Abstract
The role of the cerebellum in auditory processing is largely unknown. Recently it was shown that rats with psychophysical evidence of tinnitus had significantly elevated neural activity in the paraflocculus of the cerebellum (PFL), as indicated by functional imaging. It was further shown that PFL activity was not elevated in normal rats listening to a tinnitus-like sound. This suggests that plastic changes in the PFL may underpin chronic tinnitus, i.e., it may serve as a tinnitus generator. Using a rat model of acoustic-trauma-induced tinnitus, the role of the cerebellum was further examined in a series of experiments: The PFLwas surgically ablated in animals with established tinnitus; the PFL was surgically ablated in animals before induction of tinnitus; the PFL was reversibly inactivated by chronic lidocaine infusion into the subarcuate fossa of animals with established tinnitus. It was found that PFL ablation eliminated established tinnitus without altering auditory discrimination. Similar to the ablation results, PFL inactivation with lidocaine reversibly eliminated existing tinnitus. In contrast however, PFL ablation before tinnitus induction attenuated, but did not completely eliminate, tinnitus. In a rat model of noise-induced chronic tinnitus, the cerebellar PFL may serve as a sufficient but non-obligatory generator of tinnitus.
Keywords: tinnitus, cerebellum, paraflocculus, flocculus, noise-trauma, behavioral model, lidocaine
1.1 Introduction
Chronic tinnitus may develop because insults to the peripheral auditory system produce maladaptive changes in susceptible brain areas that serve as trigger zones. Trigger zones may in turn produce a cascade of events affecting other brain areas that then serve as permanent generator sites (Brozoski et al., 2005). Generator sites may cause tinnitus in one or more ways by homeostatically compensating, or overcompensating, for abnormal activity in a trigger zone. The generator mechanism may reflect a release from tonic inhibitory control or it may be a reactive response to elevated excitatory drive.
Multiple sites along the auditory neuraxis may be considered potential tinnitus generators. Candidate loci include the dorsal cochlear nucleus (DCN), the inferior colliculus (IC), and auditory cortex (AC) (Bauer et al., 2008; Brozoski et al., 2002; Norena et al., 2003; Salvi et al., 1990; Tan et al., 2007; Yang et al., 2007). Emerging data also suggest that non-lemniscal auditory structures and non-auditory regions should not be discounted as potential generators. The medial geniculate body (MGB), secondary auditory cortex (SAC), and nucleus accumbens (NA) have been considered (Eggermont et al., 1998; Rauschecker et al., 2010). However to date, convincing causal evidence has not implicated any one site as a necessary tinnitus generator. Although there is considerable evidence implicating the DCN in chronic tinnitus (Kaltenbach et al., 2008), bilateral ablation of the DCN in rats with established tinnitus did not eliminate psychophysical evidence of tinnitus (Brozoski et al., 2005). However, identical DCN ablation prior to tinnitus induction was sufficient to prevent acoustic trauma induction of tinnitus (Brozoski et al., 2012), even though auditory thresholds and discrimination were unaffected. This would suggest that the DCN at least serves as a necessary trigger zone for tinnitus.
There is a need to identify critical or necessary site(s) underpinning chronic tinnitus. Identification of these sites would permit detailed investigation of the synaptic and cellular mechanisms. This in turn should facilitate the development of rationally targeted therapies.
Recent work has identified a novel site that may be involved in modulating or generating chronic noise-induced tinnitus. The paraflocculus (PFL) of the cerebellum (CBL) showed significantly elevated neural activity in rats with evidence of tinnitus, as indicated by manganese-enhanced magnetic resonance imaging (MEMRI) (Brozoski et al., 2007a). Increased uptake Mn++ primarily at synaptic Ca++ channels, while evident in rats with chronic tinnitus, was not evident in normal control rats exposed to an external 90 dB SPL tinnitus-like sound. These observations suggest that the PFL may play a unique role in processing auditory information and the pathology of tinnitus. Although the data are limited, acoustically responsive neurons in the PFL have been identified (Azizi et al., 1990; Azizi et al., 1985; Mortimer, 1975). There is both descending acoustic input to the PFL from SAC, via the pons, and direct ascending input to the FL from the cochlea (Azizi et al., 1985; Eisenman, 1980; Morest et al., 1997; Rasmussen, 1990). The objective of the present study was to further investigate the potential role of the PFL as a tinnitus generator. The effect of permanent surgical ablation and reversible chemical inactivation of the PFL was investigated in a rat model of chronic tinnitus. (434 wds)
2. Materials and Methods
2.1 Subjects
Sixty-nine young adult male Long-Evans rats (Harlan, Indianapolis, IN), 4 months old at study entry, were used in the combined experiments. All animals were maintained at 25°C on a 12/12h reversed light/dark schedule and housed in individual cages. During psychophysical training and testing, the animals were maintained on a food restricted diet with a lower boundary limit of 80 percent of free feeding weight.
2.2 Experimental Design
The experiments were approved by the SIU Laboratory Animal Care and Use Committee and met the ethical standards established by the National Academy of Sciences. A series of three experiments were conducted. In all experiments tinnitus was induced by a single unilateral exposure to high-level band-limited noise, and quantified using a procedure shown to be sensitive to tinnitus (Bauer et al., 2001; Brozoski et al., 2008).
Experiment 1: Tinnitus induction by sound exposure and psychophysical assessment were followed by unilateral ablation of the PFL ipsilateral to the sound exposed ear; after ablation, tinnitus was re-assessed. Experiment 2: PFL ablation ipsilateral to the exposed ear preceded sound exposure by two weeks; tinnitus was then assessed as in Experiment 1. Experiment 3: Tinnitus induction and psychophysical assessment preceded ipsilateral deactivation of the PFL by chronic lidocaine infusion. Tinnitus was re-assessed both during and after PFL deactivation. In all experiments unexposed control rats were run in parallel with exposed rats. Sham surgery involved anesthesia and exposure of the subarcuate fossa (containing the PFL), but a craniotomy and ablation were not performed. Sham noise exposure involved anesthesia and hearing threshold determination using the auditory brainstem evoked response (ABR). Table 1 summarizes the number of subjects allocated to each experiment and each manipulation.
TABLE 1.
Procedural summary for Experiments 1 – 3.
| Experiment & Treatment Group | Sequence of Manipulations | Prediction | n |
|---|---|---|---|
| 1 Unexposed Control | Sham sound exposure, behavioral training and testing, ipsilateral PFL ablation, re-test | No tinnitus; no effect of PFL ablation on psychophysical performance | 4 |
| 1 Exposed | Sound exposure; behavioral training and testing, ipsilateral PFL ablation, re-test | Post-exposure tinnitus reduced by PFL ablation; no general effect of ablation | 7 |
| 2 Sham ablated & Unexposed | Sham ablation, sham sound exposure, behavioral training and testing | No tinnitus | 6 |
| 2 Ablated & Exposed | Ipsilateral PFL ablation, sound exposure, behavioral training and testing | Protection against tinnitus | 10 |
| 2 Sham ablated & Exposed | Sham ablation, sound exposure, behavioral training and testing | Tinnitus | 12 |
| 2 Ablated & Sham Exposed | Ipsilateral PFL ablation, sham sound exposure, behavioral training and testing | No tinnitus and normal psychophysical performance | 6 |
| 3 Unexposed | Sham sound exposure, behavioral training and testing, sham surgery / sham lidocaine | No tinnitus | 8 |
| 3 Exposed & Lidocaine | Sound exposure, behavioral training and testing, testing for 2 weeks with osmotic pump + lidocaine, retesting after pump removal & lidocaine washout | Initial tinnitus, drug-mediated attenuation of tinnitus, post-drug return of tinnitus | 16 |
2.2.1 Traumatic acoustic exposure
Unilateral traumatic acoustic exposure and sham exposure was done under ketamine-xylazine anesthesia in Experiments 1 and 3, and under isoflurane inhalation anesthesia in Experiment 2. The rats were placed in a modified stereotaxic head frame with a speaker driver (FT17H, Fostex, Tokyo, Japan) attached to a metal speculum placed at the entrance of the right ear canal. They were unilaterally exposed once for 1 hr to band-limited noise, with a peak level of 116 - 120 dB (SPL) centered at 16 kHz, falling to ambient levels at 8 kHz and 24 kHz. ABR thresholds were obtained before and immediately afterward. Details of the trauma exposure have been previously published (Brozoski et al., 2005; Brozoski et al., 2011).
2.2.2 Tinnitus Assessment
Tinnitus was determined using a psychophysical procedure shown to be sensitive to tinnitus (Bauer et al., 2001; Bauer et al., 1999) and described in detail in open-source publication (Brozoski et al., 2012). Briefly, an operant conditioned-suppression procedure was used to determine the animal's perception of test tones and silent periods embedded in an ambient of low level (60 dB, SPL) broad-band noise (BBN). The most important feature of the procedure is the required discrimination between the presence and absence of sound, when tested with a variety of sounds varying in composition, frequency, and level. By definition, tinnitus cannot sound like silence. The animals were tested daily in commercial operant test chambers (Lafayette Instruments, Mod. 80001, Lafayette, IN, USA) equipped with lid-mounted speakers. Speaker-off periods (i.e., silence) acquired a special significance because lever pressing during the silent periods led to a foot shock at the conclusion of the period. The behavior of interest was lever pressing during randomly presented test sounds (1 min duration) that substituted for some of the speaker-off presentations (also 1 min duration). Assessment sessions consisted of ten randomly inserted, non-contiguous, presentations. Two of the ten were always silent (i.e., speaker off) periods. The remaining 8 presentations were of a randomly-selected tone or noise, with different levels in each presentation. Lever pressing was quantified using a relative rate measure, the suppression ratio (R). R was determined as a running measure for successive 1-min segments of each session using the formula R = B/(A+B), where A was the number of lever presses in the preceding 1-min segment and B the number of lever presses in the current 1-min segment. R can vary between 0 and 1. A value of 0 is attained when lever pressing in the current minute is 0, a value of 0.5 when lever pressing in the current minute is equal to that of the previous minute, and a value of 1 when lever pressing in the previous minute is zero. R provided a running index of behavior, in 1-min segments, and enabled a quantitative comparison between subjects as well as unbiased compilation of group data. R is a useful index of perceptual performance in that it is very sensitive to short-term behavioral effects, such as those produced by sensory events, but it is very insensitive to gradual behavioral effects, such as those produced by changes in motivational status, for example, satiation.
Exposed and unexposed rats were treated identically and tested in parallel. Individual animal and group discrimination functions were derived from the final 3 to 5 sessions of each test series, where performance variance was minimal. Stimuli were tested across a range of levels from low to high audibility. Evidence of tinnitus was determined by the divergence of group discrimination functions. For animals with tinnitus, test stimuli that resembled their tinnitus served as a signal for response (lever press) suppression. In contrast, for unexposed animals without tinnitus, the signal for suppression was silence. Test stimuli with sensory features resembling tinnitus therefore produced greater suppression (i.e., fewer lever presses) in animals with tinnitus, although individuals with combined tinnitus and hyperacusis can display a significant function upshift. Previous research (Bauer et al., 2001) has shown that Long–Evans adult rats, unilaterally exposed to high level band-limited noise centered at 16 kHz, show evidence of tinnitus in a range between 10 and 30 kHz.
2.2.3 PFL Ablation (Experiments 1 and 2)
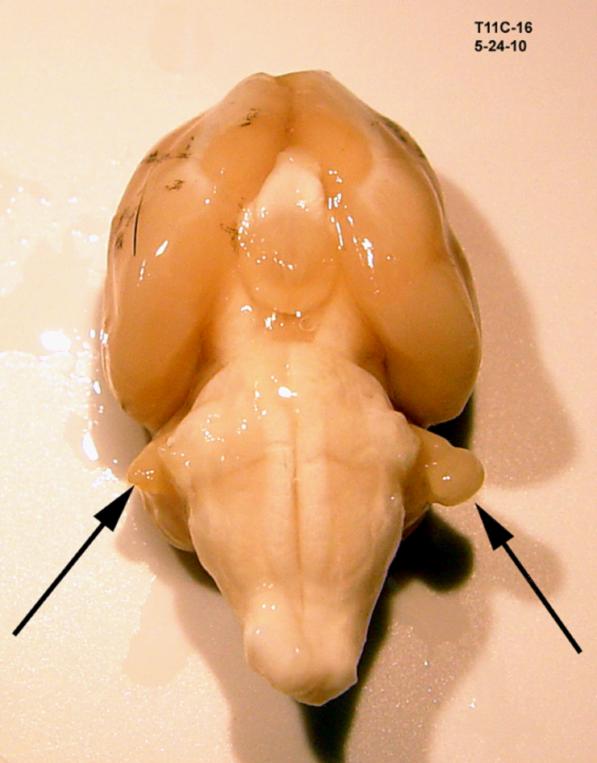
Rats were anesthetized to a surgical level using either an isoflurane/O2 mixture (Aerrane, Baxter Healthcare Corp., Deerfield, IL, USA), or intramuscular ketamine/xylazine mixture (24.6 and 3 mg/kg, respectively; ketamine, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA; xylazine, Anased, Lloyd Laboratories, Shenandoah, IA), and placed in a modified stereotaxic head frame. The dorsum of the skull and cervical region was shaved and sterilely prepared with betadine solution. The procedure was done using a surgical microscope. A 1 cm incision over the caudal scalp exposed the occipital bone; the temporalis and occipital muscles were reflected off the temporal ridge. A 2.3 mm craniotomy was made using a diamond bur. To gain access to the PFL, the subarcuate fossa was opened without violating the vestibular labyrinth, and the PFL was identified and ablated using thermal cautery. Ablation was limited to the cerebellar tissue within the subarcuate fossa and did not involve the cochlear nucleus. Typically a stalk of the PFL would remain after ablation (Fig.1). Sterile Gelfoam (Pfizer, New York) was placed into the fossa and the craniotomy closed with bone wax. The skin was closed with wound clips and the rat was allowed to recover for a minimum of 48 hrs before behavioral testing resumed.
Figure 1.
A typical unilateral PFL ablation. The remnant of the right PFL stalk is evident in this ventral view of an excised brain, 3 months after thermal ablation of the PFL.
2.2.4 Lidocaine infusion into subarcuate fossa (Experiment 3)
Rats were anesthetized with isoflurane, as described above, and placed in a modified stereotaxic head frame. The right subarcuate fossa was exposed as described in Section 2.2.2. Small gauge polyethylene tubing was attached to an osmotic infusion pump (Alzet 2002, Durect Corp., Cupertino, CA) and the tip gently inserted into the fossa. The catheter tip was stabilized in position using bone wax. The catheter tubing was secured to the posterior face of the temporal fossa using fiberglass mesh and cyanoacrylate. The osmotic pump, filled with 4% lidocaine, was placed in a subcutaneous pocket beneath the skin overlying the right scapula. The skin was closed with wound clips and animals allowed to recover in their home cage for 48 hrs before behavioral testing resumed. The pumps delivered the lidocaine solution at 0.5 μl/hr for 14 days. The psychophysical test series was completed in 12 days. At the conclusion of the drug test period, catheter placement was confirmed, the pumps removed, and post-drug behavioral testing was resumed after 7 days.
2.2.5 MEMRI
Twenty hours prior to imaging animals were pre-treated with a subcutaneous injection of 80 mg/kg MnCl2 dissolved in sterile saline. The method was similar to that shown to be sensitive to tinnitus-associated increases in auditory pathway activity (Brozoski et al., 2007a; Holt et al., 2010). The animals, in their home cage, were then held in a double-wall sound insulated booth without food but with freely available water. The objective was to equate and minimize the baseline acoustic environment for all subjects. The sound floor in this environment across the audible frequency range for rats was less than 10 dB (SPL). Immediately before imaging, animals were given a lethal dose of anesthetic (Euthasol, Virbac, Ft. Worth, TX), decapitated, the mandible removed, and excess muscle tissue dissected away from the skull. The head was placed in a polyethylene holder along with a 1 mm diameter glass capillary filled with CuSO4 (3 mM). The image phantom of the capillary indexed the left hemisphere, making laterality unambiguous. Images were obtained using a vertical bore Varian Unity/Inova 600 mHz NMR with a 14.1 T magnet. Contiguous transverse (i.e., coronal) slices, 0.5 mm thick (26 μm planar resolution), were obtained, extending 13 mm caudally from Bregma (26 slices total). The planar scans were exported as TIFF images and imported into Image J (ver. 1.44p, http://imagej.nih.gov/ij). for analysis. The intensities of areas of interest were quantified on an 8 bit scale with respect to the nearest muscle mass. Muscle levels served as the reference for each image, thereby equilibrating field brightness between scans. Each brain was imaged twice, with a 180° rotation between image sets. This further compensated for field anisotropy. The results from each scan set, per animal, were averaged for each area of interest. The areas of interest quantified were: the DCN, anterior ventral cochlear nucleus, posterior ventral cochlear nucleus, the cerebellar paraflocculus, the inferior colliculus, the medial geniculate body, the primary auditory cortex, and the amygdala.
2.2.6 Auditory threshold testing
ABR measurements were obtained using an IHS Smart EP System (Intelligent Hearing Systems, Miami, FL) running IHS High-Frequency Software (v. 2.33) and equipped with IHS High-Frequency transducers (HFT9911-20-0035). Hearing thresholds, obtained under ketamine-xylazine anesthesia, were defined by the lowest stimulus level to evoke a visually distinct waveform in a 10 msec. window following stimulus onset. Details of the ABR procedure and calibration have been described elsewhere (Brozoski et al., 2005; Brozoski et al., 2011).
2.2.7 Statistical analyses
The psychophysical results were analyzed using mixed ANOVAs, with treatment groups (e.g., exposed vs. unexposed) as the independent group comparison, and stimulus level as the repeated-measures comparison. Only stimulus levels above speaker off were included in the analysis. Different test conditions, e.g., 10 kHz tones, 20 kHz tones, were analyzed as independent experiments. When treatment group size was unequal, uncorrected independent t tests were used to compare the performance of independent treatment groups. MEMRI image data were analyzed using mixed ANOVAs, with treatment groups as the independent group comparison and ipsilateral/contralateral as the repeated-measures comparison. Different brain regions were analyzed independently.
3 Results
3.1 General behavioral effects of ablation and lidocaine
General psychophysical performance, as indicated by tests with control stimuli such as broad-band noise, was not affected by either unilateral ablation of the PFL or infusion of lidocaine into the subarcuate fossa. Vestibular symptoms were observed in two of four unexposed subjects and two of seven exposed subjects in Experiment 1, immediately after PFL ablation. Animals exhibited a head tilt towards the operated ear. The symptom resolved within 3 days and did not affect subsequent performance. Lidocaine infusion did not produce vestibular symptoms at any time.
3.2 Effect of PFL ablation on established tinnitus (Experiment 1)
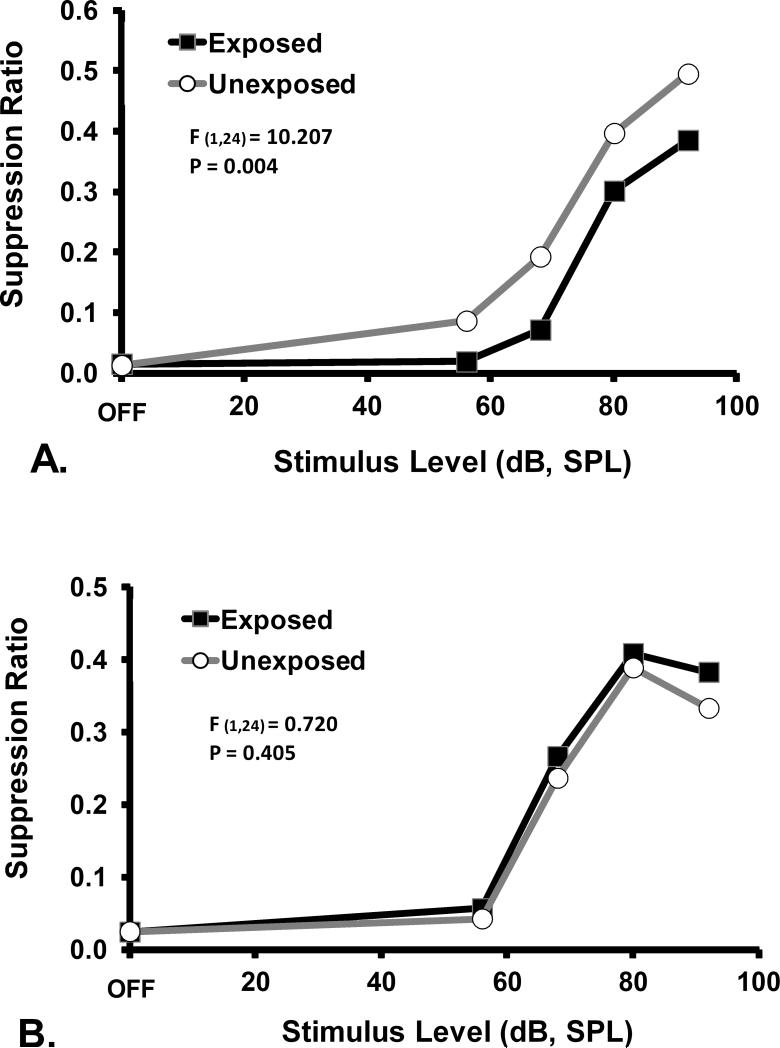
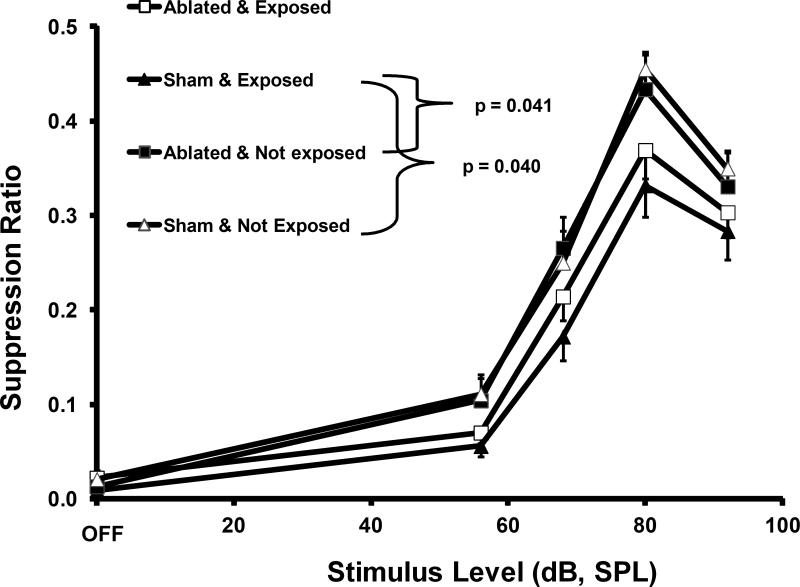
PFL ablation effectively eliminated established tinnitus. Prior to ablation, noise-induced tinnitus was evident as a down-shift in the 16 kHz and 20 kHz psychophysical functions of exposed subjects relative to controls (F1,24 = 10.207; p=0.004; Fig. 2A). After PFL ablation, there was complete elimination of the down-shift. Psychophysical performance in response to 16 kHz test stimuli was identical for exposed and unexposed subjects (F1,24 = 0.72; p=0.405; Fig. 2B).
Figure 2.
Psychophysical functions for the tinnitus diagnostic stimulus of 16 kHz tones, prior to (A) and after (B) ablation of the PFL ipsilateral to noise exposure. Discrimination performance, as indicated by the suppression ratio (R) is plotted on the y axis. The significant downshift of the exposed group, prior to ablation, is indicative of tinnitus. The statistical summary in each panel shows the difference between groups at stimulus levels above OFF. Ablation abolished the indication of tinnitus.
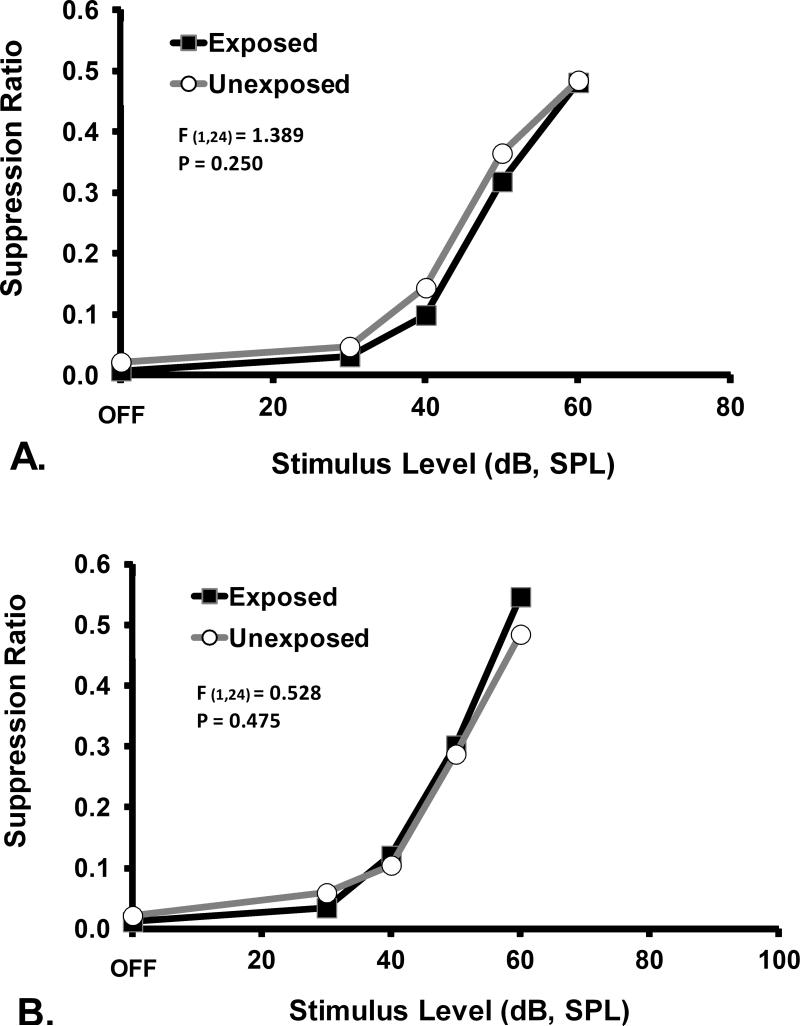
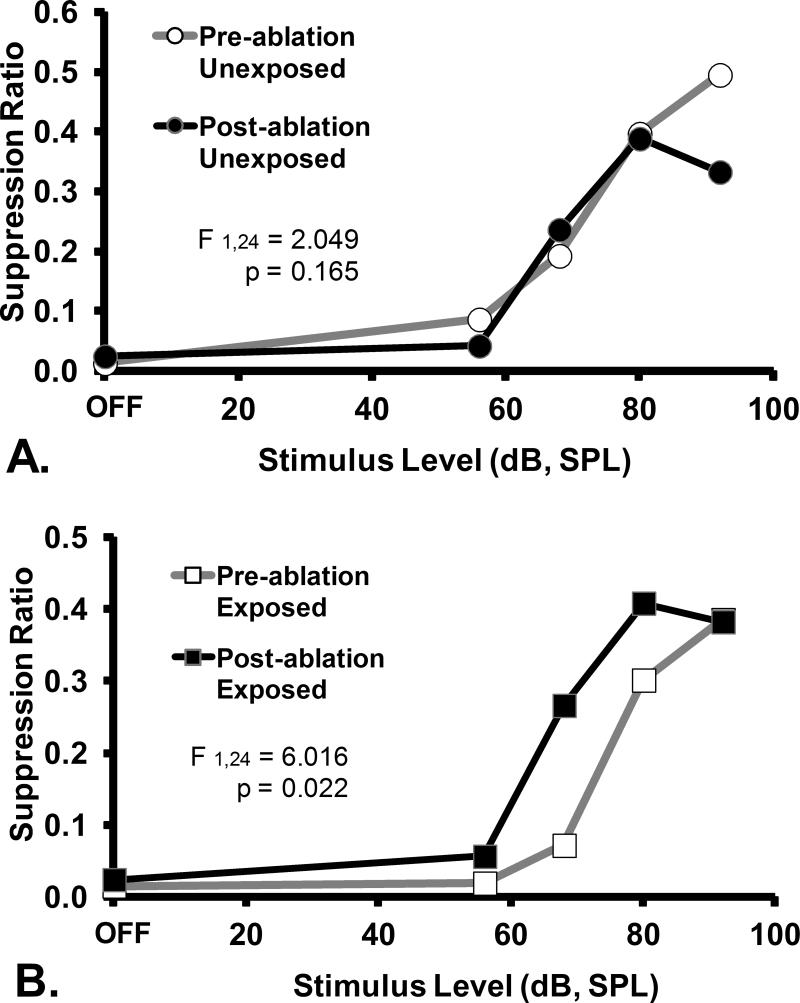
The therapeutic effect of PFL ablation was frequency specific and evident only in the exposed rats. Ablation had no psychophysical effect on control-stimulus performance (Fig. 3). Within-group comparisons further elucidated the ablation effect. Ablation had no effect on unexposed rats, as shown by tonal (16 kHz, Fig. 4A) or noise (Fig. 5A) test performance. In contrast, a frequency-specific post-ablation therapeutic effect was clearly seen for exposed rats (16 kHz, Fig. 4B), although the ablation had no effect on their control-stimulus performance (noise, Fig. 5B).
Figure 3.
Psychophysical functions for the non-diagnostic stimulus of broad-band noise, prior to (A) and after (B) ablation of PFL ipsilateral to noise exposure. Subjects and statistical tests as in Fig. 2. Ablation had no significant general effect on psychophysical performance.
Figure 4.
A within group comparison of the effects of ipsilateral PFL ablation on the diagnostic stimulus (16 kHz tones) performance of A. unexposed and B. exposed rats. Data depicted in Fig. 2 have been re-plotted to show that there was no ablation effect on the unexposed animals (A), but significant effect on the exposed animals (B). Statistics and axes as in preceding figures.
Figure 5.
A within group comparison of the effects of ipsilateral PFL ablation on the non-diagnostic stimulus (broad-band noise) performance of A. unexposed and B. exposed rats. Data depicted in Fig. 3 have been re-plotted to show that there was no ablation effect on the general psychophysical performance of either group. Statistics and axes as in preceding figures.
3.3 Effect of PFL ablation prior to noise-exposure and tinnitus induction (Experiment 2)
Although ipsilateral PFL ablation eliminated established tinnitus, the question remained as to whether the PFL was obligatory for tinnitus. To answer this question rats had the ipsilateral PFL ablated before sound exposure. As summarized in Table 1, positive controls had sham ablation before exposure, while negative controls had either sham ablation or ipsilateral ablation, but were unexposed. If the ipsilateral PFL is an obligatory site for chronic tinnitus, then ablation prior to exposure should greatly reduce or eliminate tinnitus. Contrary to this hypothesis, the ablated and exposed group developed tinnitus, although not dramatically so. This is shown in the 16 kHz psychophysical functions (Fig. 6) where the ablated-before-exposure group (open square points) was not statistically different from animals that developed tinnitus, i.e., the sham-ablated-exposed rats (solid triangular points) with tinnitus (p = 0.380). However the results for the ablated-before-exposure group were somewhat ambiguous, since they were not statistically distinct from either of the negative control groups that did not have tinnitus (unexposed ablated, p = 0.201, solid square points; unexposed sham ablated, open triangular points, p = 0.083). Therefore these intermediate results indicate that while the unilateral PFL does not appear to be obligatory for the development of acoustic-trauma-induced tinnitus, it nevertheless contributes to the magnitude of the effect.
Figure 6.
The effect of ipsilateral PFL ablation prior to high-level noise exposure. Psychophysical performance for the diagnostic stimulus of 16 kHz tones. Intact exposed animals showed the function downshift typical of animals with tinnitus (sham exposed, solid triangular data points). Unexposed animals, whether or not ablated, did not show evidence of tinnitus. Ipsilateral ablation did not completely protect animals exposed to high-level noise after the ablation (ablated exposed, open square data points). The ablated exposed animals were not significantly different statistically than the other positive or negative control groups. Significance levels were for uncorrected independent t tests. Error bars show the standard error of the mean.
3.3.1 Residual tinnitus in PFL ablated animals
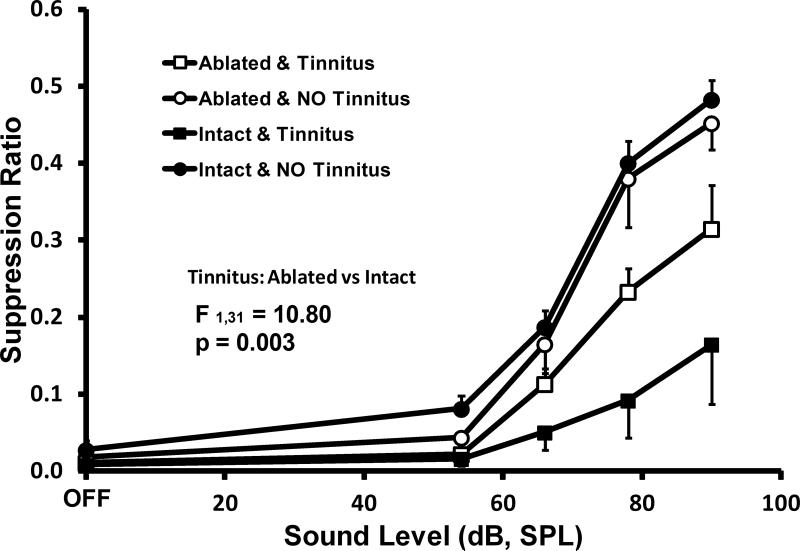
In order to further understand these results, a subset of exposed subjects, individually identified as either having or not having tinnitus, were functionally imaged at the completion of behavioral testing using MEMRI. The objective was to survey areas that may have been responsible for the residual tinnitus in the absence of the PFL. Both ablated and sham ablated rats (all exposed) were imaged in this factorial experiment: 4 ablated with tinnitus, 4 ablated without tinnitus, 4 sham ablated with tinnitus, and 4 sham ablated without tinnitus. The last group was included because not all intact (sham ablated) exposed rats develop tinnitus. The psychophysical data for this subset of exposed subjects showed that ablated rats that developed the maximum tinnitus for their treatment group, still had less tinnitus than intact rats with tinnitus (F1,31 = 10.8, p=0.003; Fig. 7).
Figure 7.
Tinnitus diagnostic performance for 20 kHz test tones of exposed animals selected from Experiment 2. All animals were exposed; 8 ablated and 8 intact (sham ablated). Each of these groups were equally divided two groups of 4 each, that showed either maximum tinnitus (square data points) or minimum tinnitus (circular data points). Not all exposed rats developed significant tinnitus (filled vs solid circular points). Of animals developing tinnitus, intact consistently showed greater evidence (filled square point) than ablated (open square points). At the conclusion of testing, all animals were functionally brain imaged using MEMRI. Error bars show the standard error of the mean. The statistical summary shows the difference between ablated and intact groups at stimulus levels above OFF
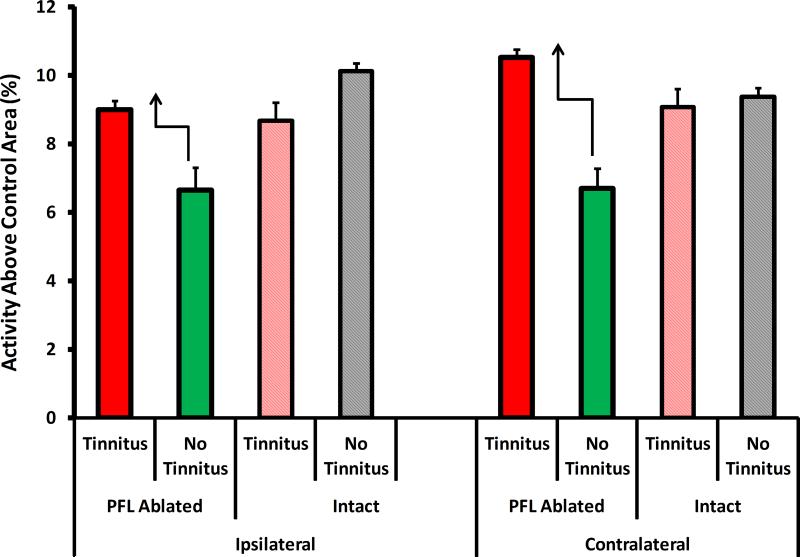
MEMRI indicated that, in general, neural activity was equivalently elevated in the auditory areas (see method for list) of both the ablated and intact animals with tinnitus, compared to those without tinnitus. An exception was the auditory thalamus (Fig. 8). Ablated rats with residual tinnitus showed bilaterally elevated MGB activity, with respect to ablated non-tinnitus controls. This elevation was not seen in intact rats with tinnitus. Furthermore the effect was more pronounced in the direct pathway from the exposed ear, i.e., the contralateral MGB. This suggests that the auditory thalamus may significantly contribute to the tinnitus signal. Alternatively, in ablated animals with tinnitus, the MGB may be more effectively gating a tinnitus signal to the cortex from a distributed network of affected areas that do not include the PFL.
Figure 8.
Summary of the MEMRI imaging results of Experiment 2 animals depicted in Fig. 7. The only consistent activity pattern differences, tinnitus vs. no-tinnitus, that appeared between intact and ablated animals, was evident in the MGB. Ablated rats that developed tinnitus had elevated MGB activity with respect to ablated rats that did not develop tinnitus (dark red vs. dark green bars). In contrast, intact rats that developed tinnitus had the same level of MGB activity as intact rats without tinnitus (light red vs gray bars). The activity pattern of other auditory areas was similar across all four groups (not shown, see text). Error bars shown the mean deviation.
In an attempt to further account for residual tinnitus in PFL ablated animals, a volume analysis of tissue remaining in the subarcuate fossa after ablation was determined using the brain scans. This analysis quantified the thoroughness of ablation. In order to equate brain volumes between animals, an ipsilateral (ablated) / contralateral (intact) ratio was used. There was no statistical difference in the ipsi/contra volume ratios for the two groups (t-test, p=0.496). Therefore residual tinnitus of the ablated animals could not be explained by residual PFL tissue, i.e., thoroughness of ablation.
3.4 Reversible deactivation of the ipsilateral PFL (Experiment 3)
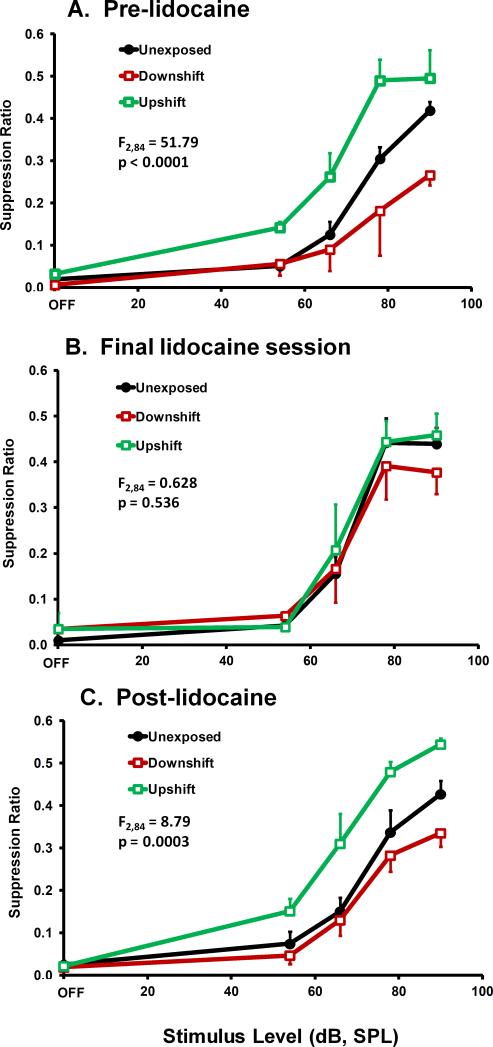
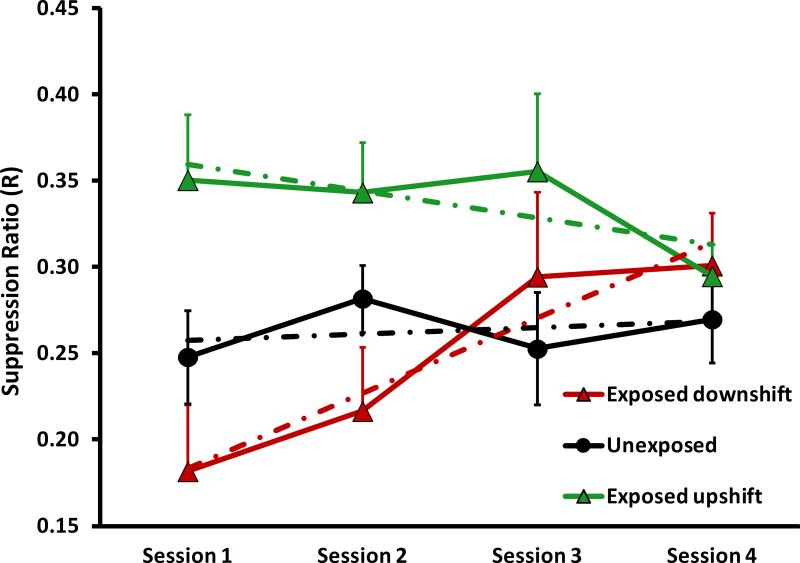
To confirm the ablation results of Experiment 1, rats were tested before, during, and after 14 days of 4% lidocaine infused directly into the ipsilateral subarcuate fossa. The objective was to reversibly deactivate the PFL in animals with chronic tinnitus. Twenty-six rats were noise exposed prior to psychophysical testing; of these, 8 with the best evidence of tinnitus (indicated by a function downshift) and 8 with the best evidence of hyperacusis-and-tinnitus (indicated by a function upshift) were selected for lidocaine treatment (Fig. 9A). Lidocaine was infused at 0.5 μl/hr into the ipsilateral subarcuate fossa using an osmotic pump (see Section 2.2.3); 8 control rats were not noise exposed and were not treated with lidocaine. At the conclusion of the lidocaine infusion, the location and patency of catheters was determined. Four animals in each of the exposed groups had patent and accurately placed catheter tips within the subarcuate fossa. Data from the 8 animals with dislocated catheters were not included in the analysis because it was not known when they dislodged from the fossa.
Figure 9.
Reversible attenuation of tinnitus and hyperacusis (potentially with tinnitus) by chronic infusion of 4% lidocaine into the subarcuate fossa. The diagnostic test stimulus was 20 kHz tones. Group psychophysical functions are shown before infusion (A), at the conclusion of infusion (B) and after a washout period (C). Statistical summary and axes as in previous figures.
Lidocaine treatment of the ipsilateral PFL eliminated the psychophysical evidence of tinnitus and hyperacusis in the exposed rats (Fig. 9B). The therapeutic effect appeared gradually over the 14 day treatment period (Fig. 10), suggesting a progressive deactivation of the PFL by the chronic lidocaine. After a 6 week washout period both the tinnitus and hyperacusis returned, although not quite to pretreatment levels (Fig. 9C). This suggests that some irreversible damage to the PFL may have accompanied the two week lidocaine treatment.
Figure 10.
Mean 20 kHz time series performance across lidocaine treatment sessions, of the groups depicted in Fig. 9. Downshifted data reflected tinnitus, while upshifted data indicated hyperacusis with tinnitus. Error bars show the standard error of the mean. Broken lines show the least-mean-square regression line for each function. As treatment progressed, the psychophysical evidence of both tinnitus and hyperacusis, progressively decreased.
4. Discussion
Chronic tinnitus is most likely a central phenomenon. The sensation persists after complete deafferentation, for example after surgical destruction of the cochlea or transection of the cochleovestibular nerve (Berliner et al., 1992). Tinnitus is most commonly associated with some form of peripheral auditory injury or damage, such as age-related hearing loss or noise trauma (Nondahl et al., 2002). However, the prevalence of tinnitus among the hearing impaired, including occupational hearing loss, ranges between 20 and 58 percent (Alberti, 1987; Griest et al., 1998). This suggests that tinnitus likely involves altered central functions that are distinct from those of hearing loss. A fundamental knowledge gap lies in the details of this central pathology. A working hypotheis is that de-afferentation results in a loss of inhibition within the auditory brainstem. This could result in increased or altered neural activity at more rostral sites, including auditory cortex, with the modified activity perceived as sound (Brozoski et al., 2007b; Eggermont et al., 2004).
Identification of brain structures that generate tinnitus is an important goal. Indeed, developing effective treatments depends on this. Animal models of noise-induced tinnitus have implicated the DCN as a candidate site involved in the pathologic mechanisms that result in tinnitus. Increased spontaneous activity and increased driven activity have been demonstrated in rodents with tinnitus derived from cochlear injury caused by noise exposure (Brozoski et al., 2002; Kaltenbach et al., 2004). However, recent work has shown that the DCN is not an obligatory site for maintaining chronic tinnitus. Bilateral DCN ablation failed to eliminate behavioral evidence of established tonal tinnitus in rats (Brozoski et al., 2005). This suggests that other brain areas participate in generating tinnitus, particularly once it is established as a chronic condition.
Evidence for tinnitus-specific pathology, as opposed to noise-exposure pathology, in structures rostral to the cochlear nuclei has been sparse. Single-unit activity in the inferior colliculus (IC) was evaluated in chinchilla after tinnitus induction using three different forms of cochlear injury (acoustic trauma, round window application of cisplatin or carboplatin). A pattern of abnormal neural activity characterized by high-frequency spiking within bursts and decreased inter-spike-interval variance, was identified in animals with tinnitus (Bauer et al., 2008). Noise-exposed mice with behavioral evidence of tinnitus showed altered tonotopic organization in their auditory cortex (Yang et al., 2011). Systemic salicylate-induced tinnitus in rats has been associated with increased neural activation in inferior colliculus and temporal cortex (Paul et al., 2009; Yang et al., 2007). Although these data are suggestive, the role of rostral areas in generating the tinnitus is uncertain. While the inferior colliculus and cortex may participate in tinnitus perception, they may not necessarily be signal generators. Neural reorganization and abnormal activity in rostral structures could represent reactions to altered neural activity more proximal in the auditory pathway. Direct, and ideally reversible, manipulation of neural activity in each structure, accompanied by tinnitus assessment at each stage, is required to establish the causal links in the cascade of brain events responsible for tinnitus.
Functional brain imaging is a powerful tool for surveying brain activity associated with pathological conditions such as tinnitus (Lanting et al., 2009). Using activity maps as a guide, specific areas can be targeted for direct manipulation and/or therapeutic intervention (Langguth et al., 2010). Manganese (Mn), a paramagnetic element taken up through calcium channels at active synapses, serves as an activity-dependent contrast agent when used in magnetic resonance imaging (MEMRI) (Pautler, 2006; Silva et al., 2004). MEMRI has been shown to be sensitive to stimulus driven activation of central auditory areas in rodents (Yu et al., 2005), and tinnitus-associated increases in collicular activity in rats (Holt et al., 2010). Using MEMRI, the PFL was identified as an area of greatly elevated activity in rats with behavioral evidence of noise-induced tinnitus (Brozoski et al., 2007a). Similar enhancement was not evident in a control cohort that received Mn during a 7 hr exposure to an external sound of ‘artificial tinnitus.’ In addition, rats with chronic tinnitus, but pretreated with vigabatrin, a compound shown to be an effective tinnitus therapeutic in rats (Brozoski et al., 2007b; Yang et al., 2011), also failed to show elevated PFL activity. These results suggest that the cerebellum, and in particular the PFL, may be an important structure in the tinnitus pathway. The primary objective of the present research was to further elucidate the role of the cerebellum in tinnitus. Specifically, could it be a necessary generator site or trigger zone?
4.1 The cerebellum as an auditory structure
The cerebellum has traditionally been viewed as a vestibular-motor engine for coordinated activity (Moruzzi, 1958). Recent work has expanded the functional role of the cerebellum. It is now considered a multimodal analyzer involved in cognition, emotion, learning and sensory processing, including nociception (Ito, 2006; Saab et al., 2003; Schmahmann, 1991; Schmahmann, 1996). Current concepts of cerebellar function emphasize its role in the representation of behavior via internal models (Ito, 2008). The cerebellum is integrated within a large-scale network involving pre-frontal cortex and temporo-parietal cortex. Activation of this network during mental tasks, including auditory, verbal and language tasks, is evident in neuroimaging studies (Fiez et al., 1996; Schlosser et al., 1998). When investigating cerebellar contributions to sensory processing, however, the potential confound of including a motor reaction is not trivial. In a meta-analysis that pooled data from 15 functional imaging studies using auditory stimulation without a motor response, the brain region that was the most active, following primary auditory cortex (in 12 of 15 studies), was the cerebellum (Petacchi et al., 2005).
4.2.1 The cerebellum involved in pathological auditory states
Several functional imaging studies in humans have reported cerebellar involvement in tinnitus. Activation of the cerebellum in association with severely disturbing tinnitus was first noted by Shulman using single photon emission computerized tomography (PET) measuring regional cerebral perfusion. In ten patients with severe tinnitus, significantly increased blood flow was found in the right and left cerebellum compared with non-tinnitus controls (Shulman et al., 1999). Mirz et al. (1999) studied twelve subjects with severely disturbing tinnitus using PET imaging co-registered with MRI. Subjects were imaged while listening to their tinnitus and under conditions of tinnitus suppression using masking sounds and lidocaine infusion. The structure with the largest difference in regional cerebral blood flow between baseline and masking with sound and lidocaine was, again, the cerebellum (Mirz et al., 1999).
Osaki et al. (2005) compared regional cerebral blood flow using PET in subjects with bilateral tinnitus associated with profound hearing loss and normal hearing control subjects without tinnitus. Tinnitus subjects with unilateral cochlear implants were imaged while listening to their tinnitus with the implant turned off and again after 15 min of cochlear implant stimulation and induction of residual inhibition. The control subjects were imaged during monaural listening to 75 dB white noise and again during silence without external sound stimulation. Notably, two tinnitus-related central sites were identified in the cochlear implant subjects. Regional blood flow was significantly increased in the right cerebellum during tinnitus perception. During the period of residual inhibition of the tinnitus there was enhanced blood flow in the right anterior temporal lobe.
4.2.2 Electrophysiologic and anatomic evidence for cerebellar auditory function
Investigations of the anatomy and neurophysiology of cerebellar auditory circuitry are sparse. Rasmussen (1990) demonstrated direct projections to the flocculus (FL) from the cochlea in chinchilla, cat and monkey (Rasmussen, 1990). Morest reported neural degeneration extending from the cochlea into the FL after acoustic trauma in chinchilla, and remarked on the potential significance of this poorly investigated connection (Morest et al., 1997). Azizi et al. (1985) recorded excitation followed by prolonged inhibition in 20 percent of PFL Purkinje neurons in response to electrical excitation of auditory cortex. A significant percentage of non-Purkinje units, presumably small interneurons in the molecular layer, responded to both auditory cortex and inferior tectum stimulation. Notably, frequency dependent changes in complex spike activity in response to free field sound stimulation were recorded in the PFL (Azizi et al., 1985). Although sparse, it would appear that there are cochleocerebellar and corticocerebellar networks involving the PFL and the FL of the cerebellum. Further details of these potentially significant neural connections remain to be investigated.
4.3 Theoretical mechanism for PFL modulation of tinnitus
There are several potential mechanisms by which the cerebellum may be involved in tinnitus. The cerebellum integrates somatosensory input from multiple sources (Sawtell, 2010; Voogd et al., 1998). It is possible that integration capacity is involved in somatic modulation of tinnitus, which may be present in significant proportion of people with tinnitus (Levine et al., 2003; Sanchez et al., 2002). The cerebellum may also perform adaptive signal processing by serving as a comparator of anticipated perception events with received sensory input. A decrease in sensory input may trigger compensatory feed forward excitation that attempts to normalize input to rostral circuits through increased gain. This would be consistent with the cerebellum encoding internal models or neural representations. Damaged, or maladjusted circuitry in the cerebellum could result in inappropriate dynamic modulation of the internal representation of silence. In this scenario, elevated activity in a trigger zone – distinct from the PFL – could elicit an inappropriate compensatory change in a generator site, such as the PFL, resulting in a perceived sound. As noted by Ito, differentiation between a self-produced stimulus and an externally produced stimulus may be based on the successful cancellation of self-produced stimuli by accurate prediction mediated by an internal model (Ito, 2008). Following auditory damage, a cerebellar comparator may be fed conflicting information from a damaged cochlea, with attendant decreased eighth nerve activity (Salvi et al., 1982), combined with enhanced activity in a trigger zone such as the DCN (Brozoski et al., 2002; Kaltenbach et al., 1999). In this scenario, with a mismatch between externally and internally generated sensations, operation of the cerebellar cancellation model could be distorted so that external silent conditions are no longer accurately represented. Alternatively, there may exist a cochlea-cerebellar circuit involving direct ascending sensory input from the cochlea into the PFL and FL, and a recurrent efferent feedback loop to the DCN. Signal modulation at the level of the DCN would then be transmitted to the auditory cortex and perceived as a sensation.
Ablation of the PFL was successful in eliminating chronically established tinnitus but less successful in preventing the de novo onset of tinnitus. This suggests that the PFL may be an important, but non-obligatory tinnitus generator. However the reversible therapeutic effect of lidocaine infusion into the subarcuate fossa, strengthens the conclusion that once established as a tinnitus generator, the PFL becomes a necessary component of the central tinnitus network. Ablation studies are useful as first attempts in establishing the role of a particular site in a process. Consistency of lesion volume and elimination of collateral damage are challenging, and these considerations temper conclusions drawn from such studies. The observed incomplete protection from noise-induced tinnitus by PFL ablation may be related less to the ablation volume than the number and type of cells that remain.
A unique cell type located in the PFL, FL, and DCN, may play an important role in the pathology of tinnitus (Mugnaini et al., 1994). The unipolar brush cell (UBC) is an excitatory glutamatergic interneuron that receives direct mossy fiber input in the cerebellum and synapses onto granule cells and Purkinje cells (Dino et al., 2000). This cellular network is capable of feed-forward amplification of a neural signal (Mugnaini et al., 2011). Anatomically, the largest density of UBCs appear in the transition zone between the PFL and the FL, as well as in the dorsomedial DCN. Unfortunately, it is unknown if the auditory inputs from cochlea and SAC terminate within input fields that would impact the UBCs, although this would be a very intriguing direction for future study. The anatomic and physiologic data on the auditory input to the PFL and FL are also very sparse, with most information suggesting the auditory inputs involve the ventral PFL. Interestingly, in addition to the DCN, the UBCs are most heavily concentrated in the FL and the ventral PFL (Manohar et al., 2012).
The PFL and FL may function as a sensory comparator that serves as a gain control circuit. It receives direct input from the cochlea via the auditory nerve and descending input from the auditory cortex (Morest et al., 1997; Rasmussen, 1990). De-afferentation would result in decreased direct auditory input to the PFL and FL. UBC's provide a mechanism for highly amplified feed-forward excitation. This might comprise a source of aberrant information that is interpreted by auditory cortex as sound.
5.1 Conclusions
The functional significance of connections between the PFL, FL, the cochlea and more rostral auditory structures are not well understood. However, neurophysiological data, human imaging data, and anatomical evidence linking the cochlea and the PFL and FL are tantalizing. The potential role of the cerebellum in chronic tinnitus should be further examined.
Highlights: The Cerebellum as a Novel Tinnitus Generator.
The paraflocculus of the cerebellum may be a sufficient, but non-obligatory, generator of acoustic-trauma-induced tinnitus.
Parafloccular ablation eliminated pre-existing psychophysically indicated tinnitus in rats.
Inactivation of the paraflocculus, with lidocaine, reversibly eliminated pre-existing tinnitus and hyperacusis in rats.
Pre-emptive parafloccular ablation blunted, but did not prevent the onset of acoustic-trauma-induced tinnitus in rats.
Acknowledgement
Supported by the National Institute on Deafness and Other Communication Disorders, # 1R01DC009669-01.
Abbreviations
- ABR
auditory brainstem response
- AC
auditory cortex
- CBL
cerebellum
- DCN
dorsal cochlear nucleus
- FL
flocculus
- IC
inferior colliculus
- MEMRI
manganese enhanced magnetic resonance imaging
- MGB
medial geniculate body
- NA
nucleus accumbens
- PFL
paraflocculus
- SAC
secondary auditory cortex
- SPL
sound pressure level
- UBC
unipolar brush cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alberti PW. Tinnitus in occupational hearing loss: nosological aspects. J Otolaryngol. 1987;16:34–5. [PubMed] [Google Scholar]
- Azizi SA, Woodward DJ. Interactions of visual and auditory mossy fiber inputs in the paraflocculus of the rat: a gating action of multimodal inputs. Brain Res. 1990;533:255–62. doi: 10.1016/0006-8993(90)91347-j. [DOI] [PubMed] [Google Scholar]
- Azizi SA, Burne RA, Woodward DJ. The auditory corticopontocerebellar projection in the rat: inputs to the paraflocculus and midvermis. An anatomical and physiological study. Exp Brain Res. 1985;59:36–49. doi: 10.1007/BF00237663. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J of the Assoc for Res in Otolaryngol. 2001;2:54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg. 1999;121:457–62. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–78. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner KI, Shelton C, Hitselberger WE, Luxford WM. Acoustic tumors: effect of surgical removal on tinnitus. Am J Otol. 1992;13:13–7. [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206:227–36. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. Learning about tinnitus from an animal model. Seminars in Hearing. 2008;29:242–258. [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–90. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI). Hear Res. 2007a;228:168–79. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Spires TJ, Bauer CA. Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol. 2007b;8:105–18. doi: 10.1007/s10162-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Wisner KW, Sybert LT, Bauer CA. Bilateral Dorsal Cochlear Nucleus Lesions Prevent Acoustic-Trauma Induced Tinnitus in an Animal Model. J Assoc Res Otolaryngol. 2011 doi: 10.1007/s10162-011-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Wisner KW, Sybert LT, Bauer CA. Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J Assoc Res Otolaryngol. 2012;13:55–66. doi: 10.1007/s10162-011-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dino MR, Schuerger RJ, Liu Y, Slater NT, Mugnaini E. Unipolar brush cell: a potential feedforward excitatory interneuron of the cerebellum. Neuroscience. 2000;98:625–36. doi: 10.1016/s0306-4522(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Kenmochi M. Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex. Hear Res. 1998;117:149–60. doi: 10.1016/s0378-5955(98)00008-2. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Eisenman LM. Pontocerebellar projections to the paraflocculus in the rat. Brain Res. 1980;188:550–4. doi: 10.1016/0006-8993(80)90053-0. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME, Balota DA, Tallal P, Petersen SE. PET activation of posterior temporal regions during auditory word presentation and verb generation. Cereb Cortex. 1996;6:1–10. doi: 10.1093/cercor/6.1.1. [DOI] [PubMed] [Google Scholar]
- Griest SE, Bishop PM. Tinnitus as an early indicator of permanent hearing loss. A 15 year longitudinal study of noise exposed workers. AAOHN J. 1998;46:325–9. [PubMed] [Google Scholar]
- Holt AG, Bissig D, Mirza N, Rajah G, Berkowitz B. Evidence of key tinnitus-related brain regions documented by a unique combination of manganese-enhanced MRI and acoustic startle reflex testing. PLoS One. 2010;5:e14260. doi: 10.1371/journal.pone.0014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–13. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Heffner HE. Spontaneous activity in the dorsal cochlear nucleus of hamsters tested behaviorally for tinnitus. (Abstract).. Association for Research in Otolaryngology Midwinter Research Meeting; St. Petersburg Beach, FL. 1999. [Google Scholar]
- Kaltenbach JA, Godfrey DA. Dorsal cochlear nucleus hyperactivity and tinnitus: are they related? Am J Audiol. 2008;17:S148–61. doi: 10.1044/1059-0889(2008/08-0004). [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355:121–5. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Langguth B, Kleinjung T, Landgrebe M, de Ridder D, Hajak G. rTMS for the treatment of tinnitus: the role of neuronavigation for coil positioning. Neurophysiol Clin. 2010;40:45–58. doi: 10.1016/j.neucli.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153:643–8. doi: 10.1007/s00221-003-1747-3. [DOI] [PubMed] [Google Scholar]
- Manohar S, Paolone NA, Bleichfeld M, Hayes SH, Salvi RJ, Baizer JS. Expression of doublecortin, a neuronal migration protein, in unipolar brush cells of the vestibulocerebellum and dorsal cochlear nucleus of the adult rat. Neuroscience. 2012;202:169–83. doi: 10.1016/j.neuroscience.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirz F, Pedersen B, Ishizu K, Johannsen P, Ovesen T, Stodkilde-Jorgensen H, Gjedde A. Positron emission tomography of cortical centers of tinnitus. Hear Res. 1999;134:133–44. doi: 10.1016/s0378-5955(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Morest DK, Kim J, Bohne BA. Neuronal and transneuronal degeneration of auditory axons in the brainstem after cochlear lesions in the chinchilla: cochleotopic and non-cochleotopic patterns. Hear Res. 1997;103:151–68. doi: 10.1016/s0378-5955(96)00172-4. [DOI] [PubMed] [Google Scholar]
- Mortimer JA. Cerebellar responses to teleceptive stimuli in alert monkeys. Brain Res. 1975;83:369–90. doi: 10.1016/0006-8993(75)90831-8. [DOI] [PubMed] [Google Scholar]
- Moruzzi R.D.a.G. The physiology and pathology of the cerebellum. University of Minnesota Press; Minneapolis: 1958. [Google Scholar]
- Mugnaini E, Floris A. The unipolar brush cell: a neglected neuron of the mammalian cerebellar cortex. J Comp Neurol. 1994;339:174–80. doi: 10.1002/cne.903390203. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Sekerkova G, Martina M. The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev. 2011;66:220–45. doi: 10.1016/j.brainresrev.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BE, Tweed TS. Prevalence and 5-year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J Am Acad Audiol. 2002;13:323–31. [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183:137–53. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Paul AK, Lobarinas E, Simmons R, Wack D, Luisi JC, Spernyak J, Mazurchuk R, Abdel-Nabi H, Salvi R. Metabolic imaging of rat brain during pharmacologically-induced tinnitus. Neuroimage. 2009;44:312–8. doi: 10.1016/j.neuroimage.2008.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautler RG. Biological applications of manganese-enhanced magnetic resonance imaging. Methods Mol Med. 2006;124:365–86. doi: 10.1385/1-59745-010-3:365. [DOI] [PubMed] [Google Scholar]
- Petacchi A, Laird AR, Fox PT, Bower JM. Cerebellum and auditory function: an ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp. 2005;25:118–28. doi: 10.1002/hbm.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen G. Remarks on the cochleo-cerebellar connections. Research Notebooks, History of Medicine Division, National Library of Medicine; Washington D.C.: 1990. pp. 1–42. [Google Scholar]
- Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–26. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab CY, Willis WD. The cerebellum: organization, functions and its role in nociception. Brain Res Brain Res Rev. 2003;42:85–95. doi: 10.1016/s0165-0173(03)00151-6. [DOI] [PubMed] [Google Scholar]
- Salvi R, Perry J, Hamerink RP, Henderson D. Relationships between cochlear pathologies and auditory nerve and behavioral responses following acoustic trauma. In: Hamerink RP, Henderson D, Salvi R, editors. New Perspective on Noise-Induced Hearing Loss. Raven Press; New York: 1982. pp. 165–188. [Google Scholar]
- Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear Res. 1990;50:245–57. doi: 10.1016/0378-5955(90)90049-u. [DOI] [PubMed] [Google Scholar]
- Sanchez TG, Guerra GC, Lorenzi MC, Brandao AL, Bento RF. The influence of voluntary muscle contractions upon the onset and modulation of tinnitus. Audiol Neurootol. 2002;7:370–5. doi: 10.1159/000066155. [DOI] [PubMed] [Google Scholar]
- Sawtell NB. Multimodal integration in granule cells as a basis for associative plasticity and sensory prediction in a cerebellum-like circuit. Neuron. 2010;66:573–84. doi: 10.1016/j.neuron.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998;64:492–8. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–87. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–98. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Shulman A, Strashun A. Descending auditory system/cerebellum/tinnitus. Int Tinnitus J. 1999;5:92–106. [PubMed] [Google Scholar]
- Silva AC, Lee JH, Aoki I, Koretsky AP. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR Biomed. 2004;17:532–43. doi: 10.1002/nbm.945. [DOI] [PubMed] [Google Scholar]
- Tan J, Ruttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Kopschall I, Rohbock K, Knipper M. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007;145:715–26. doi: 10.1016/j.neuroscience.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21:370–5. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–53. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A. 2011;108:14974–9. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wadghiri YZ, Sanes DH, Turnbull DH. In vivo auditory brain mapping in mice with Mn-enhanced MRI. Nat Neurosci. 2005;8:961–8. doi: 10.1038/nn1477. [DOI] [PMC free article] [PubMed] [Google Scholar]