0%) | 7,747 (19.0%) | 0.17 |
| H2-receptor antagonists | 1,227 (11.7%) | 3,757 (9.2%) | 0.08 |
| Other gastroprotective medications | 442 (4.2%) | 1,361 (3.3%) | 0.05 |
| Proton pump inhibitors | 1,668 (15.8%) | 5,535 (13.6%) | 0.06 |
| SSRI antidepressants | 1,481 (14.1%) | 3,873 (9.5%) | 0.15 |
| Corticosteroids | 747 (7.1%) | 1,990 (4.9%) | 0.1 |
| Other medication use within 14 days before index date | |||
| Cotrimoxazole | 243 (2.3%) | 244 (0.6%) | 0.18 |
| Metronidazole | 67 (0.6%) | 79 (0.2%) | 0.08 |
| Other oral antibiotic | 1,268 (12.0%) | 2,204 (5.4%) | 0.27 |
IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug; SSRI, selective serotonin receptor inhibitor.
Standardized differences <0.10 are generally not considered meaningful.
In the primary analysis, we found no increase in the risk of hemorrhage among older patients receiving warfarin who initiated levothyroxine in the previous 30 days (adjusted odds ratio (OR) 1.11, 95% confidence interval 0.67–1.86; Table 2). As expected, we found no significant association with more distant exposures (adjusted OR 0.76, 95% confidence interval 0.26–2.25 for patients initiating levothyroxine 31–60 days before the index date; adjusted OR 0.67, 95% confidence interval 0.15–3.01 for patients initiating levothyroxine 61–90 days before bleeding; Table 2).
Table 2.
Association between hospitalization for hemorrhage and new use of levothyroxine among continuous warfarin users
| New levothyroxine exposure | Cases (n = 10,532) | Controls (n = 40,693) | Unadjusted odds ratio (95% confidence interval) | Adjusteda odds ratio (95% confidence interval) |
|---|---|---|---|---|
| Reference (no new use) | 10,505 | 40,595 | N/A | N/A |
| 0–30 days prior to index date | 21 | 63 | 1.26 (0.76–2.07) | 1.11 (0.67–1.86) |
| 31–60 days prior to index date | ≤5 | 22 | 0.73 (0.251–2.11) | 0.76 (0.26–2.25) |
| 61–90 days prior to index date | ≤5 | 13 | 0.62 (0.14–2.73) | 0.67 (0.15–3.01) |
N/A, not applicable.
Adjusted for income quintile, long-term care residence status, history of atrial fibrillation and chronic kidney disease, number of drugs prescribed during the past year, and recent medication use.
DISCUSSION
In this population-based study spanning 16 years, we found no significantly increased risk of hospitalization for hemorrhage following the initiation of levothyroxine in older patients already acclimatized to warfarin therapy. This is the first large-scale exploration of this potential drug interaction to study a clinically relevant outcome. Although hundreds of drugs can modify the response to warfarin and increase the risk of bleeding in clinical practice,2,13,14 our findings suggest that levothyroxine is not among them.
It is possible that an interaction does indeed exist between levothyroxine and warfarin but that it registers no discernible hemorrhage signal in clinical practice because of the gradual introduction of levothyroxine, a slow onset of clinically apparent interaction, and periodic adjustment of the warfarin dose, which would offset the risk of bleeding. Although this possibility cannot be excluded, it argues further against a clinically important interaction.
Although many drug interaction textbooks and other clinical tools identify the interaction between levothyroxine and warfarin as serious, little evidence supports a clinically meaningful interaction. Only a single case report describes a patient with hemorrhage presumed to be the result of concomitant therapy with the two drugs,30 and no controlled studies of this interaction have examined a clinically relevant outcome such as bleeding. Indeed, some of the concern about the potential drug interaction may reflect extrapolation of an apparent drug–disease interaction between warfarin and thyrotoxicosis, in which the response to warfarin appears to have been exaggerated.25,26,31,34–36
Several limitations of our study merit emphasis. Clinicians may already exercise caution when prescribing these drugs together because of existing warnings, which would attenuate the risk of adverse outcomes, and we could not account for preemptive warfarin dose adjustment among patients commencing levothyroxine treatment. Moreover, because many physicians initiate levothyroxine therapy gradually in older patients, this would tend to lessen the clinical impact of any potential interaction. We have no data regarding use of nonprescription acetylsalicylic acid, acetaminophen, or anti-inflammatory drugs, which may increase the risk of hemorrhage during warfarin therapy. However, these should be equally distributed regardless of thyroxine use and should not influence our results. Finally, we have no direct measure of bleeding not leading to hospitalization, drug adherence, or laboratory data, such as international normalized ratio levels, and, because our results derive from patients aged 66 years and older, the generalizability of our findings to younger patients is unknown.
In conclusion, we found no evidence that the initiation of levothyroxine was associated with an increased risk of hospitalization for hemorrhage in a large cohort of older patients receiving warfarin. Our findings suggest that concerns about a clinically meaningful levothyroxine–warfarin interaction may be misplaced. This observation may help inform future iterations of drug interaction compendia and other electronic tools that currently characterize this interaction as clinically important. For front-line physicians and pharmacists, these results provide reassurance that no particular additional vigilance is necessary when levothyroxine is initiated in patients already receiving warfarin.
METHODS
Setting
We conducted a population-based nested case–control study among Ontario residents aged 66 years or older treated with warfarin between 1 July 1994 and 31 December 2009. The study was approved by the research ethics board of Sunnybrook Health Sciences Centre.
Data sources
We examined the computerized prescription records of the Ontario Public Drug Program, which contains comprehensive records of prescription medications dispensed to Ontario residents 65 years of age and older. We identified hospital admissions using the Canadian Institute for Health Information’s Discharge Abstract Database, which contains detailed diagnostic and procedural information about all hospital admissions in the province. We used the Ontario Health Insurance Plan database to identify claims for physician services and the Registered Persons Database to obtain demographic information. These databases were linked in an anonymous fashion using encrypted health-card numbers and are regularly used to study drug safety, including the consequences of drug interactions.37–41
Identification of patients
The study design is outlined in Figure 1. For each patient, we identified a period of continuous warfarin use beginning with the first prescription for warfarin following the patient’s 66th birthday. For a patient to be defined as a continuous user of warfarin, we required successive prescription claims within 120 days. We excluded patients with a single warfarin prescription, those with fewer than 90 days of total warfarin use, and those hospitalized for bleeding within 90 days of their first warfarin prescription, to allow for an initial period of acclimatization to warfarin therapy. Patients were censored upon death, discontinuation of warfarin, hospitalization for hemorrhage or the end of the study period (31 March 2010), whichever occurred first. For patients who discontinued warfarin, observation was continued for 60 days from the date of their last prescription to identify adverse events that may have precipitated cessation of therapy.
Figure 1.
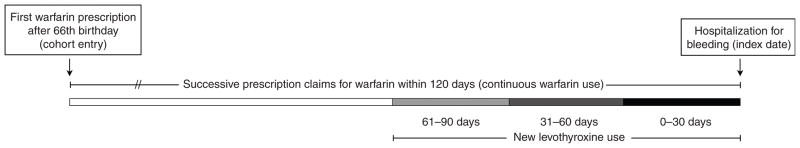
Description of the study design. We conducted a population-based, case–control study nested in a cohort of continuous warfarin users. Patients were excluded from the cohort if they had (i) only one warfarin prescription, (ii) <90 days of total warfarin use, or (iii) bleeding within 90 days of their first warfarin prescription. For cases, the index date was the date of hospitalization with a diagnosis of hemorrhage. Controls were continuous warfarin users who were not admitted to hospital for hemorrhage. Once cases and controls were identified, we looked back to assess new exposure to levothyroxine (initiated 0–30, 31–60, or 61–90 days before the index date). Patients who did not receive a new prescription for levothyroxine constituted the reference group in our analysis.
Outcomes
Hospital admissions for hemorrhage were identified using the International Classification of Disease and Related Health Problems, 9th and 10th revisions (see Supplementary Data online). For each case, the index date was defined as the date of the first hospital admission for hemorrhage following cohort entry. We identified up to four randomly selected controls who were taking warfarin but free of hemorrhage on the index date, matching on age (within 3 years), sex, and any previous hospitalization with hemorrhage (in the prior 3 years). Controls were assigned the same index date as their matched case. When four matched controls could not be obtained, the matching criteria were not altered and all available controls were analyzed. Each control could be matched to only one case, and we excluded cases without at least one matched control.
Exposure
Within the cohort of individuals receiving warfarin, we identified patients newly exposed to levothyroxine in the 90 days before their index date because the clinical consequences of this interaction are expected to manifest following the introduction of levothyroxine in patients already receiving warfarin.16–18 Patients were defined as newly exposed if they did not have another levothyroxine prescription in the previous year. As our primary analysis, we examined new levothyroxine prescriptions initiated within 30 days before the index date. We anticipated that any association would attenuate with more remote initiation of levothyroxine. As secondary analyses, we examined new levothyroxine prescriptions 31–60 days and 61–90 days before the index date.
Statistical analysis
We compared baseline characteristics of cases and controls using standardized differences, which reflect the mean difference as a percentage of the SD. This measure is not as sensitive to sample size as traditional tests such as P values; this is particularly important when analyzing large datasets. Values <0.10 suggest negligible differences between groups.
We used conditional logistic regression to estimate the OR and 95% confidence interval for the association between hemorrhage during warfarin therapy and new exposure to levothyroxine. In all analyses, patients who did not receive a new prescription for levothyroxine in the 90 days before their index date constituted the reference group.
We adjusted for income quintile (estimated from residential postal codes), residence in a long-term care facility, history of atrial fibrillation or chronic kidney disease, and the number of drugs prescribed in the past year.42 We also adjusted for the receipt of other drugs that might modify the risk of warfarin-related hemorrhage, including antiplatelet agents, nonsteroidal anti-inflammatory drugs, cyclo-oxygenase 2 inhibitors, prescription acetaminophen compounds, gastroprotective medications, selective serotonin receptor inhibitors and corticosteroids dispensed within 60 days preceding the index date, along with sulfamethoxazole/trimethoprim, metronidazole, and other oral antibiotics dispensed within 14 days of the index date (see Supplementary Data online). We used SAS version 9.2 for all analyses (SAS Institute, Cary, NC) and a two-tailed type 1 error rate of 0.05 as the threshold for statistical significance.
Supplementary Material
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Several authoritative reference sources suggest that a significant drug interaction exists between warfarin and levothyroxine, increasing the risk of hemorrhage. However, the mechanism of this interaction is speculative, and little evidence supports a clinically meaningful drug interaction.
WHAT QUESTION DID THIS STUDY ADDRESS?
We examined the clinical consequences of the possible drug interaction between levothyroxine and warfarin.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
Only a single case report describes a patient with hemorrhage presumed to be the result of concomitant therapy with the two drugs, and no controlled studies of this interaction have examined a clinically relevant outcome such as bleeding. In this large-scale controlled study, we found that older patients taking warfarin faced no increase in the risk of hospitalization for hemorrhage in the month after initiating levothyroxine.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS
Our findings suggest that concerns about a clinically meaningful levothyroxine–warfarin drug interaction are not justified. Drug interaction resources that currently characterize this interaction as important should reevaluate this classification.
Acknowledgments
We thank Brogan Inc., Ottawa, for use of their Drug Product and Therapeutic Class Database. We are indebted to Philip Hansten for detailed comments on an earlier draft of the manuscript.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
AUTHOR CONTRIBUTIONS
D.P., T.G., C.H., and D.N.J. wrote the manuscript. D.P., T.G., C.H., J.M.P., M.M.M., and D.N.J. designed research. H.Z. analyzed data.
CONFLICT OF INTEREST
J.M.P. and D.N.J. received salary support from the Institute for Clinical Evaluative Sciences, a nonprofit research institute funded by the Ontario Ministry of Health and Long-Term Care. Over the past 3 years M.M.M. has served on advisory boards for Hoffmann–La Roche, GlaxoSmithKline, Pfizer, Novartis, Lilly, AstraZeneca, Boehringer Ingelheim, and Novo-Nordisk. T.G. received salary support from the Ontario Drug Policy Research Network, a nonprofit research group funded by the Ontario Ministry of Health and Long-Term Care. This study was supported by a grant from the Canadian Institutes of Health Research and by the Institute for Clinical Evaluative Sciences, a nonprofit research institute funded by the Ontario Ministry of Health and Long-Term Care. The study sponsors had no role in designing the study; collecting, analyzing, or interpreting the data; writing the report; or the decision to submit the article for publication. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by the Institute for Clinical Evaluative Sciences or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred.
References
- 1.Greenblatt DJ, von Moltke LL. Interaction of warfarin with drugs, natural substances, and foods. J Clin Pharmacol. 2005;45:127–132. doi: 10.1177/0091270004271404. [DOI] [PubMed] [Google Scholar]
- 2.IMS Institute for Healthcare Informatics. The Use of Medicines in the United States: Review of 2010. 2011. [Google Scholar]
- 3.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414–1419. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- 4.Schulman S, Beyth RJ, Kearon C, Levine MN. American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:257S–298S. doi: 10.1378/chest.08-0674. [DOI] [PubMed] [Google Scholar]
- 5.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Hirsh J, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S–21S. doi: 10.1378/chest.119.1_suppl.8s. [DOI] [PubMed] [Google Scholar]
- 7.Kamali F, Wynne H. Pharmacogenetics of warfarin. Annu Rev Med. 2010;61:63–75. doi: 10.1146/annurev.med.070808.170037. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Ge W, Yu F, Zhu H. Impact of VKORC1 gene polymorphism on interindividual and interethnic warfarin dosage requirement–a systematic review and meta analysis. Thromb Res. 2010;125:e159–e166. doi: 10.1016/j.thromres.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Lindh JD, Holm L, Andersson ML, Rane A. Influence of CYP2C9 genotype on warfarin dose requirements–a systematic review and meta-analysis. Eur J Clin Pharmacol. 2009;65:365–375. doi: 10.1007/s00228-008-0584-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89–295. doi: 10.1080/03602530902843483. [DOI] [PubMed] [Google Scholar]
- 11.Zhou SF, Zhou ZW, Huang M. Polymorphisms of human cytochrome P450 2C9 and the functional relevance. Toxicology. 2010;278:165–188. doi: 10.1016/j.tox.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 12.International Warfarin Pharmacogenetics Consortium et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holbrook AM, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095–1106. doi: 10.1001/archinte.165.10.1095. [DOI] [PubMed] [Google Scholar]
- 14.Juurlink DN. Drug interactions with warfarin: what clinicians need to know. CMAJ. 2007;177:369–371. doi: 10.1503/cmaj.070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs LG. Warfarin pharmacology, clinical management, and evaluation of hemorrhagic risk for the elderly. Clin Geriatr Med. 2006;22:17–32. vii. doi: 10.1016/j.cger.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Stockley IH. Stockley’s Drug Interactions: A Source Book of Interactions, Their Mechanisms, Clinical Importance and Management. 6. Pharmaceutical Press; London: 2002. [Google Scholar]
- 17.Tatro DS. Drug Interaction Facts. Lippincott; St. Louis: 2009. [Google Scholar]
- 18.Hansten PD. Drug Interactions Analysis and Management: A Clinical Perspective and Analysis of Current Developments. Facts and Comparisons; St Louis: 2000. [Google Scholar]
- 19.Hansten PD. Oral anticoagulants and drugs which alter thyroid function. Drug Intell Clin Pharm. 1980;14:331–334. [Google Scholar]
- 20.Weser JK, Sellers E. Drug interactions with coumarin anticoagulants. 2. N Engl J Med. 1971;285:547–558. doi: 10.1056/NEJM197109022851005. [DOI] [PubMed] [Google Scholar]
- 21.Schrogie JJ, Solomon HM. The anticoagulant response to bishydroxycoumarin. II The effect of D-thyroxine, clofibrate, and norethandrolone. Clin Pharmacol Ther. 1967;8:70–77. doi: 10.1002/cpt196781part170. [DOI] [PubMed] [Google Scholar]
- 22.Solomon HM, Schrogie JJ. Change in receptor site affinity: a proposed explanation for the potentiating effect of D-thyroxine on the anticoagulant response to warfarin. Clin Pharmacol Ther. 1967;8:797–799. doi: 10.1002/cpt196786797. [DOI] [PubMed] [Google Scholar]
- 23.Walters MB. The relationship between thyroid function and anticoagulant thrapy. Am J Cardiol. 1963;11:112–114. doi: 10.1016/0002-9149(63)90043-2. [DOI] [PubMed] [Google Scholar]
- 24.Owens JC, Neely WB, Owen WR. Effect of sodium dextrothyroxine in patients receiving anticoagulants. N Engl J Med. 1962;266:76–79. doi: 10.1056/NEJM196201112660205. [DOI] [PubMed] [Google Scholar]
- 25.Kellett HA, Sawers JS, Boulton FE, Cholerton S, Park BK, Toft AD. Problems of anticoagulation with warfarin in hyperthyroidism. Q J Med. 1986;58:43–51. [PubMed] [Google Scholar]
- 26.Vagenakis AG, Cote R, Miller ME, Braverman LE, Stohlman F., Jr Enhancement of warfarin-induced hypoprothrombinemia by thyrotoxicosis. Johns Hopkins Med J. 1972;131:69–73. [PubMed] [Google Scholar]
- 27.Owens JC, Neely WB, Owen WR. Effect of sodium dextrothyroxine in patients receiving anticoagulants. Clin Med (Northfield Il) 1962;69:2283–2284. [PubMed] [Google Scholar]
- 28.Clark BB, Spitalny M. Prothrombinopenic activity of the salicylates and pharmacologically related drugs. Fed Proc. 1946;5:171. [PubMed] [Google Scholar]
- 29.Lowenthal J, Fisher LM. The effect of thyroid function on the prothrombin time response to warfarin in rats. Experientia. 1957;13:253–254. doi: 10.1007/BF02157446. [DOI] [PubMed] [Google Scholar]
- 30.Costigan DC, Freedman MH, Ehrlich RM. Potentiation of oral anticoagulant effect by L-thyroxine. Clin Pediatr (Phila) 1984;23:172–174. doi: 10.1177/000992288402300308. [DOI] [PubMed] [Google Scholar]
- 31.Kurnik D, Loebstein R, Farfel Z, Ezra D, Halkin H, Olchovsky D. Complex drug-drug-disease interactions between amiodarone, warfarin, and the thyroid gland. Medicine (Baltimore) 2004;83:107–113. doi: 10.1097/01.md.0000123095.65294.34. [DOI] [PubMed] [Google Scholar]
- 32.Rice AJ, McIntosh TJ, Fouts JR, Brunk SF, Wilson WR. Decreased sensitivity to warfarin in patients with myxedema. Am J Med Sci. 1971;262:211–215. doi: 10.1097/00000441-197110000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Self T, Weisburst M, Wooten E, Straughn A, Oliver J. Warfarin-induced hypoprothrombinemia. Potentiation by hyperthyroidism. JAMA. 1975;231:1165–1166. [PubMed] [Google Scholar]
- 34.Woeber KA, Warner I. Potentiation of warfarin sodium by amiodarone-induced thyrotoxicosis. West J Med. 1999;170:49–51. [PMC free article] [PubMed] [Google Scholar]
- 35.Self TH, Straughn AB, Weisburst MR. Effect of hyperthyroidism on hypoprothrombinemic response to warfarin. Am J Hosp Pharm. 1976;33:387–389. [PubMed] [Google Scholar]
- 36.Simone JV, Abildgaard CF, Schulman I. Blood coagulation in thyroid dysfunction. N Engl J Med. 1965;273:1057–1061. doi: 10.1056/NEJM196511112732001. [DOI] [PubMed] [Google Scholar]
- 37.Juurlink DN, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 38.Juurlink DN, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180:713–718. doi: 10.1503/cmaj.082001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652–1658. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 40.Park-Wyllie LY, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354:1352–1361. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 41.Wright AJ, Gomes T, Mamdani MM, Horn JR, Juurlink DN. The risk of hypotension following co-prescription of macrolide antibiotics and calcium-channel blockers. CMAJ. 2011;183:303–307. doi: 10.1503/cmaj.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38:1103–1120. doi: 10.1111/1475-6773.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


