Abstract
Background
Extensively drug-resistant (XDR) tuberculosis has spread among hospitalized patients in South Africa, but the epidemic-level impact of hospital-based infection control strategies remains unknown.
Methods
We investigated the effect of administrative, environmental, and personal infection control measures on the epidemic trajectory of XDR tuberculosis in a rural South African community. Assessments were performed with a mathematical model, which incorporated inpatient airborne tuberculosis transmission and community tuberculosis and HIV transmission.
Results
If no new interventions are introduced, 1,300 cases of XDR tuberculosis are predicted to occur in the area of Tugela Ferry by the end of 2012. Over half of these cases are likely to be nosocomially transmitted. Mask use would avert less than 10% of overall cases, due to long inpatient exposure times and real-world face-seal leakage rates, but could reduce a significant proportion of hospital staff XDR tuberculosis cases. Combining mask use with reduced hospitalization time and a shift to outpatient therapy, however, could prevent nearly one-third of XDR tuberculosis cases. Supplementing this approach with improved ventilation, rapid drug resistance testing, HIV treatment, and tuberculosis isolation facilities could avert 48% of XDR tuberculosis cases (range 34-50%) by the end of 2012. Involuntary detention, however, could result in an unexpected rise in incidence, given limited isolation capacity.
Conclusions
In the face of rising XDR tuberculosis incidence, prevalence and burden on the health care system, a synergistic combination of available nosocomial infection control strategies may prevent nearly half of XDR tuberculosis cases, even in a resource-limited setting. XDR tuberculosis transmission will continue in the community in spite of such efforts, however, indicating the need to develop parallel community-based programs.
Keywords: Tuberculosis, Drug-Resistant; Tuberculosis, Multidrug-Resistant; Tuberculosis, Extensively Drug-Resistant; Tuberculosis, MDR; Tuberculosis, XDR; Mathematical Model; Nosocomial Infections; Hospital Infections; South Africa; Infection Control
Introduction
Multidrug-resistant (MDR) tuberculosis—tuberculosis resistant to at least isoniazid and rifampin—is an increasingly important public health and clinical problem, particularly in countries with a high burden of HIV.1 Recent reports of MDR tuberculosis isolates resistant to second-line drugs have amplified these concerns. In March 2006, the first data were published on the worldwide occurrence of tuberculosis with resistance to second-line drugs, termed “extensively” drug-resistant (XDR) tuberculosis.2 Of 17,690 isolates, 20% were MDR and 2% were XDR tuberculosis. XDR tuberculosis has since been redefined as resistance to isoniazid, rifampin, any fluoroquinolone, and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin).3 Thirty-seven countries have reported XDR tuberculosis cases as of May 2007,4 and isolates have been identified from all regions of the world.5
South Africa has been a focus of attention regarding XDR tuberculosis, as the largest cluster of cases to date has been reported there.6 XDR tuberculosis has been diagnosed in every province of the country,7 with over 200 cases reported from 40 independent sites in the KwaZulu-Natal province alone as of November 2006.8 The first 53 cases were reported from the rural town of Tugela Ferry, where infected patients had a median survival time of only 16 days from sputum collection, and experienced a 98% mortality rate.6 All of those tested were HIV-positive. The majority of XDR tuberculosis patients had not been previously treated for tuberculosis, and none had been exposed to the second-line drugs to which they were resistant. However, 67% had been recently hospitalized, and two of the patients were healthcare workers, suggesting that nosocomial transmission of XDR tuberculosis may be a driver of this epidemic.
Nosocomial MDR tuberculosis outbreaks have occurred in both poor and wealthy nations, typically among HIV-infected patients.9-12 Transmission has been reduced in industrialized countries through a combination of infection control strategies, including comprehensive treatment protocols and staff training programs (administrative measures); ventilation, isolation, air filtration, and ultraviolet germicidal irradiation (environmental controls); and the use of respiratory masks (personal protection).12-14 Similar strategies have been proposed to reduce nosocomial transmission of tuberculosis in low-income settings,14-16 but the optimal implementation of such measures in the context of resource-constrained facilities with crowded multi-person wards, little or no isolation capacity, and limited budgets for technologic interventions is unknown. Thus, little data have been available to guide policymakers, clinicians and administrators in selecting interventions to address the potential nosocomial transmission of XDR tuberculosis.
We estimated the epidemic trajectory of XDR tuberculosis in a rural South African setting, and examined the likely proportion of cases due to nosocomial transmission. We then modeled the impact of various hospital-based infection control strategies on the future burden of XDR tuberculosis.
Methods
We constructed a mathematical model to simulate tuberculosis transmission in a high HIV prevalence, rural area of South Africa. Data for the construction, calibration and validation of the model were derived from Tugela Ferry, KwaZulu-Natal and the Church of Scotland Hospital, which serves a community of ~150,000 people of the Msinga district. Similar to other rural district hospitals in South Africa, this hospital has congregate 30-40 bed tuberculosis wards. Forty percent of hospital beds are occupied by HIV-infected patients and 25% of women presenting for antenatal care are HIV-infected.6 An outpatient tuberculosis treatment program, utilizing community-based directly observed therapy, has existed since 1993 and an HIV antiretroviral therapy (ARV) program since 2004.17
Model
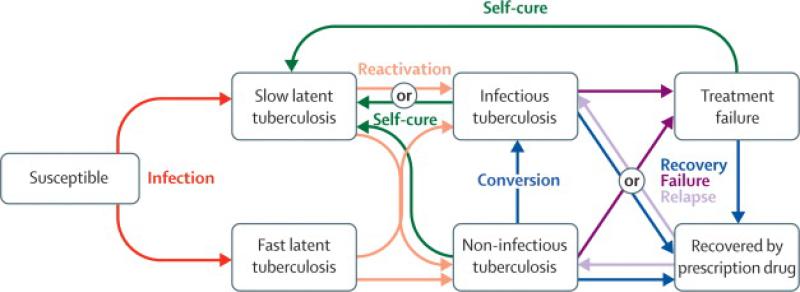
The model describes two environments: the hospital's tuberculosis ward and its catchment community population. Individuals in either of the two environments experience different risks of tuberculosis transmission. An airborne tuberculosis transmission model—incorporating the rate of ventilation, duration of exposure, and risk of inhaling infectious particles—simulates the risk of infection on the hospital ward. The rate of community-based transmission is proportional to the prevalence of tuberculosis in the community. The model uses a “compartmental” approach--describing the transitions made by infected persons as they move between disease states and environments (Figure 1). The model equations are detailed in the Appendix, which also provides details of the model calibration and validation process.
Figure 1. Model of tuberculosis pathogenesis.
Mortality is not depicted, but occurs from all compartments. HIV status and receipt of antiretroviral therapy further modify the pathogenesis of tuberculosis; transitions between tuberculosis strains due to amplified and acquired resistance or exogenous re-infection also occur.
The model includes individuals who are HIV-infected, including those receiving ARV therapy, accounting for the impact of these factors on tuberculosis pathogenesis. Drug-susceptible, MDR and XDR tuberculosis strains are modeled, including acquired and amplified resistance during therapy, the transmission of resistant strains, self-cure, strain fitness, and exogenous re-infection.
Local parameter values were obtained from a case control study of 170 patients, including 57 non-MDR, 52 MDR and 61 XDR tuberculosis patients.18 The contact rate, strain fitness, case detection rate, and acquired and amplified resistance rates in the model were fitted to longitudinal inpatient and community-based surveillance data (Appendix Figures A1-A4), and the model predictions were subsequently compared to multiple HIV and tuberculosis studies in Tugela Ferry to assess the model's predictive accuracy (Appendix Table A3).6,17-20 To account for how uncertainties in parameter values generate uncertainties in the model outcomes, Latin Hypercube Sampling was used to perform uncertainty and sensitivity analysis.21
Infection control strategies
We compared administrative, environmental and personal strategies to reduce nosocomial airborne tuberculosis transmission.14
Administrative interventions
Administrative measures included reduced hospitalization time to 5 days among admitted tuberculosis patients (the current average is 21 days). A 5-day duration of stay was considered acceptable to allow patients to become stable before initiating community-based therapy.22,23 We also simulated deferral of admission for 25% of patients, who underwent community-based therapy; such a strategy may be difficult for some patients due to the severity of their illness.22
Other administrative measures included implementation of rapid drug-susceptibility assays. Four diagnostic technologies were modeled: phage-based assays,24 the line probe assay,25 the GenoType Mycobacteria Direct (GTMD) assay,26 and the microscopic-observation drug-susceptibility (MODS) assay.27 Rifampicin-resistant patients were switched to second-line tuberculosis therapy, which may decrease infectiousness.28,29 The rapid tests were compared to conventional culture and drug-susceptibility testing using Middlebrook agar and the proportional method, which takes 4-8 weeks to produce results. The model simulated the impact of the sensitivity, specificity, and turnaround time of these assays, and included the potential amplification of resistance caused by diagnostic delay.
The final administrative measure considered was that of involuntary detention for confirmed XDR tuberculosis cases who refuse or default from therapy.30 These patients were not discharged from the facility until death or successful treatment completion. Various combinations of rapid resistance testing and isolation were combined with this intervention, to determine their aggregate impact.
Environmental interventions
We simulated improved natural ventilation through fans and open windows,31 modeled the use of a mechanical ventilation system and the impact of high-efficiency particulate air (HEPA) filters and ultraviolet germicidal irradiation (UVGI).32
Isolation facilities could provide additional infection control. While 40 patients are typically hospitalized together on the ward, other hospital areas or trailer park homes could provide new space for isolation. We modeled individual isolation as well as 5- and 10-patient isolation clusters. We also modeled the combination of isolation with rapid drug-susceptibility testing to more quickly identify and isolate MDR tuberculosis cases.
Personal interventions
Personally-protective measures included N95 respirators for staff and surgical masks for patients. We incorporated the mask efficiency, face-seal leakage rate, and adherence to this intervention.33 We also simulated the use of a dedicated nurse who enforces adherence to mask use.
Personal protective measures also included HIV voluntary counseling and testing for admitted patients, with ARV therapy for those who qualify. The proportion of patients accepting an HIV test and being eligible for ARV therapy was varied, and therapy was initiated at the completion of the intensive phase of tuberculosis treatment. We did not model the impact of immune reconstitution syndrome, given uncertainties about its incidence and impact in this context.34
The final personal protective measure simulated was the effect of encouraging HIV testing among healthcare staff, with redeployment of HIV-infected staff to to lower their risk of tuberculosis infection.
We modeled plausible combinations of the above interventions.
Results
Epidemic projections
After calibration to two years of longitudinal data concerning the epidemic trajectory of XDR tuberculosis in this area (Appendix Figures A1-A4), the model was found to reliably predict epidemic data from multiple subsequent studies in Tugela Ferry (Appendix Table A3). The model predicted that in the absence of new interventions, the annual number of XDR tuberculosis cases arising in Tugela Ferry would increase from 194 in 2007 to 234 in 2012, generating 1,302 cases by the end of 2012. The annual number of all MDR tuberculosis cases would grow from 352 in 2007 to 425 in 2012 (Figure 2a). We estimate that 72–96% of new XDR tuberculosis cases would be among HIV-infected persons.
Figure 2. Epidemiological structure of the model.
Tuberculosis states are further characterised by the sub-states of infectious tuberculosis, non-infectious tuberculosis, or treatment failure, as shown in figure 1. One inpatient setting is simulated, representing the tuberculosis ward of the Church of Scotland Hospital in Tugela Ferry. However, susceptible, latently-infected, and recovered patients could be admitted to this ward as false positive tuberculosis cases, and health-care workers also constitute part of the susceptible inpatient population. Mortality is not depicted.
Our calibration of the model to longitudinal community-based and inpatient data allowed the model to infer the transmission dynamics of XDR tuberculosis in both the hospital and community setting, which includes the likely proportion of cases that have arisen in the hospital and community. This process suggested that almost nine out of every ten XDR tuberculosis infections occur in the hospital at present, but this proportion would decline to four out of ten in 2012, with an increase in community-based transmission. We simulated the case in which all nosocomial transmission was completely eliminated starting from 2007. The long-term steady-state was 42 incident cases of XDR tuberculosis per year (range 26 to 58; i.e., transmission was sustained in the community when the model was repeatedly run across the uncertainty ranges of the parameter values).
Our model suggested that the burden of XDR tuberculosis on the health system would increase if no new interventions are introduced. Fifty-one percent of inpatients with tuberculosis in 2007 have some form of MDR tuberculosis in the model, with 29% having XDR tuberculosis. By the end of 2012, these proportions rose to 78% and 48%, respectively (Figure 2b).
The model's projections corresponded closely to current events at the Church of Scotland Hospital and Tugela Ferry (see Appendix Table A3 for validation details). A complete sensitivity analysis is also provided in the Appendix (Table A4). The model's results were most sensitive to natural history parameters: the proportion of patients who rapidly advance to active disease and the fraction who become infectious.
Interventions
Administrative measures
When implemented alone, administrative measures would prevent less than 10% of future XDR tuberculosis cases over the next 5 years (Figure 3a). Discharging patients after 5 days of admission would avert 6% of XDR tuberculosis cases on average (78 cases; range 3-9%, or 39 to 117 cases). The benefit of removing patients from the risk of nosocomial transmission was partially offset not only by increased community risk, but also by the rapid entry of new patients to fill the emptied beds. If 25% of currently admitted patients were deferred from admission, 7% of XDR tuberculosis cases (91 cases; range 4-10%, or 52 to 130 cases) would be averted. The rapid drug-susceptibility assays did not differ significantly from each other in efficacy when implemented alone, indirectly preventing between 2 and 4% of XDR tuberculosis cases (26 to 52 cases).
Figure 3. Incident cases of drug-resistant tuberculosis.
Number of XDR tuberculosis cases increased from 194 in 2007 (range 122–316) to 234 in 2012 (147–380), whereas the total number of MDR tuberculosis cases rises from 352 in 2007 (223–531) to 425 in 2012 (269–640). Incident cases of overall tuberculosis increased from 1780 in 2007 (1287–2313) to 1890 in 2012 (1397–2423). Error bars in all figures indicate maximum and minimum values obtained through uncertainty analysis, in which the values of parameters were varied across the range of possible values (tables 1 and 2 in the webappendix).
Involuntary detention in the congregate tuberculosis ward would increase XDR tuberculosis by 3% between 2007 and the end of 2012 (39 cases; range 2-4%, or 26 to 52 cases) if the intervention was implemented alone in the absence of individual patient isolation facilities. The risk of nosocomial transmission outweighs the reduced community risk from removing XDR tuberculosis patients from the community. When combined with both rapid resistance testing and individual isolation for those confirmed to have XDR tuberculosis (see below), involuntary detention would avert less than 1% of cases (1.7 cases; range: 0-2%, or 0 to 26 cases) beyond those already averted by individual isolation and rapid testing.
Environmental measures
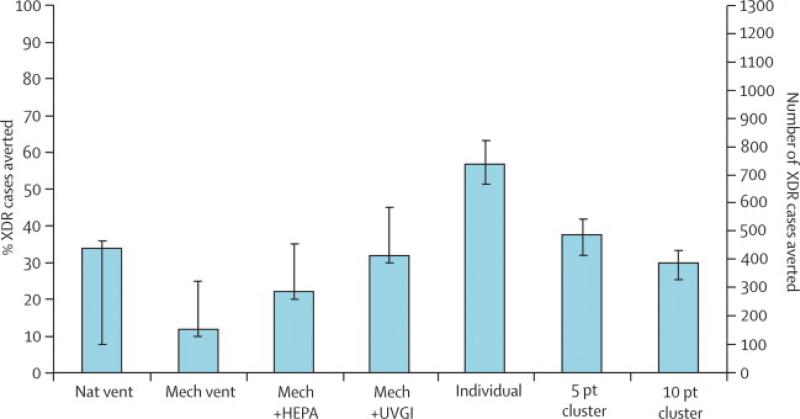
Improvements in ventilation would be among the most effective measures if implemented alone (Figure 3b). Improvements to natural ventilation would prevent 33% of XDR tuberculosis cases (430 cases) on average, but could vary widely in efficacy (from 8-35%, or 104 to 456 cases), given the variability of wind patterns. Mechanical ventilation would prevent 12% of cases alone (156 cases; range 10-25%, or 130 to 326 cases), with a high upper bound given the improved efficacy of some ventilation systems.31 Supplementation of mechanical ventilation with HEPA filters could effect a further 10% reduction in incidence (a total of 286 cases; range 20-35%, or 260 to 456 cases). With UVGI supplementation, mechanical ventilation would avert 32% of cases (417 cases; range 30-45%, or 391 to 586 cases).
Greater partitioning of the inpatient ward into smaller units would also be an effective strategy, if the partitioned ward maintained the same ventilation rate as the full ward. Individual isolation facilities could prevent 57% of future XDR tuberculosis cases (742 cases; range 51-63%, or 664 to 820 cases). Clustering patients into 5 patient units would prevent 37% of cases (482 cases; range 32-41%, or 417 to 534 cases). Increasing this number to 10 patients per cluster would still effect a 30% drop in cases (391 cases; range 27-35%, or 352 to 456 cases). A perverse increase in XDR tuberculosis incidence by 26% (339 cases; range: 21-33%, or 273 to 430 cases) would occur if the capacity of the tuberculosis ward were increased from 40 to 100 beds without new isolation facilities or other interventions.
Personal protective measures
On average, 3% of all XDR tuberculosis cases (39 cases; range 2-6%, or 26 to 78 cases) occur among hospital staff in this model. If implemented in the absence of other interventions, respiratory mask use would prevent 2% of total cases and nearly two-thirds of XDR tuberculosis cases among staff (26 cases; range: 1-3%, or 13 to 39 cases). Five percent of XDR tuberculosis infections (65 cases; range 2-10%, or 26 to 130 cases) would be averted if patients were additionally provided with surgical masks. The impact of this intervention is limited by the long duration of exposure, low effective filter efficiency (e.g., face-seal leakage), and varying levels of adherence to mask use (Figure 3c). Enforcement of adherence would improve the number of averted XDR tuberculosis cases by only 1% on average (range 0-2%), due to low effective filter efficiency and prolonged exposure. However, mask use is far more effective when combined with other strategies (see below).
VCT and the initiation of ARV therapy would prevent only 1% of XDR tuberculosis cases when implemented exclusively to eligible patients admitted to the tuberculosis ward (13 cases; range: 1-2%, or 13 to 26 cases). The effectiveness of VCT and ARV therapy increases dramatically if offered at the community level; 24% of XDR tuberculosis cases (312 cases; range: 17-30% or 221 to 391 cases) would be averted (data not shown). One-third of staff infections, representing 1% of all XDR tuberculosis cases (13 cases; range: 0-2%, or 0 to 26 cases), would be averted if staff were tested for HIV, and those testing positive were redeployed to work away from the tuberculosis unit.
Available combinations
Several combinations of interventions that are immediately available at district hospitals in South Africa were modeled in concert, to determine their aggregate effects on nosocomial transmission. Some interventions that were minimally effective when implemented alone were found to have large effects when implemented in concert (Figure 3d). For example, reducing the length of inpatient stay or enforcing staff and patient respiratory mask use would each avert less than 10% of all XDR tuberculosis cases. However, when implemented together, these strategies would avert 28% of XDR tuberculosis cases (365 cases; range 21-33%, or 273 to 430 cases).
If these two interventions were combined with natural ventilation improvements, cases of XDR tuberculosis would drop by an additional 9%, resulting in a cumulative reduction of 37% (482 cases; range 26-40%, or 339 to 521 cases). In total, the combination of enforced mask use, reduced length of stay with outpatient continuation therapy, improved natural ventilation, MODS testing, hospital-based VCT with ARV provision, and isolation of patients in 5 bed units would prevent 48% of future XDR tuberculosis cases (625 cases; range 34-50%, or 443 to 651 cases) (Figure 3d).
Discussion
Without new interventions, a total 1,302 cases of XDR tuberculosis may arise in Tugela Ferry, KwaZulu-Natal by the end of 2012—most of which are due to nosocomial transmission. Many individual infection control measures would have limited epidemic-level impact if implemented alone. In concert, however, these strategies may have significant synergy. A combination of infection control strategies that can be rapidly implemented in South African district hospitals—improved natural ventilation, reduced hospitalization with the provision of outpatient therapy, mask use, rapid drug-susceptibility testing, hospital-based HIV testing with ARV therapy, and isolation of patients in 5-person units—could avert half of XDR tuberculosis cases (651 cases) in Tugela Ferry over the next 5 years.
Limiting inpatient exposure to infectious tuberculosis droplet nuclei was found to be crucial to affecting the epidemic trajectory of XDR tuberculosis. Creating individual isolation facilities and installing UVGI-supplemented mechanical ventilation systems were among the most successful strategies for preventing future XDR tuberculosis cases. Unfortunately, these interventions are costly and not immediately available in most resource-limited district hospitals in South Africa. However, additional simple strategies, when used in combination, may produce effective results at an epidemic level. We observed synergy between interventions that reduced the concentration of tuberculosis droplet nuclei in hospital wards, the likelihood and duration of exposure for susceptible inpatients, and the population in congregate tuberculosis wards.
In contrast, interventions that increase the number of XDR tuberculosis patients in congregate hospital wards—through prolonged hospitalization or involuntary detention in the absence of sufficient isolation facilities—could perversely increase the incidence of XDR tuberculosis. The practical implementation of deferring hospitalization for community-based therapy is therefore an important subject for further study.23
Of note, available strategies, including N95 respirator mask use and staff HIV testing and work assignment redeployment, can significantly reduce the number of XDR tuberculosis cases occurring among hospital staff. Although the total number of cases averted is small, the critical nature of hospital staff availability and safety highlights important system-level benefits from strategies that may not have immediate epidemic-level impact.
Our results are consistent with prior studies of airborne tuberculosis transmission,32,35-38 although this study is the first to estimate the epidemic-level impact of hospital-based strategies. We recognize several limitations of this study. Given that this is a modeling study, our conclusions are based on the model assumptions. Although our model was validated against multiple data sources, other models may be constructed to effectively simulate XDR tuberculosis transmission; therefore, our results should be viewed as a starting point for further investigation, rather than as mathematical “proof” of any particular concept discussed here. Although we performed extensive uncertainty analysis, all unknowns could not be incorporated. We varied the efficacy of different interventions over a broad range of possible values.36,39 We did not include HIV as increasing the risk of infection with MDR tuberculosis over non-MDR tuberculosis, as this remains uncertain.40 Similarly, little is known about the future potency of treatment for XDR tuberculosis. We projected a wide range of possible treatment efficacy, from totally ineffective to the 54% successful treatment rate observed among Peruvian XDR tuberculosis patients, who were largely HIV-negative.41 Furthermore, the airborne transmission model employed for these projections is based on the even distribution of infectious particles in a room, and does not account for how the probability of infection is modified by complexities such as the distance between individual patients (a subject under active investigation).36,42
Our study was based upon data largely collected from one site in rural South Africa and thus, it may not be fully generalizable. Nevertheless, this setting is typical of those throughout rural areas of South Africa and other resource-poor settings where tuberculosis and HIV are highly prevalent. Additionally, recent data suggest that common strains of XDR tuberculosis are widely distributed throughout this province, and perhaps all of South Africa.43,44
Our study also focused on hospital-based interventions, and did not systematically address community-based interventions. Some programs, such as HIV testing and ARV therapy, may have a greater impact at the community level than when implemented in the hospital alone (24% versus 1% of XDR tuberculosis cases averted, respectively). Furthermore, we did not simulate the impact of infection control strategies outside of the tuberculosis ward, in locations such as HIV clinics, or how resource-strapped health districts may prioritize interventions based upon their respective costs.
Although these limitations suggest the need for further research, our current projections highlight the need for immediate action in addressing the XDR tuberculosis epidemic. The burden of XDR tuberculosis on the health system is already high in this area, and is expected to rise dramatically over the next few years. Effective hospital-based strategies to limit the transmission of XDR tuberculosis are within reach, even in resource-limited settings. Combined administrative, environmental and personal infection control measures to reduce nosocomial transmission, could prevent nearly half of future XDR tuberculosis cases. Such comprehensive programs must be rapidly implemented throughout KwaZulu-Natal and the rest of South Africa. Additional community-based strategies should also be developed in parallel, since hospital-based efforts alone may not fully curtail XDR tuberculosis transmission.
Figure 4. Burden of drug-resistant tuberculosis on the hospital.
Without new interventions, the proportion of inpatients with any form of MDR tuberculosis is predicted to increase from 51% in 2007 to 78% in 2012, whereas those with XDR tuberculosis increased from 29% to 48%. XDR tuberculosis consisted of 57% of all MDR tuberculosis inpatients in 2007, increasing to 62% in 2012.
Figure 5. XDR tuberculosis cases averted through administrative measures, 2007–2012.
LOS=reducing the length of inpatient stay to 5 days (from the current average of 21 days); use of rapid rifampicin resistance tests for the diagnosis of MDR tuberculosis. GTMD=GenoType Mycobacteria Direct, HAIN Diagnostics. MODS=microscopic-observation drug-susceptibility assay. Detention=enforcing involuntary detention of confirmed XDR tuberculosis patients who refuse therapy, without the implementation of new isolation facilities.
Figure 6. XDR tuberculosis cases averted through environmental measures to reduce nosocomial transmission, 2007–2012.
Nat vent=improvements in natural ventilation. Mech=using a mechanical ventilation system. HEPA=high-efficiency particulate air filters. UVGI=ultraviolet germicidal irradiation. Individual=providing individual isolation facilities. Cluster=separation of patients into units of five patients or ten patients (from the baseline 40-patient unit).
Figure 7. XDR tuberculosis cases averted through personal protective measures to reduce nosocomial transmission, 2007–2012.
Staff mask=provision of N95 masks to staff members. Staff+patient mask=additionally providing patients with basic surgical masks; Enforce=maintaining a dedicated infection control officer to enforce adherence to mask use. VCT+ARV=provision of voluntary counselling and testing, with subsequent antiretroviral therapy to those admitted patients who qualify.
Figure 8. Efficacy of rapidly-available combinations of strategies to reduce nosocomial transmission.
Mask=both staff N95 respirators and patient masks with adherence enforcement. LOS=reducing average length of stay to 5 days. Vent=improvements in natural ventilation. MODS=microscopic observed drug susceptibility assay. VCT=voluntary counselling and testing in admitted patients, with subsequent antiretroviral therapy to those who qualify. 5 pt=isolating patients in groups of five patients.
Acknowledgements
Thanks to Kristina Talbert-Slagle and the six anonymous reviewers of this manuscript for their helpful insights and commentary. The authors acknowledge the challenging and outstanding work of the staff of the Church of Scotland Hospital, and appreciate support from the Doris Duke Charitable Foundation (JA, NRG, NSS, AM, GHF), the Irene Diamond Fund (JA, NRG, NSS, AM, GHF), the Guggenheim Foundation (APG), the Institute for Advanced Study (APG), the James S. McDonnell Foundation (SB, EP, APG), the Notsew Orm Sands Foundation (APG), the National Institutes of Health (SB), and the National Science Foundation (SB, EP, APG). SB is also supported by the National Medical Scientist Training Program for M.D./Ph.D. students, NIH 5 T32 GM07205-32.
Funding sources: The authors are funded by the Doris Duke Charitable Foundation, the Irene Diamond Fund, the Guggenheim Foundation, the Institute for Advanced Study, the James S. McDonnell Foundation, the Notsew Orm Sands Foundation, the National Institutes of Health, and the National Science Foundation.
Role of the funding source
The funders had no role in the study design, implementation, data analysis, or in the writing of the manuscript or decision to submit it for publication.
Appendix
1 Model assumptions and equations
The model structure we employed is based upon prior simulations of tuberculosis transmission in the context of multiple drug-resistant strains, HIV, airborne nosocomial transmission and exogenous re-infection [1-13]. The ordinary differential equations detailed below (Equations 1-21) describe transitions between multiple health states that the simulated people can experience, from susceptibility to tuberculosis through treatment failure. People in any of the health states can reside in each of the two environments in this model (the community of Tugela Ferry and the inpatient TB unit of the Church of Scotland Hospital). The two environments are connected by admission and discharge, as detailed later in this Appendix. The two environments have different transmission dynamics, described by Equations 22 and 23. Some of the treatment parameters will also differ between the two environments, as inpatients may receive more extensive therapy than those in the community (as detailed further below).
Within each environment, the same stages of TB pathogenesis occur. Susceptible persons (S) can become infected with TB and move to periods of latent infection of either short (E) or long (L) duration [14]. Latently-infected persons may then experience reactivation of their disease to manifest active infectious (Ti) or non-infectious (Tn) TB, where the latter may convert over time to an infectious state [6, 7]. Active TB may be detected and treated, with some persons recovering (R) while others may fail therapy (F). A few active TB patients also experience self-cure [5]. Patients who recover via therapy may relapse [5], while those in treatment may experience acquired or amplified resistance [15]. Latently-infected and recovered persons can be exogenously re-infected with any strain, contingent upon their partial immunity and the time-dependent risk of infection with that strain (which includes the fitness and prevalence of the strain; Equations 22 and 23) [16]. TB pathogenesis will be described by new rates if a person is HIV-infected, and further modified by ARV therapy (conditional upon patient adherence) [17, 18]. Tables A1 and A2 (listed at the end of this Appendix) describe the values used to parameterize the model.
Because people in both environments can experience the same health states, the same series of ordinary differential equations (below) is repeated twice, once for each environment. The two sets of equations differ in some parameters (listed in Tables A1 and A2). In the model equations below, superscript c refers to an individual's HIV status (0 signifies HIV-negative, 1 signifies HIV-positive but not on antiretroviral therapy, and 2 signifies HIV-positive and receiving antiretroviral therapy), while subscript m refers to the TB type (non-MDR, non-XDR MDR, or XDR).
Equations 1 through 3 describe susceptible persons. New susceptible HIV-negative persons enter the model via birth (b), which is equal to deaths at each time step in this closed population model. Susceptible persons may be infected with any of the three TB types at a time-specific risk of infection λm(t). We simulated the three TB types which have been found in Tugela Ferry: a drug-sensitive type; a MDR type resistant to isoniazid, rifampin and streptomycin; and a 6-drug resistant XDR type (isoniazid, rifampin, ethambutol, streptomycin, ciprofloxacin, and kanamycin) [19-21]. We included XDR-TB transmission to both HIV-infected and HIV-uninfected persons, given reports of XDR-TB cases among HIV-negative patients in KwaZulu-Natal [22]. Susceptible individuals may be infected with HIV (at a rate hiv) or die from non-TB-related causes at background mortality rate μ. Some HIV-infected susceptibles may receive ARV therapy (at rate arv). HIV and ARV therapy were modeled in this simple manner, similar to prior models [3, 5, 6, 17]. Access to treatment for HIV is centralized through the facility we model here [23, 24]. A more complex HIV model was judged to offer little insight for the purposes of simulating nosocomial XDR transmission over a brief time period. It has been previously established that HIV can affect the probability of progressive primary disease in those infected, the rate at which latent infections become TB cases by endogenous reactivation, and the death rate for infectious and non-infectious TB [17]. These factors are incorporated into the parameters in Table A1. We follow the work of previous authors, who calculated the degree to which ARV therapy modifies TB, producing the parameters for the ARV-treated HIV-infected patients, also listed in Table A1 [18]. We assume that HIV infection does not occur during an inpatient stay, so the hiv parameter equals zero for the hospital environment. To improve the readability of the differential equations, the time-dependence indicator (t) is removed from state variables (S, L, E, Ti, Tn, F, R) and from the dynamic parameter λm.
| (1) |
| (2) |
| (3) |
A proportion of infected patients (1-p) who do not experience primary progressive disease enter slow latency, described by Equations 4 through 6. This population can be affected by exogenous re-infection, which is contingent upon a person's degree of susceptibility despite prior infection (x) [7, 14]. A fitness “cost” parameter for drug-resistant strains is built-in to the risk of infection (see Equations 22 and 23); following Cohen and Murray [7], we describe infection and re-infection as conditional upon this parameter, rather than defining all possible mixed latent states, from which regression and progression have not been well-defined and are under active investigation [13, 25, 26]. People who naturally self-cure from active TB states, at rate σ per year, return to slow latent status, as in prior models [4, 6, 7, 16, 27]. Reactivation of disease occurs at rate v. HIV infection, ARV induction, and non-TB-related death are experienced by latently-infected persons in the same manner as with susceptible persons.
| (4) |
| (5) |
| (6) |
Movement to and from the rapid latent state (Equations 7 through 9) are analogous to that to and from the slow latent state, except that individuals (proportion p of those infected) enter this state via primary progression, while no persons enter via self-cure from active TB [7]. Rapid latents experience reactivation of disease at the rate τ.
| (7) |
| (8) |
| (9) |
A proportion f of those persons who have reactivated from latent states to active TB are infectious [14] (Equations 10 through 12), while non-infectious active TB patients may also convert to infectious status at a rate w [5], and patients who have recovered due to chemotherapy may relapse to active disease at rate δ [7]. Patients move from active TB status to that of failure or recovery at rate d, or experience self-cure (σ). They may also die of TB (at rate μT), in addition to facing the background non-TB mortality rate μ.
| (10) |
| (11) |
| (12) |
Individuals who have active non-infections forms of TB (Equations 13 through 15) follow an analogous path to those with active infectious TB. A proportion of reactivating patients (1-f) enter the non-infectious state, and from this state they may also become infectious at rate w.
| (13) |
| (14) |
| (15) |
Treatment failure (Equations 16 through 18) occurs for a proportion of patients (1-k), some of whom (proportion α, calibrated to longitudinal data as described later in this Appendix) experience acquired or amplified resistance to the next higher category of resistant TB [7, 14]. Failures may still experience re-treatment, and therefore may continue to experience further treatment failure episodes at the risk of acquiring (more) drug resistance [14]. Some of these individuals may self-cure [7]. A proportion are also infectious [5], as discussed further below. In the notation used in these equations, αm+1 refers to the development of resistance that leads to the next higher category of drug-resistant TB. Since there is no further classification beyond XDR in this model, this term is ignored for XDR patients, who continue to be classified as XDR cases if they amplify or acquire further resistance. Similarly, αm refers to the proportion of patients who have developed resistance through treatment for the lower-category type m-1. There is no lower category than drug-susceptible TB, so this term is ignored for that group.
| (16) |
| (17) |
| (18) |
The proportion (k) of patients who recover from therapy (Equations 19 through 21) may still experience re-infection (with any strain), according to the degree to which partial immunity does not protect them (x). The parameter k is taken from local data when available, as cited. For XDR TB patients, we adopted a range of possible values given the existing studies on XDR TB treatment in other countries (see Table A1). The mode of the triangular distribution of possible treatment success values is a factor lower for those infected with HIV than for those who are HIV-negative. We applied the same factor decrease to HIV-positive XDR patients versus HIV-negative XDR patients, as to general HIV-positive MDR TB patients compared to HIV-negative MDR patients. However, we also varied the possible treatment efficacy for XDR TB over the entire range of values observed to date, in uncertainty and sensitivity analyses. Patients who have recovered via chemotherapy may also relapse (δ) to active disease.
| (19) |
| (20) |
| (21) |
To define the risks of TB infection in each environment (λm(t)), we used two sets of equations. In the community, the risk of infection with each type of TB is the product of the transmissibility coefficient (β, calibrated to local data as described below), the fitness “cost” of resistance in terms of decreased virulence (fitm, which is 0 for drug-susceptible TB), and the number of infectious TB cases at time t (Ti(t)+φF(t); where φ is the proportion of treatment failures who are infectious) [14]:
| (22) |
The inpatient risk of infection is defined by a model of airborne contagion from Gammaitoni and Nucci [8], which produces the following expression [12] (a validated modification of the Wells-Riley model of airborne transmission [10, 11]), in which q is the number of infectious quanta produced per hour by infectious persons (varied stochastically across a triangular distribution with mode 1.3 and edges 0 to 60 [11, 12, 28, 29]); κ is the pulmonary ventilation rate of susceptible individuals (assumed uniform between 0.48 and 0.6 m3/h [12, 30]); ω is the room volume (m3); and ψ is the ventilation rate (air changes/h). The latter two values and their modifying factors are discussed in detail in the “environmental measures” section of this Appendix (below).
| (23) |
The two environments are connected by admission and discharge rates. Particularly in the context of rural South Africa, admission to the hospital is dependent upon the availability of beds [31]. There is no waiting list at the Church of Scotland Hospital (rejected patients remain in the community and can try to receive admission later), hence a transient triage situation for each time step, rather than a permanent queueing state, can be used to simulate this environment. If z equals the beds available, then z times the current service rate (γ(t)) will be the total possible flow into the inpatient TB ward. Following Kaplan et al. [32], each subpopulation Pj of patients who make up the total demanding population P will be admitted at rate zγ(t)Pj/P if the system is overburdened (admission proportionate to the relative demand if z<P). Hence admission occurs at the rate γ(t)Pjmin(1,z/P) from each demanding outpatient population Pj into the corresponding inpatient compartment. The demanding populations include a calibrated fraction of patients from each active disease compartment (accounting for those receiving outpatient therapy or having no access to the hospital) and 0.05% of the non-diseased compartments. The latter constitute “wrong” admissions to the TB ward (patients with TB symptoms who get admitted under the false premise of having active TB, but who do not have the disease) [33]. The service rate is the time-specific weighted average of discharge rates among inpatients, which is 22.82/yr for drug-susceptible patients, 45.63/yr for uninfected patients, 13.04/yr for non-XDR MDR-TB patients, and 15.87/yr for XDR-TB patients [20, 23, 34].
Equations were confirmed by three of the authors, and by an automated algorithm which checked for missing states and flows. Four of the authors also independently reviewed the parameter values.
2 Calibration and validation
We calibrated the model to all available longitudinal data from Tugela Ferry (Figures A1 to A4; [33-35]), and validated it against all available cross-sectional data (Table A3). In all cases, the most current XDR definition is used [36]. It is inappropriate to calibrate every parameter of such a large model to limited data, as such calibration would violate principles of unique parameter identifiability, generating numerous spurious parameter values that could “fit the curve” without necessarily having real-world validity [37]. We therefore calibrated only the small set of parameters that could not be immediately or accurately evaluated through empirical studies (listed with results in Table A1), and checked their optimality as described below. The calibration procedure allowed us to determine the transmission dynamics of this environment, and thereby estimate factors that are difficult to manually determine (e.g., the effect of local socioeconomics on the dynamics of infectious contact and subsequent TB transmission, or what proportion of XDR-TB cases were arising from community-based transmission versus nosocomial transmission).
Simulated annealing was used for calibration [38, 39]. The term “annealing” is derived from metallurgy, in which materials were observed to become malleable at high temperatures, but conform to a hardened shape as they cool. Analogously, the simulated annealing algorithm starts at a “high temperature”, meaning that the algorithm searches across the broad possible values to attribute to the modeled system, then slowly “cools” as it finds optimal explanations for the data, eventually settling into the global optimum after exploring the search space. Hence, this optimization procedure is insensitive to initial parameter estimates, which was further analyzed by varying initial estimates over the range displayed in Table A1 to confirm their ultimate convergence onto the optimized values. The procedure involves finding the mean and range of the calibrated parameters while varying the estimates of the non-calibrated parameters across their uncertainty ranges. The algorithm has been described in great detail elsewhere [38, 39].
Initial population values were obtained by running the 150,000 person population in Tugela Ferry to equilibrium in the absence of TB treatment or HIV. We then introduced case detection and inpatient TB treatment on a linearly increasing schedule from 1966 and outpatient therapy from 1993 [40]. HIV was introduced in 1983 [41] using incidence estimates calculated with the procedure described on p. 4 of the Appendix to [17], fitting to within 10% of the annual provincial HIV prevalence estimates since 1995 with a single logistic curve [42]. ARVs were introduced in 2004 [23]. XDR TB was also introduced in an HIV-infected inpatient in 2004 [35]. The least squares fit explained (R2) over 95% of the variance in the data.
Of interest to modelers, the calibrated fitness “costs” of MDR strains in this area (the degree to which the acquisition of drug-resistance confers a decrease in virulence) were small but negative--indicating that the specific strains circulating in the area are possibly equally or slightly more infectious than drug-susceptible TB, in the context of the model's assumptions. This finding is consistent with prior laboratory studies and modeling experiments of effectively-transmitted MDR strains [7, 43]. However, given the complexities of compensatory evolution and heterogeneity in fitness as a function of the drug-resistance mutation [43, 44], we would suggest further research on these strains to investigate our finding, and have provided only short-term epidemic projections, given that longer-term dynamics are subject to great uncertainties regarding strain evolution.
3 Intervention scenarios
Interventions were categorized into three groups: personally protective, environmental, and administrative [45, 46].
3.1. Personally protective measures
3.1.A
Respiratory protection
The efficiency of an N95 respirators is 95%, with a real-world face-seal leakage rate of 0-39% [8, 47-50]. Adherence ranges between 44% and 97% [51-53]. To simulate the use of these respirators, we calculated the effective filter efficiency by sampling from a uniform distribution across the adherence range and a triangular distribution across the leakage range (with mode 10% [8]), then calculating adherence*(efficiency-efficiency*leakage) [8]. One minus this effective filter efficiency was multiplied by the pulmonary ventilation rate within the calculation of inpatient risk (Equation 23), to adjust the inpatient risk for the effective number of inhaled infectious particles [8, 12]. To simulate the impact of staff respirator use, the new risk is applied to the population of 5 susceptible staff members, who do not experience discharge unless infected and reactivating with active TB, after which they move from staff to patient status. We renewed the staff population by withdrawing from the community susceptible populations when staff became ill (assuming HIV prevalence among staff parallels that of the community population). We also compared the efficacy of staff respirators to the estimated efficacy of additionally having patients use less effective barriers such as inexpensive surgical masks and handkerchiefs. These barriers have a leakage rate sampled from a triangular distribution from 0 to 100% with mode 20%, and an efficiency sampled from a triangular distribution with mode 50% (range 0% to 75%) [8, 50, 54-57]. When an enforcement nurse was added to the ward, we estimated adherence to shift to a uniform 70 to 100% distribution for both staff and patients [58].
3.1.B
Increased HIV voluntary counseling and testing, with subsequent antiretroviral provision to qualified patients, was simulated by sampling from a uniform distribution of 50 to 95%, representing the estimated proportion of untreated HIV-positive inpatients willing to get tested and who are eligible for and adherent to treatment [23]. ARV therapy for this group then converted patients from ARV-untreated to ARV-treated status over a 2 month timeframe, simulating the gradual benefits of therapy and the delay among patients who complete the intensive phase of TB treatment before initiating ARV therapy [20, 23, 59].
3.2. Environmental measures
3.2.A
Ventilation improvements alter the denominator of the inpatient risk equation (Equation 23). Air changes per hour are measured using a tracer gas concentration-decay technique following carbon dioxide release [60]. The baseline absolute ventilation (room volume times air changes per hour, ωψ) was estimated to be 121 m3/h (range 40-205), with improvements in natural ventilation (e.g., opening windows, introducing fans) producing a new ventilation rate of 2,477 m3/h (range 240-3,349) [61]. A mechanical ventilation system in this ward was estimated to produce 402 m3/h (range 330-1,209), with high-efficiency particulate air (HEPA) supplementation further increasing effective ventilation by a factor of 3.37 and ultraviolet germicidal irradiation (UVGI) supplementation increasing effective ventilation by a factor of 10.31 above the unsupplemented system [8, 61-65]. Upper-room UVGI in a waiting area alone was not simulated because of the absence of data that could be extrapolated to the simulated hospital [66]. Triangular distributions were constructed for sampling, according to the mean and range of the ventilation rate.
3.2.B
Isolation
Currently, 40 patients are kept together in the ward. We simulated the case in which park homes or trailers are purchased to partition patients into smaller units of equivalent ventilation to the baseline ward. Individual isolation removes patients from the numerator of Equation 23. To simulate the case in which patient load exceeds trailer capacity for individual isolation, we also simulated 5 patient or 10 patient clusters by adjusting Equation 23 to one-eighth or one-fourth of its baseline value to create new inpatient compartments with smaller populations, so that susceptible patients were exposed to a smaller pool of infectors of each type. Since drug-susceptibility tests require 5 weeks on average to return, profile-based cohorting of all MDR- or XDR-TB subjects to separate these patients from susceptible patients prior to the drug-resistant patients’ death is unlikely (death, on average, occurs in 22 and 14 days from admission, respectively, for non-XDR MDR-TB and XDR-TB patients [34]). Therefore, the number of infectors of each type (drug-susceptible TB, MDR-TB, XDR-TB) was distributed evenly among the various new wards if this intervention is used alone. We also simulated the case in which one of the clusters would be for rifampin-resistant cases only (if rapid rifampin testing was used, as discussed below) by subtracting the inpatient population of these drug-resistance isolation wards from Equation 23 when calculating the inpatient risk to others. The effect of this ward partitioning measure was determined by sampling from a triangular distribution corresponding to the mean and range of the ventilation rate (Section 3.2.A, above).
3.3. Administrative measures
3.3.A
Reducing the length of inpatient stay among TB patients was simulated by decreasing the average stay among TB patients to 5 days [34], with continuation of SLD based therapy for MDR patients at the cure rates listed in Table A1. Continuation therapy was simulated by discharging patients to Equations 10 through 18. The patients represented by these equations first experienced the lower cure rate corresponding to standard therapy initiated during their inpatient admission (Table A1); they then decayed into a second set of identical equations, where the therapeutic cure rate was replaced by the higher rate corresponding to second-line therapy (also in Table A1). The decay rate was set equal to the reciprocal length of time required for drug-resistance testing results to return (listed below). We assumed 10-14% default or refusal [21, 67] from outpatient second-line therapy [68].
3.3.B
Involuntary detention was simulated by preventing XDR patients who refused or defaulted from therapy (10-14% of the population, based on case-control and cohort studies of the frequency of default in the area [21, 67]) from being discharged from the hospital. These patients with XDR were put into separate compartments without discharge until cure or death occurred, subject to the timing and sensitivity of the drug-resistance testing results (see below). We also combined this intervention with isolation facilities and rapid resistance tests to determine the impact of these interventions in concert.
3.3.C
Improvements in drug-susceptibility testing were simulated by modeling rapid rifampin resistance testing assays that may be considered pragmatic in a high-prevalence setting with some basic laboratory capacity but high volume and notable budget constraints. Patients who test positive for rifampin resistance are presumed MDR [69], and are switched to SLD-based therapy after the number of days corresponding to the estimated turnaround time of the test at the Church of Scotland Hospital (see below). The different testing methods were simulated by having MDR patients exposed to the value describing the efficacy of standard therapy, k (in Table A1), during the period in which testing was taking place. This was changed to the higher, second-line therapy efficacy value (also listed in Table A1) after the results of testing returned, indicating the switch to second-line drugs. To perform this simulation, the differential equations specified above were repeated twice. The first set of equations used the standard treatment efficacy value, and the second set used the SLD value. The population of inpatients started in the first set of equations, which decayed into the second set at a rate equal to the reciprocal of the testing time (below). False-negatives (one minus sensitivity, multiplied by the population tested) were subjected to the standard therapy efficacy value. Furthermore, MDR-TB patients receiving second-line treatment reverted to non-infectious status over the course of 5.5 weeks, which is simulated by inserting this rate of conversion into Equations 10 through 18 [70].
The baseline Middlebrook agar and proportional method of drug-susceptibility testing requires 5 weeks, and has 84% sensitivity (range 82.5 to 89%) and 100% specificity (99.9 to 100%) for rifampin resistance [71]. For phage-based assays, we simulated a turnaround time of 3 days [72-82], and 98% sensitivity (range 81 to 100%) and 93% specificity (range 73 to 100%) [79]. Line probe assays were simulated with a turnaround time of 3 days, and with 91% sensitivity (range 90% to 100%) and 96% specificity (range 91% to 100%) [83-85]. The GenoType Mycobacteria Direct (GTMD) assay was simulated with a turnaround time of 2 days, and with 92% sensitivity (range 87% to 97%) and 100% specificity (range 95% to 100%) [86]. The microscopic-observation drug-susceptibility (MODS) assay was simulated with a turnaround time of 11 days, and a 97.8% sensitivity (range 97.3 to 97.9%) and 99.6% specificity (range 99.2 to 100%) for rifampin resistance detection [71]. Triangular distributions are sampled from, corresponding to the range and mean of the listed sensitivity and specificity values.
4 Uncertainty and sensitivity analysis
Latin Hypercube Sampling (LHS) was used to draw parameter estimates from the probability distributions, which were used for simulation, uncertainty and sensitivity analyses [87, 88]. The formulation described by Stein [88] was used to incorporate the co-variation of the HIV-related parameters, by varying the underlying relative risk of TB progression across its uncertainty range; this risk defines the impact of HIV on the natural history parameters, as described on p.13 of the Appendix to [17]. The error bars in Figures 1-3 of the manuscript text reflect the limits of the outcome estimates obtained through this sampling strategy. The output from sensitivity analysis is displayed in Table A4.
Table A1.
Parameters used to describe TB/HIV epidemiology in rural KwaZulu-Natal. Values in parentheses describe the ranges used in sensitivity and uncertainty analyses.
| Variable | Definition | HIV- | HIV+ (no ARV) | HIV+ (ARV) | Source |
|---|---|---|---|---|---|
| β | transmissibility coefficient | 0.000051 (0.000045-0.000056) | Calibration | ||
| fitm, fitx | fitness cost of resistance, non-XDR MDR and XDR TB | -0.12, -0.16 (-0.2-0.7) | Calibration; range from [7] | ||
| μ | background death rate | 0.023 (0.022- 0.024) | 0.098(0.093-0.103) | 0.051(0.045-0.056) | [18, 89] |
| hiv | HIV incidence | dynamic* | N/A | N/A | [24] |
| arv | ARV initiation rate | N/A | dynamic* | N/A | [23] |
| d | rate of therapeutic initiation and sterilization | 0.77 (0.69-0.85; outpt); 9.45 (8.51-10.40; inpt non-MDR); 6.08 (5.47-6.69; inpt MDR) |
Calibration; [90-93] | ||
| k | proportion of treated patients who recover from non-MDR TB | 0.73 (0.65-0.88) | 0.58 (0.52-0.74) | 0.67(0.6-0.85) | [7, 20, 21, 34, 35, 67, 94-97] |
| proportion of treated patients who recover from non-XDR MDR TB; (std Rx: standard therapy; SLD: second-line therapy) |
0.47 (0.18-0.58; std Rx); 0.67 (0.33-0.80; SLD) |
0.30 (0.16-0.36; stdRx); 0.45 (0.25-0.51; SLD) |
0.40 (0.17-0.48; std Rx); 0.60 (0.33-0.68;SLD) |
[7, 20, 21, 34, 35, 67, 94-97] | |
| proportion of treated patients who recover from XDR TB | 0 (0-0; std Rx); 0.54 (0-0.54; SLD) |
0 (0-0; std Rx); 0.36 (0-0.54; SLD) |
0 (0-0; std Rx); 0.48 (0-0.54; SLD) |
[17, 20, 59,96] | |
| μT | active TB death rate | 0.30 (0.20-0.40); 0.21 (0.18-0.25) if non-infectious | 1.0 (0.75-1.0) | 0.76 (0.63-0.76) | [5, 17, 18] |
| αm, αx | proportion of treated patients who acquire or amplify resistance (non- MDR to non-XDR MDR, and non-XDR to XDR MDR TB) | 0.038 (0.025-0.050); 0.030 (0.025-0.050) | Calibration; [7] | ||
changes over time; see Appendix text.
Table A2.
Natural history parameters. The parameters for the ARV category are given by the relative risk calculation detailed on p.13 of the Appendix to ref. [17], using the data from [18].
| Variable | Definition | HIV- | HIV+ (no ARV) | HIV+ (ARV) | Source |
|---|---|---|---|---|---|
| p | proportion of infected people who develop primary progressive TB | 0.14 (0.08-0.25) | 0.67 (0.36-0.80) | 0.61 (0.32-0.74) | [5, 17] |
| x | degree of susceptibility despite prior infection | 0.35 (0.10-0.60) | 0.75 (0.50-1.00) | 0.69 (0.45-1.00) | [5, 17] |
| α | rate of natural self-cure | 0.020 (0.15-0.25) | [5] | ||
| v | rate at which slow latent infecteds progress to active TB | 0.00011 (0.00010-0.00030) | 0.17 (0.04-0.20) | 0.13 (0.03-0.14) | [5, 17] |
| τ | rate at which fast latent infecteds progress to active TB | 0.88 (0.76-0.99) | 12.0 (10.4-13.5) | 9.1 (8.7-9.4) | [5, 6,17] |
| f | proportion of reactivating TB cases that become infectious | 0.65 (0.50-0.65) | 0.30 (0.19-0.40) | 0.36 (0.22-0.49) | [5, 17] |
| w | rate of conversion from non-infectious to infectious TB | 0.015 (0.007-0.020) | [5] | ||
| δ | rate of relapse from chemotherapeutic cure | 0.0010 (0-0.010) | [7] | ||
| ϕ | proportion of treatment failures who are infectious | 0.50 (0.25-0.75) | [5, 14] | ||
Table A3.
Model validation. After calibration, the model's outputs were compared to the data shown below, to assess the model's ability to estimate quantities considered relevant to this simulation.
| Statistic | Model | Data | Source |
|---|---|---|---|
| Community TB prevalence, Jan 2007 | 0.0100 | 0.0100 | [34] |
| Community MDR prevalence, 2001 | 0.00029 | 0.00028 | [98] |
| Community MDR prevalence, 2005 | 0.00172 | 0.00165 | [23] |
| MDR admissions (including XDR), 2005-2006 | 387 | 396 | [33] |
| XDR admissions, 2005-2006 | 212 | 214 | [33] |
| Av. monthly admitted XDR cases, 2005 | 8.22 | 9.67 | [35] |
| Av. monthly admitted XDR cases, 2006 | 9.48 | 8.17 | [35] |
| Av. monthly admitted non-XDR MDR cases, 2005 | 5.15 | 6.08 | [35] |
| Av. monthly admitted non-XDR MDR cases, 2006 | 9.41 | 9.08 | [35] |
| XDR/non-XDR MDR relative incidence rate, 2005-2006 | 1.22 | 1.18 | [35] |
| ARV-treated population, 2005 | 1353 | 1300 | [20] |
| Proportion of community cases that are MDR, 2005 | 15% | 10% | [35] |
| Proportion of community cases that are XDR, 2005 | 4% | 6% | [35] |
| MDR admissions that are XDR, Jan 2007 | 57% | 55% | [33] |
| Proportion of non-XDR MDR cases that are HIV+, Aug 2006 | 86% | 77% | [34] |
| Proportion of XDR cases that are HIV+, Aug 2006 | 96% | 93% | [34] |
Table A4.
Sensitivity analysis displayed as partial rank correlation coefficients (PRCC) to TB incidence.
| Variable | PRCC | Definition |
|---|---|---|
| fitm | 0.00170 | fitness cost of resistance, non-XDR MDR |
| fitx | 0.00512 | fitness cost of resistance, XDR TB |
| μ | -0.0197 | background death rate |
| hiv | 0.505 | HIV infection rate |
| arv | -0.0370 | ARV initiation rate |
| d | -0.445 | rate of case detection/treatment |
| k | -0.526 | proportion of treated patients who recover from non-MDR TB |
| -0.0144 | proportion of treated patients who recover from non-XDR MDR TB | |
| -0.00153 | proportion of treated patients who recover from XDR TB | |
| μT | -0.485 | active TB death rate |
| αm | 0.00148 | proportion of treated patients who acquire or amplify resistance: non-MDR to non-XDR MDR |
| αx | 0.00107 | proportion of treated patients who acquire or amplify resistance: non-XDR to XDR MDR TB |
| p | 0.937 | proportion of infected people who develop primary progressive TB |
| x | 0.00129 | degree of susceptibility despite prior infection |
| α | 0.00800 | rate of natural self-cure |
| v | 0.00317 | rate at which slow latent infecteds progress to active TB |
| τ | 0.417 | rate at which fast latent infecteds progress to active TB |
| f | 0.913 | proportion of reactivating TB cases that become infectious |
| w | 0.00762 | rate of conversion from non-infectious to infectious TB |
| δ | 0.00370 | rate of relapse from chemotherapeutic cure |
| ϕ | 0.0228 | proportion of treatment failures who are infectious |
Figures A1-A4.
Model calibration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions
All authors participated in the study design, data interpretation, and writing of this manuscript. SB, EMP and APG constructed and implemented the model. JA, NRG, NSS, AM, and GHF collected primary data from the study site.
Conflict of Interest Statements
The authors have no conflicts of interest to declare.
References
- 1.Zignol M, Hosseini MS, Wright A, et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis. 2006;194(4):479–85. doi: 10.1086/505877. [DOI] [PubMed] [Google Scholar]
- 2.Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs--worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006;55(11):301–5. [PubMed] [Google Scholar]
- 3.Notice to Readers: Revised Definition of Extensively Drug-Resistant Tuberculosis. MMWR. 2006;55(43):11176. [Google Scholar]
- 4.World Health Organization . Global map and information on XDR-TB. WHO; Geneva: 2007. Available online: http://www.who.int/tb/xdr/en/index.html. [Google Scholar]
- 5.Shah NS, Wright A, Bai G-H, et al. Worldwide Emergence of Extensively Drug-resistant Tuberculosis. Emerg Infect Dis. 2007;13(3) doi: 10.3201/eid1303.061400. Available online: http://www.cdc.gov/EID/content/13/3/380.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 7.South African Press Association . XDR-TB found in all provinces. SAPA; Durban: Feb 12, 2007. [Google Scholar]
- 8.Makhaye C. Deadly TB on upward spiral in KZN. Sunday Tribune. 2007 Nov 12; [Google Scholar]
- 9.Edlin BR, Tokars JI, Grieco MH, et al. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326(23):1514–21. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 10.Narvskaya O, Otten T, Limeschenko E, et al. Nosocomial outbreak of multidrug-resistant tuberculosis caused by a strain of Mycobacterium tuberculosis W-Beijing family in St. Petersburg, Russia. Eur J Clin Microbiol Infect Dis. 2002;21(8):596–602. doi: 10.1007/s10096-002-0775-4. [DOI] [PubMed] [Google Scholar]
- 11.Sacks LV, Pendle S, Orlovic D, Blumberg L, Constantinou C. A comparison of outbreak- and nonoutbreak-related multidrug-resistant tuberculosis among human immunodeficiency virus-infected patients in a South African hospital. Clin Infect Dis. 1999;29(1):96–101. doi: 10.1086/520189. [DOI] [PubMed] [Google Scholar]
- 12.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City--turning the tide. N Engl J Med. 1995;333(4):229–33. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 13.Stroud LA, Tokars JI, Grieco MH, et al. Evaluation of infection control measures in preventing the nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis in a New York City hospital. Infect Control Hosp Epidemiol. 1995;16(3):141–7. doi: 10.1086/647075. [DOI] [PubMed] [Google Scholar]
- 14.Bock N, Jensen PA, Miller B, Nardell E. Tuberculosis Infection Control in Resource-limited Settings in the Era of Expanding HIV Care and Treatment. Journal of Infectious Disease. 2007 doi: 10.1086/518661. in press. Available online: www.cdc.gov/nchstp/od/gap/docs/6x9TB%20Booklet_1-4-07.pdf. [DOI] [PubMed]
- 15.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(17):1–141. [PubMed] [Google Scholar]
- 16.World Health Organization . Guidelines for the prevention of tuberculosis in health care facilities in resource-limited settings. WHO; Geneva: 1999. [Google Scholar]
- 17.Gandhi N, Moll A, Pawinski R, Zeller K, Lalloo U, Friedland G. Favorable outcomes of integration of TB and HIV treatment in a rural South Africa: The Sizonq'oba study. XVI International Conference on AIDS; Toronto: 2006. [DOI] [PubMed] [Google Scholar]
- 18.Andrews J. Clinical Predictors of Multidrug Resistance and Mortality Among Tuberculosis Patients in a Rural South African Hospital: A Case-Control Study. Yale University School of Medicine M.D. Thesis.; New Haven: 2007. [Google Scholar]
- 19.Weyer K, Lancastre J, Brand J, van der Walt M, Levin J. National survey of tuberculosis drug resistance in South Africa, 2001-2002. MRC; Pretoria: 2003. Available online: http://www.sahealthinfo.org/tb/natsurvey.pdf. [Google Scholar]
- 20.Vella V, Marra C, Maluleke M, Hlope H, Peer A, Tefera A. Surveillance of XDR and MDR at COSH, 2005-2006. KwaZulu-Natal Epidemiology Bulletin. 2007 Mar;:15. [Google Scholar]
- 21.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission. International Statistical Review. 1994;2:229–243. [Google Scholar]
- 22.Moll A, Gandhi N, Sturm AW, et al. XDR TB as Cause of Death in TB/HIV Coinfected Patients in Rural South Africa. (MOPE0181).. XVI International AIDS Conference; Toronto. 2006. [Google Scholar]
- 23.Rich ML. The PIH Guide to the Medical Management of Multidrug-Resistant Tuberculosis. Harvard University; Boston: 2003. [Google Scholar]
- 24.da Silva PA, Boffo MM, de Mattos IG, et al. Comparison of redox and D29 phage methods for detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis. Clin Microbiol Infect. 2006;12(3):293–6. doi: 10.1111/j.1469-0691.2005.01355.x. [DOI] [PubMed] [Google Scholar]
- 25.Cooksey RC, Morlock GP, Glickman S, Crawford JT. Evaluation of a line probe assay kit for characterization of rpoB mutations in rifampin-resistant Mycobacterium tuberculosis isolates from New York City. J Clin Microbiol. 1997;35(5):1281–3. doi: 10.1128/jcm.35.5.1281-1283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco-Alvarez de Luna F, Ruiz P, Gutierrez J, Casal M. Evaluation of the GenoType Mycobacteria Direct assay for detection of Mycobacterium tuberculosis complex and four atypical mycobacterial species in clinical samples. J Clin Microbiol. 2006;44(8):3025–7. doi: 10.1128/JCM.00068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355(15):1539–50. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iseman MD. A Clinician's Guide to Tuberculosis. Lippincott Williams & Wilkins; Hagerstown: 2000. [Google Scholar]
- 29.Riley RL, Wells WF, Mills CC, Nyka W, McLean RL. Air hygiene in tuberculosis: quantitative studies of infectivity and control in a pilot ward. Am Rev Tuberc. 1957;75(3):420–31. doi: 10.1164/artpd.1957.75.3.420. [DOI] [PubMed] [Google Scholar]
- 30.Singh JA, Upshur R, Padayatchi N. XDR-TB in South Africa: No Time for Denial or Complacency. PLoS Med. 2007;4(1):e50. doi: 10.1371/journal.pmed.0040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4(2):e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gammaitoni L, Nucci MC. Using a mathematical model to evaluate the efficacy of TB control measures. Emerg Infect Dis. 1997;3(3):335–42. doi: 10.3201/eid0303.970310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noakes CJ, Beggs CB, Sleigh PA, Kerr KG. Modelling the transmission of airborne infections in enclosed spaces. Epidemiol Infect. 2006;134(5):1082–91. doi: 10.1017/S0950268806005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. Aids. 2007;21(3):335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 35.Nardell EA, Keegan J, Cheney SA, Etkind SC. Airborne infection. Theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144(2):302–6. doi: 10.1164/ajrccm/144.2.302. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Leung G, Tang J, Yang X, Chao C. Role of ventilation in airborne transmission of infectious agents in the built environment—A multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 37.Biscotto CR, Pedroso ER, Starling CE, Roth VR. Evaluation of N95 respirator use as a tuberculosis control measure in a resource-limited setting. Int J Tuberc Lung Dis. 2005;9(5):545–9. [PubMed] [Google Scholar]
- 38.Fennelly KP. Personal respiratory protection against Mycobacterium tuberculosis. Clin Chest Med. 1997;18(1):1–17. doi: 10.1016/s0272-5231(05)70352-x. [DOI] [PubMed] [Google Scholar]
- 39.Fennelly KP. Personal respiratory protection and prevention of occupational tuberculosis. Int J Tuberc Lung Dis. 2005;9(5):476. [PubMed] [Google Scholar]
- 40.Espinal MA, Laserson K, Camacho M, et al. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberc Lung Dis. 2001;5(10):887–93. [PubMed] [Google Scholar]
- 41.World Health Organization . Report of the meeting of the WHO Global Task Force on XDR-TB. WHO; Geneva: 2006. [Google Scholar]
- 42.Beggs CB, Noakes CJ, Sleigh PA, Fletcher LA, Siddiqi K. The transmission of tuberculosis in confined spaces: an analytical review of alternative epidemiological models. Int J Tuberc Lung Dis. 2003;7(11):1015–26. [PubMed] [Google Scholar]
- 43.Weyer K. Transmission of Extensively Drug-resistant TB in South Africa and Implications for Infection Control in Health Care Settings.. Conference on Retroviruses and Opportunistic Infections; Los Angeles. 2007. [Google Scholar]
- 44.South Africa Department of Health . The KZN “outbreak”. DoH; Pretoria: 2006. Available online: http://www.doh.gov.za/docs/reports/2006/kzn/xdr.pdf. [Google Scholar]
- 1.Blower SM, Chou T. Modeling the emergence of the ‘hot zones’: tuberculosis and the amplification dynamics of drug resistance. Nat Med. 2004;10(10):1111–6. doi: 10.1038/nm1102. [DOI] [PubMed] [Google Scholar]
- 2.Blower SM, et al. The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med. 1995;1(8):815–21. doi: 10.1038/nm0895-815. [DOI] [PubMed] [Google Scholar]
- 3.Porco TC, Small PM, Blower SM. Amplification dynamics: predicting the effect of HIV on tuberculosis outbreaks. J Acquir Immune Defic Syndr. 2001;28(5):437–44. doi: 10.1097/00042560-200112150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Currie CS, et al. Tuberculosis epidemics driven by HIV: is prevention better than cure? Aids. 2003;17(17):2501–8. doi: 10.1097/01.aids.0000096903.73209.ac. [DOI] [PubMed] [Google Scholar]
- 5.Dye C, et al. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet. 1998;352(9144):1886–91. doi: 10.1016/s0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 6.Cohen T, et al. Beneficial and perverse effects of isoniazid preventive therapy for latent tuberculosis infection in HIV-tuberculosis coinfected populations. Proc Natl Acad Sci U S A. 2006;103(18):7042–7. doi: 10.1073/pnas.0600349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen T, Murray M. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat Med. 2004;10(10):1117–21. doi: 10.1038/nm1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gammaitoni L, Nucci MC. Using a mathematical model to evaluate the efficacy of TB control measures. Emerg Infect Dis. 1997;3(3):335–42. doi: 10.3201/eid0303.970310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterling TR, Lehmann HP, Frieden TR. Impact of DOTS compared with DOTS-plus on multidrug resistant tuberculosis and tuberculosis deaths: decision analysis. Bmj. 2003;326(7389):574. doi: 10.1136/bmj.326.7389.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley EC, Murphy G, Riley RL. Airborne spread of measles in a suburban elementary school. Am J Epidemiol. 1978;107(5):421–32. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- 11.Beggs CB, et al. The transmission of tuberculosis in confined spaces: an analytical review of alternative epidemiological models. Int J Tuberc Lung Dis. 2003;7(11):1015–26. [PubMed] [Google Scholar]
- 12.Noakes CJ, et al. Modelling the transmission of airborne infections in enclosed spaces. Epidemiol Infect. 2006;134(5):1082–91. doi: 10.1017/S0950268806005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues P, Gomes MG, Rebelo C. Drug resistance in tuberculosis--a reinfection model. Theor Popul Biol. 2007;71(2):196–212. doi: 10.1016/j.tpb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Dye C, Williams BG. Criteria for the control of drug-resistant tuberculosis. Proc Natl Acad Sci U S A. 2000;97(14):8180–5. doi: 10.1073/pnas.140102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee JS, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004;363(9407):474–81. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 16.Cohen T, et al. Exogenous re-infection and the dynamics of tuberculosis epidemics: local effects in a network model of transmission. J R Soc Interface. 2006 doi: 10.1098/rsif.2006.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams BG, et al. The impact of HIV/AIDS on the control of tuberculosis in India. Proc Natl Acad Sci U S A. 2005;102(27):9619–24. doi: 10.1073/pnas.0501615102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301(5639):1535–7. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi N, et al. High prevalence and mortality from extensively-drug resistant (XDR) TB in TB/HIV coinfected patients in rural South Africa. (THLB0210). XVI International AIDS Conference; Toronto. 2006. [Google Scholar]
- 20.Gandhi NR, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 21.South Africa Department of Health . The KZN “outbreak”. DoH; Pretoria: 2006. Available online: http://www.doh.gov.za/docs/reports/2006/kzn/xdr.pdf. [Google Scholar]
- 22.Weyer K. Transmission of Extensively Drug-resistant TB in South Africa and Implications for Infection Control in Health Care Settings. Conference on Retroviruses and Opportunistic Infections; Los Angeles. 2007. [Google Scholar]
- 23.Gandhi N, et al. Favorable outcomes of integration of TB and HIV treatment in a rural South Africa: The Sizonq'oba study. XVI International Conference on AIDS; Toronto: 2006. [Google Scholar]
- 24.Health Systems Trust . HIV incidence. HST; Durban: 2007. [Google Scholar]
- 25.van Rie A, et al. Reinfection and mixed infection cause changing Mycobacterium tuberculosis drug-resistance patterns. Am J Respir Crit Care Med. 2005;172(5):636–42. doi: 10.1164/rccm.200503-449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behr MA. Tuberculosis due to multiple strains: a concern for the patient? A concern for tuberculosis control? Am J Respir Crit Care Med. 2004;169(5):554–5. doi: 10.1164/rccm.2401001. [DOI] [PubMed] [Google Scholar]
- 27.Resch SC, et al. Cost-Effectiveness of Treating Multidrug-Resistant Tuberculosis. PLoS Med. 2006;3(7):e241. doi: 10.1371/journal.pmed.0030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escombe AR, et al. The Detection of Airborne Transmission of Tuberculosis from HIV-Infected Patients, Using an In Vivo Air Sampling Model. Clin Infect Dis. 2007;44(10):1349–57. doi: 10.1086/515397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riley RL, et al. Infectiousness of air from a tuberculosis ward. Am Rev Respir Dis. 1962;85:511–25. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 30.Nardell EA, et al. Airborne infection. Theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144(2):302–6. doi: 10.1164/ajrccm/144.2.302. [DOI] [PubMed] [Google Scholar]
- 31.Shmueli A, Sprung CL, Kaplan EH. Optimizing admissions to an intensive care unit. Health Care Manag Sci. 2003;6(3):131–6. doi: 10.1023/a:1024457800682. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EH, Craft DL, Wein LM. Emergency response to a smallpox attack: the case for mass vaccination. Proc Natl Acad Sci U S A. 2002;99(16):10935–40. doi: 10.1073/pnas.162282799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferry Tugela. Records of the Church of Scotland Hospital. KwaZulu-Natal; South Africa: 2007. [Google Scholar]
- 34.Andrews J. Clinical Predictors of Multidrug Resistance and Mortality Among Tuberculosis Patients in a Rural South African Hospital: A Case-Control Study. Yale University School of Medicine M.D. Thesis; New Haven: 2007. [Google Scholar]
- 35.Vella V, et al. Surveillance of XDR and MDR at COSH, 2005-2006. KwaZulu-Natal Epidemiology Bulletin. 2007 Mar;15 [Google Scholar]
- 36.Notice to Readers: Revised Definition of Extensively Drug-Resistant Tuberculosis. MMWR. 2006;55(43):11176. [Google Scholar]
- 37.Jacquez JA. Identifiability and Model Distinguishability, in Compartmental Analysis in Biology and Medicine. University of Michigan Press; Ann Arbor: 1985. [Google Scholar]
- 38.Kirkpatrick S, Gelatt CD, Vecchi MP. Optimization by Simulated Annealing. Science. 1983;220(4598):671–80. doi: 10.1126/science.220.4598.671. [DOI] [PubMed] [Google Scholar]
- 39.Zabinsky Z. Stochastic Adaptive Search for Global Optimization. Springer; New York: 2003. pp. 11–18. [Google Scholar]
- 40.Whalen CC. Failure of directly observed treatment for tuberculosis in Africa: a call for new approaches. Clin Infect Dis. 2006;42(7):1048–50. doi: 10.1086/501022. [DOI] [PubMed] [Google Scholar]
- 41.Horwitz S. Imaging HIV/AIDS. Wellcome Unit for the History of Medicine; Oxford: 2006. [Google Scholar]
- 42.Health Systems Trust . HIV prevalence (%) (total population) HST; Durban: 2007. [Google Scholar]
- 43.Gagneux S, et al. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312(5782):1944–6. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 44.Cohen T, Sommers B, Murray M. The effect of drug resistance on the fitness of Mycobacterium tuberculosis. Lancet Infect Dis. 2003;3(1):13–21. doi: 10.1016/s1473-3099(03)00483-3. [DOI] [PubMed] [Google Scholar]
- 45.Bock N, et al. Tuberculosis Infection Control in Resource-limited Settings in the Era of Expanding HIV Care and Treatment. Journal of Infectious Disease. 2007 doi: 10.1086/518661. (in press) [DOI] [PubMed] [Google Scholar]
- 46.Bock N, et al. Tuberculosis Infection Control in the Era of Expanding HIV Care and Treatment. WHO; Geneva: 2006. Available online: http://www.who.int/tb/publications/2006/tbhiv_infectioncontrol_addendum.pdf. [DOI] [PubMed] [Google Scholar]
- 47.Coffey CC, et al. Fitting characteristics of eighteen N95 filtering-facepiece respirators. J Occup Environ Hyg. 2004;1(4):262–71. doi: 10.1080/15459620490433799. [DOI] [PubMed] [Google Scholar]
- 48.Biscotto CR, et al. Evaluation of N95 respirator use as a tuberculosis control measure in a resource-limited setting. Int J Tuberc Lung Dis. 2005;9(5):545–9. [PubMed] [Google Scholar]
- 49.Lee K, Slavcev A, Nicas M. Respiratory protection against Mycobacterium tuberculosis: quantitative fit test outcomes for five type N95 filtering-facepiece respirators. J Occup Environ Hyg. 2004;1(1):22–8. doi: 10.1080/15459620490250026. [DOI] [PubMed] [Google Scholar]
- 50.Lawrence RB, et al. Comparison of performance of three different types of respiratory protection devices. J Occup Environ Hyg. 2006;3(9):465–74. doi: 10.1080/15459620600829211. [DOI] [PubMed] [Google Scholar]
- 51.Tokars JI, et al. Use and efficacy of tuberculosis infection control practices at hospitals with previous outbreaks of multidrug-resistant tuberculosis. Infect Control Hosp Epidemiol. 2001;22(7):449–55. doi: 10.1086/501933. [DOI] [PubMed] [Google Scholar]
- 52.LoBue P, Catanzaro A. Healthcare worker compliance with nosocomial tuberculosis control policies. Infect Control Hosp Epidemiol. 1999;20(9):623–4. doi: 10.1086/501684. [DOI] [PubMed] [Google Scholar]
- 53.Sutton PM, et al. Evaluating the control of tuberculosis among healthcare workers: adherence to CDC guidelines of three urban hospitals in California. Infect Control Hosp Epidemiol. 1998;19(7):487–93. doi: 10.1086/647851. [DOI] [PubMed] [Google Scholar]
- 54.Nicas M. Respiratory protection and the risk of Mycobacterium tuberculosis infection. Am J Ind Med. 1995;27(3):317–33. doi: 10.1002/ajim.4700270302. [DOI] [PubMed] [Google Scholar]
- 55.Barnhart S, et al. Tuberculosis in health care settings and the estimated benefits of engineering controls and respiratory protection. J Occup Environ Med. 1997;39(9):849–54. doi: 10.1097/00043764-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Menzies D, et al. Tuberculosis among health care workers. N Engl J Med. 1995;332(2):92–8. doi: 10.1056/NEJM199501123320206. [DOI] [PubMed] [Google Scholar]
- 57.Chen CC, Willeke K. Aerosol penetration through surgical masks. Am J Infect Control. 1992;20(4):177–84. doi: 10.1016/s0196-6553(05)80143-9. [DOI] [PubMed] [Google Scholar]
- 58.Sinkowitz RL, et al. Status of tuberculosis infection control programs at United States hospitals, 1989 to 1992. APIC. Association for Professionals in Infection Control and Epidemiology. Am J Infect Control. 1996;24(4):226–34. doi: 10.1016/s0196-6553(96)90054-1. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization . Report of the meeting of the WHO Global Task Force on XDR-TB. WHO; Geneva: 2006. [Google Scholar]
- 60.Menzies R, et al. Measuring ventilation of patient care areas in hospitals. Description of a new protocol. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1992–9. doi: 10.1164/ajrccm.152.6.8520767. [DOI] [PubMed] [Google Scholar]
- 61.Escombe AR, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4(2):e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noakes CJ, Beggs CB, Sleigh PA. Modelling the Performance of Upper Room Ultraviolet Germicidal Irradiation Devices in Ventilated Rooms: Comparison of Analytical and CFD Methods. Indoor and Built Envir. 2004;13(6):477–88. [Google Scholar]
- 63.Menzies D, et al. Effect of ultraviolet germicidal lights installed in office ventilation systems on workers’ health and wellbeing: double-blind multiple crossover trial. Lancet. 2003;362(9398):1785–91. doi: 10.1016/S0140-6736(03)14897-0. [DOI] [PubMed] [Google Scholar]
- 64.Miller SL, MacHer JM. Evaluation of a Methodology for Quantifying the Effect of Room Air Ultraviolet Germicidal Irradiation on Airborne Bacteria. Aerosol Science and Technology. 2000;33(3):274–95. [Google Scholar]
- 65.Xu P, et al. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-scale studies. Atmospheric Envir. 2003;37(3):405–19. [Google Scholar]
- 66.Ko G, et al. Estimation of tuberculosis risk and incidence under upper room ultraviolet germicidal irradiation in a waiting room in a hypothetical scenario. Risk Anal. 2001;21(4):657–73. doi: 10.1111/0272-4332.214142. [DOI] [PubMed] [Google Scholar]
- 67.Holtz TH, et al. Risk factors associated with default from multidrug-resistant tuberculosis treatment, South Africa, 1999-2001. Int J Tuberc Lung Dis. 2006;10(6):649–55. [PubMed] [Google Scholar]
- 68.Mitnick C, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348(2):119–28. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization . Guidelines for the programmatic management of drug-resistant tuberculosis. WHO; Geneva: 2006. [PubMed] [Google Scholar]
- 70.Fortún J, et al. Sputum conversion among patients with pulmonary tuberculosis: are there implications for removal of respiratory isolation? Journal of Antimicrobial Chemotherapy. 2007;59:794–8. doi: 10.1093/jac/dkm025. [DOI] [PubMed] [Google Scholar]
- 71.Moore DA, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355(15):1539–50. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Albert H, et al. Simple, phage-based (FASTPplaque) technology to determine rifampicin resistance of Mycobacterium tuberculosis directly from sputum. Int J Tuberc Lung Dis. 2004;8(9):1114–9. [PubMed] [Google Scholar]
- 73.Albert H, et al. Rapid indication of multidrug-resistant tuberculosis from liquid cultures using FASTPlaqueTB-RIF, a manual phage-based test. Int J Tuberc Lung Dis. 2002;6(6):523–8. doi: 10.5588/09640569513048. [DOI] [PubMed] [Google Scholar]
- 74.Butt T, et al. Rapid detection of rifampicin susceptibility of Mycobacterium tuberculosis in sputum specimens by mycobacteriophage assay. J Pak Med Assoc. 2004;54(7):379–82. [PubMed] [Google Scholar]
- 75.da Silva PA, et al. Comparison of redox and D29 phage methods for detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis. Clin Microbiol Infect. 2006;12(3):293–6. doi: 10.1111/j.1469-0691.2005.01355.x. [DOI] [PubMed] [Google Scholar]
- 76.Eltringham IJ, et al. Evaluation of reverse transcription-PCR and a bacteriophage-based assay for rapid phenotypic detection of rifampin resistance in clinical isolates of Mycobacterium tuberculosis. J Clin Microbiol. 1999;37(11):3524–7. doi: 10.1128/jcm.37.11.3524-3527.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eltringham IJ, Wilson SM, Drobniewski FA. Evaluation of a bacteriophage-based assay (phage amplified biologically assay) as a rapid screen for resistance to isoniazid, ethambutol, streptomycin, pyrazinamide, and ciprofloxacin among clinical isolates of Mycobacterium tuberculosis. J Clin Microbiol. 1999;37(11):3528–32. doi: 10.1128/jcm.37.11.3528-3532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McNerney R, et al. Rapid screening of Mycobacterium tuberculosis for susceptibility to rifampicin and streptomycin. Int J Tuberc Lung Dis. 2000;4(1):69–75. [PubMed] [Google Scholar]
- 79.Pai M, et al. Bacteriophage-based assays for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a meta-analysis. J Infect. 2005;51(3):175–87. doi: 10.1016/j.jinf.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 80.Simboli N, et al. In-house phage amplification assay is a sound alternative for detecting rifampin-resistant Mycobacterium tuberculosis in low-resource settings. Antimicrob Agents Chemother. 2005;49(1):425–7. doi: 10.1128/AAC.49.1.425-427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watterson SA, et al. Comparison of three molecular assays for rapid detection of rifampin resistance in Mycobacterium tuberculosis. J Clin Microbiol. 1998;36(7):1969–73. doi: 10.1128/jcm.36.7.1969-1973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yzquierdo SL, et al. Evaluation of phage assay for rapid phenotypic detection of rifampicin resistance in Mycobacterium tuberculosis. Ann Clin Microbiol Antimicrob. 2006;5:11. doi: 10.1186/1476-0711-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skenders G, et al. Multidrug-resistant tuberculosis detection, Latvia. Emerg Infect Dis. 2005;11(9):1461–3. doi: 10.3201/eid1109.041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirano K, Abe C, Takahashi M. Mutations in the rpoB gene of rifampin-resistant Mycobacterium tuberculosis strains isolated mostly in Asian countries and their rapid detection by line probe assay. J Clin Microbiol. 1999;37(8):2663–6. doi: 10.1128/jcm.37.8.2663-2666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cooksey RC, et al. Evaluation of a line probe assay kit for characterization of rpoB mutations in rifampin-resistant Mycobacterium tuberculosis isolates from New York City. J Clin Microbiol. 1997;35(5):1281–3. doi: 10.1128/jcm.35.5.1281-1283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Franco-Alvarez de Luna F, et al. Evaluation of the GenoType Mycobacteria Direct assay for detection of Mycobacterium tuberculosis complex and four atypical mycobacterial species in clinical samples. J Clin Microbiol. 2006;44(8):3025–7. doi: 10.1128/JCM.00068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission. International Statistical Review. 1994:229–243. [Google Scholar]
- 88.Stein M. Large sample properties of simulations using Latin Hypercube Sampling. Technometrics. 1987;29(2):143–51. [Google Scholar]
- 89.World Health Organization . Country data: South Africa. WHO; Geneva: 2007. [Google Scholar]
- 90.Fortun J, et al. Sputum conversion among patients with pulmonary tuberculosis: are there implications for removal of respiratory isolation? J Antimicrob Chemother. 2007;59(4):794–8. doi: 10.1093/jac/dkm025. [DOI] [PubMed] [Google Scholar]
- 91.Holtz TH, et al. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med. 2006;144(9):650–9. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 92.Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119(2):183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warren RM, et al. Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med. 2004;169(5):610–4. doi: 10.1164/rccm.200305-714OC. [DOI] [PubMed] [Google Scholar]
- 94.Espinal MA, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. Jama. 2000;283(19):2537–45. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 95.Kawai V, et al. Tuberculosis mortality, drug resistance, and infectiousness in patients with and without HIV infection in Peru. Am J Trop Med Hyg. 2006;75(6):1027–33. [PMC free article] [PubMed] [Google Scholar]
- 96.Leimane V. MDR-TB and XDR-TB: Management and Treatment Outcomes in Latvia. 37th Union World Conference on Lung Health; Paris: 2006. [Google Scholar]
- 97.van der Walt M, Weyre K, Lancastre J. South Africa Medical Research Council Expert Consultation on Drug-Resistant Tuberculosis. Johannesburg: 2006. A standardised approach to management of drug-resistant TB in South Africa. [Google Scholar]
- 98.Weyer K, et al. National survey of tuberculosis drug resistance in South Africa, 2001-2002. MRC; Pretoria: 2003. Available online: http://www.sahealthinfo.org/tb/natsurvey.pdf. [Google Scholar]