Abstract
The use of placebo as a control condition in clinical trials of major depressive disorder and anxiety disorders continues to be an area of ethical concern. Typically, opponents of placebo controls argue that they violate the beneficent-based, “best proven diagnostic and therapeutic method” that the original Helsinki Declaration of 1964 famously asserted participants are owed. A more consequentialist, oppositional argument is that participants receiving placebo might suffer enormously by being deprived of their usual medication(s). Nevertheless, recent findings of potential for suicidality in young people treated with antidepressants, along with meta-analyses suggesting that antidepressants add no significant clinical benefit over placebos, warrant a re-evaluation of the arguments against placebo. Furthermore, the nature of placebo treatment in short-term clinical trials is often not well understood, and lack of understanding can foster opposition to it. This paper will show how scientific justifications for placebo use are morally relevant. The fundamental ethical importance of placebo controls is discussed in relation to several aspects of clinical trials, including detection of adverse events, accurate assessment of clinical benefit, advancing understanding of the heterogeneity of depression and anxiety disorders and respecting informed consent requirements. Prohibiting the use of placebo controls is morally concerning in that such prohibitions allow for the possibility of serious adverse public health consequences. Moral worries that research participants receiving placebo are being unduly jeopardised will be shown to be exaggerated, especially in relation to the net benefits for end-users to be gained from the quality of data resulting from using placebo controls.
Major depressive disorder (MDD) and anxiety disorders occasion the highest number of prescriptions in the United States—118 million in 2005.1 Approximately 1 in 5 adults and 1 in 12 adolescents are expected to experience at least one major depressive episode, and roughly 1 in 3 adults will develop one or more anxiety disorders.2,3 The enormity of this suffering and its associated market-place has led to the steady appearance of new antidepressant and anxiolytic medications, resulting in continuing controversies about their efficacy and side effects.
While randomised placebo-controlled trials have generally been considered the gold standard for evaluating the safety and efficacy of new medications, their implementation remains controversial. Placebo critics such as Rothman and Michels have complained that participants in such trials are owed the best proven treatment, not a biologically inert compound.4 They are distressed over the possibility that many participants entering the trial's placebo arm might experience harm by being deprived of their usual medications—a worry that might be especially pronounced among patients with MDD or anxiety disorders. Nevertheless, we will make the case that under certain trial conditions, these worries over placebo use are unfounded and fail to appreciate various benefits accruing from such trials, whose net utility, we argue, morally outweighs the benefits of trials using biologically active controls. We will advance the argument that good science is itself a morally relevant concern, and that placebo opponents often overlook how problematic the findings from equivalency trials in MDD can be, resulting in their introducing precisely the welfare risks to a drug's end-users that good science is supposed to prevent.
LEADING ARGUMENTS REGARDING THE USE OF PLACEBO CONTROLS IN MOOD AND ANXIETY DISORDERS
Some experts in clinical research into mood and anxiety disorders have maintained that placebos are a necessary comparison group for evaluating new treatments. Committees comprising researchers and bioethicists representing the National Depressive and Manic-Depressive Association in the USA and the Fourth European Expert Forum on Ethical Evaluation of Placebo-Controlled Studies in Depression maintain that the efficacy and side effect profile of any new drug can be confidently determined only if the drug is compared with an inert substance.5,6 Significantly, the US Food and Drug Administration (FDA) continues to require that new treatments demonstrate greater efficacy than placebo for these conditions.7
Arguments favouring placebo controls characterise these trials' data as having greater ability to distinguish between effective and ineffective treatments (that is, greater assay sensitivity). A second, related argument is that if an active control was itself never evaluated in a placebo-controlled trial, using it in an equivalency study begs the question of its efficacy.8 A third argument is that the ethical concerns about placebo use should revolve around the issue of risk to participants, rather than around denial of treatment, and that in the absence of a significant risk of harm, placebo treatment is acceptable. A fourth argument is that it is generally easier to achieve statistical significance in placebo-controlled trials, where effects tend to be larger, such that smaller numbers of participants need to be exposed to the investigational medication and research costs are lower.9
Opponents of placebo controls, however, have repeatedly argued against their use, primarily on ethical grounds.4,10–13 The primary ethical argument rests on article II.3 in the original Declaration of Helsinki, which asserts that every patient participating in a medical study (including patients assigned to a control condition) “should be assured of the best proven diagnostic and therapeutic method”.14 Because there are several medications proven to be more effective than placebo in the treatment of depression and anxiety, some ethicists have argued that placebo treatment is equivalent to withholding treatment, or assert that it constitutes essentially no treatment.13 Others have argued that placebo use can induce an unreasonable degree of suffering among study participants who, because of placebo orthodoxy, are denied conventional treatments that would relieve their discomfort.11 Others point to the possibility of inflicting both physical harm from placebo use (primarily suicide in depression and anxiety trials) and also social harms, such as damage to employment and relationships, and mental anguish.15 Notably, the most recent (2008) amended version of the Declaration of Helsinki permits the use of placebo when it “is necessary to determine the efficacy or safety of an intervention and the patients who receive placebo or no treatment will not be subject to any risk of serious or irreversible harm”.16
In a widely referenced article, Emanuel and Miller attempted to find a middle ground between the poles of placebo orthodoxy and its categorical prohibition.15 They suggest that three criteria need to be met in order to ethically justify the use of a placebo control: that the medical condition (1) has a high placebo response rate, (2) is characterised by a waxing-and-waning course or frequent spontaneous remissions and (3) is treated with existing therapies that are only partly effective or have very serious side effects. Emanuel and Miller condone placebo use as long as the patients' underlying condition is not unnecessarily exacerbated by discontinuing their medication (or if the medication does not appear sufficiently efficacious), such that placebo is not necessarily a worse therapeutic option. What we will argue below, however, is that precisely the conditions that Emanuel and Miller envision in favouring placebo occur more often among depressed or anxious patients entering psychopharmacologic trials than is usually appreciated. Furthermore, we will highlight some recent findings and offer additional considerations indicating how a greater appreciation of the scientific acceptability of placebo controls speak to the ethical concerns raised above.
SALIENT ASPECTS OF MAJOR DEPRESSIVE DISORDER AND ANXIETY DISORDERS
The illnesses of MDD and the anxiety disorders have unique characteristics in comparison with more classic “medical” disorders. There is currently no physiological test that can definitively prove that these disorders are present or absent, and their severity varies widely from person to person. Although the term major depressive disorder implies severe clinical depression, the criteria for the disorder require only five depressive symptoms that persist for at least 2 weeks and cause some level of distress to the individual or impact the individual's function in some way. For example, MDD may be diagnosed when patients with five depressive symptoms are bothered by a lack of desire to socialise with friends, or if trouble concentrating is reducing their work productivity. At the other end of the spectrum, the sickest MDD patients may become frankly psychotic or experience a total absence of volition, so that they no longer eat and barely move. Thus, the diagnosis of MDD is qualified as mild, moderate or severe, in order to reflect the level of distress or impairment.17 Anxiety disorders similarly vary widely in their severity and impairment.
Further complicating the clinical picture is that patients may improve with medication, psychotherapy, a combination of the two, or simply with the passage of time. Despite substantial evidence showing the efficacy of certain medications for MDD and anxiety among study populations generally, psychiatrists currently have no way to predict which specific treatment modality a particular patient requires in order to improve. These imponderables demand that treatment for these conditions be informed by data from trials that, as we argue below, often require placebo controls.
ETHICAL IMPORTANCE OF PLACEBO CONTROLS
Detection of adverse events
In October 2004, the FDA issued a public health advisory for all marketed antidepressants, warning that they may increase the risk of suicidal thoughts and self-directed harm.18 This advisory emerged from an analysis of suicide-related phenomena reported in children with either MDD or an anxiety disorder who participated in placebo-controlled trials for these conditions. Children receiving the active medications had a small but statistically significant greater rate of self-directed harm behaviours than children receiving placebo. Subsequent re-analyses of this data set and others identified that the risk was present in people up to age 24 years, and the black-box warnings on the package inserts were modified to reflect this risk.19 This finding of increased risk for potentially serious harm with antidepressant treatments would not have been detected if the phase II and III studies included in the FDA's review had been conducted with an equivalency design, in which an established drug rather than placebo is used as the comparator or control. If all antidepressants, through some aspect of their mechanism, place some depressed subjects at risk for a temporary increase in self-harm, that risk could not be reliably detected without the presence of a placebo-treated control group. Fortunately, suicide is rare in randomised trials, but it is this rarity that makes it very difficult to detect through other research designs in which multiple confounding factors may be present. Indeed, there is no certainty that these medications do not also cause other rare but important adverse events, which may be detectable only through comparison with a placebo arm.
An additional concern involving adverse events is the ability to distinguish between symptoms arising from the illness itself and side effects arising from the new treatment. For example, insomnia is a very common symptom of both depression and anxiety, and antidepressant and anti-anxiety medications can affect the neurobiology of sleep regulation.20 If we wonder whether a new medication is causing insomnia, comparing two active medications in a clinical trial cannot answer that question, as the contribution of the disease under study cannot be isolated in such a design. Only a placebo-treated control group can provide an accurate measure of the insomnia-inducing nature of the medication being tested.
One might counterargue, though, that determining whether or not a condition such as insomnia is an effect of a drug or the underlying disease is less morally pressing than maintaining the participant on a medication with proven efficacy. In other words, might the gravity of the patient's potential suffering during treatment with placebo morally override the value of knowledge gained in our example of using placebo to identify the source of the a symptom (ie, whether from the disease or from the drug)? To this question, we have two replies.
The first is that Phase II and III clinical trials of new treatments for MDD or anxiety disorders typically last for 6 to 8 weeks. During that period, participants meet with the study physician weekly in the early stages of the trial and then usually schedule visits every 2 weeks during the latter half of the trial. This frequency of physician contact greatly exceeds that which patients treated in a typical outpatient practice receive and provides substantial protection against undetected worsening of the participants' mental health. In contrast, a patient in a private practice may go 4 or 6 weeks between physician visits when starting a medication, which most clinicians would recognise as inherently more risky than systematised and frequent follow-up while on placebo. Consequently, we do not believe that these maleficent-based arguments against placebo controls are convincing, especially if the trials involve patients who are not severely ill.
Our second reply is that the therapeutic value of an active comparator may be dramatically over-rated. For example, in an influential paper that decried placebo use in bisphosponates trials for the prevention of osteoporosis, Benjamin Freedman and his colleagues wrote, “It is distressing to note that placebo-controlled studies of osteoporosis prophylaxis are still underway in North America. These studies will likely result in real harm to patients assigned to placebo …” (p248).10 The authors assumed the benefits of the current treatment, oestrogen replacement, to be so certain that only equivalency studies of bisphosphonates versus oestrogen should be performed. But, as is now widely known, a later large, randomised placebo-controlled clinical trial of oestrogen versus placebo demonstrated that oestrogen causes higher rates of stroke, blood clots and breast cancer than placebo, and the combination of oestrogen and progesterone is even more harmful.21 If bisphosphonates caused the same kind of adverse events, an equivalency trial of bisphosphonates and oestrogen would have been unlikely to detect these harmful effects, because of their use in both treatment arms. This example underscores the harms stemming from overconfidence in our presumed knowledge of all the biological effects of our existing treatments. Placebo controls sometimes serve as our strongest safeguard against such hubris.
Uncertainty of benefit
The second issue demonstrating the need for placebo controls is that of confidently assessing a drug's degree of efficacy. A recent meta-analytic study that received worldwide attention concluded that there was no clinically meaningful difference in outcome between depressed patients receiving active (and subsequently FDA-approved) antidepressant medication for MDD and those receiving placebo.22 Although there are methodological concerns about the design of this analysis, the findings nevertheless build on other meta-analytic studies suggesting that patients with mild-to-moderate forms of MDD or an anxiety disorder are less likely than sicker patients to demonstrate greater improvement with active medication than with placebo treatment.23–25 A major reason for this is the placebo effect, which presents a tremendous methodological challenge.
The tendency of certain patients to improve while on placebo treatment is a serious confounder in the design of drug trials for new treatments.26 It is altogether common, for instance, that the symptoms of the illness recede during brief periods of the illness when hope is restored, stress relieved and some “action” taken. But participating in a clinical trial is precisely such a situational opportunity for the placebo effect to occur. In fact, about half of all randomised trials of antidepressants and anxiolytics versus placebo fail to demonstrate superiority for the active medication.27,28 These findings demonstrate that receipt of placebo during participation in a clinical trial is hardly equivalent to receiving “no treatment”. From a methodological perspective, these results also reflect the great value of using a placebo control to maintain the blind in phase II and III trials, in that they indicate that clinical trial personnel are not “seeing through the blind” and, through therapeutic enthusiasm or for other reasons, not biasing results in favour of the experimental medication.i
This frequent failure of active drugs to demonstrate superiority over placebo has disturbing implications for equivalence trials comparing an established with an experimental medication, because the placebo effect increases the likelihood that the medications will be found to be equally beneficial. In such trials, it is impossible to know whether this benefit stems from the biological action of the compounds or from non-specific placebo effects. Indeed, one would expect an even greater placebo effect in an equivalency research design, because the participants' hope is in no way tempered by the thought that they might receive placebo. The ethical implications of this uncertainty in equivalency trials are obvious: medications that are actually no better than placebo could be approved for clinical use.
Heterogeneity of major depressive disorder and anxiety disorders
Another confounding factor in the study of MDD and anxiety is that these disorders are not homogeneous diseases. Their underlying biologies are poorly understood, and it is widely believed that the current classification of MDD and anxiety disorders is not congruent with the underlying biological pathways by which people become depressed or anxious.29 But if the purpose of research is to move psychiatry to the point where the drug selected for any particular patient is based specifically on the biology of their illness—rather than the current practice, in which the drug selected is based primarily on non-biological factors (eg, cost or the physician's “hunch”)—researchers must be able to separately isolate the effects of time, improvement and treatment, using techniques such as metabolomics, pharmacogenomics and functional neuroimaging. Resolving these questions is greatly hampered when the non-specific biological changes induced by placebo effects are not controlled.
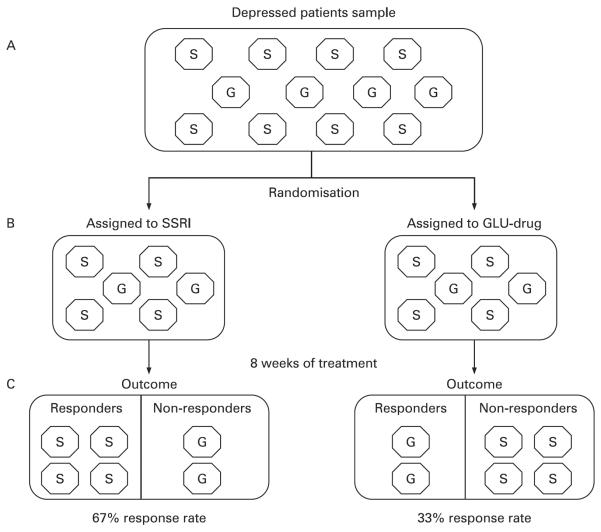
The currently existing drugs help approximately one-third of patients achieve remission and another third to achieve a good response (that falls short of a full remission of symptoms).30,31 So, if we consider “good response” to be a marker of efficacy, then about two-thirds of patients significantly benefit from existing drugs. But suppose a new drug was developed that was effective in the one-third who do not respond and it was compared against a conventional drug (eg, an SSRI) in an equivalency study. In a head-to-head trial without placebo control, the new drug will always appear to be ineffective, because it will only help one-third of participants. The more common form of MDD, represented by two-thirds of the study participants, will respond better to the comparator antidepressant, but this will leave the one-third who are resistant to existing treatments out of luck, even though that proportion of them in the study who received the experimental drug benefited. This scenario is represented pictorially in fig 1. The moral implications of this phenomenon are that insisting on an active comparator control is itself an implicit moral choice: by protecting the rights of the majority of participants (ie, SSRI-responders) to receive a proven treatment, the minority of participants (ie, glutamate responders) are prevented from reaping lasting benefit from their contribution to the study, because the experimental glutamate drug will be considered “ineffective” and will not be brought to market. Although it would be possible to design an equivalency trial comparing the experimental drug with a different type of antidepressant, in which only patients who did not respond to an SSRI could be enrolled, the same previously mentioned problems of undetected placebo response and unblinding could still produce false-positive results of efficacy for the experimental compound.
Figure 1.
Equivalency trial comparing a proven antidepressant (a selective serotonin reuptake inhibitor; SSRI) versus an investigational drug that acts through glutamate mechanisms (GLU-drug). (A) The sample of patients entering the study. Each octagon represents one person with depression. For simplicity, the figure supposes that there are two biological subtypes of depression (which cannot be clinically separated by any existing test): one due to altered serotonin signalling, identified by an “S” in the octagon, the other due to disrupted glutamate signaling, marked by a “G” in the octagon. Assume the serotonin form of depression is twice as common as the glutamate form. Also assume that SSRIs are fully effective in treating serotonin depressions and the GLU-drug is fully effective in treating glutamate depressions. (B) Through randomisation, equal proportions of each subtype of depression are assigned to treatment with the two drugs. (C) After 8 weeks of treatment, all serotonin-type depressions treated with SSRI have responded, and all glutamate-type depressions treated with GLU-drug have responded. Because the serotonin form of depression is more common than the glutamate form, there is a higher response rate in the SSRI treatment arm than the GLU-drug treatment arm. The conclusion from this equivalency trial design would be that the GLU-drug was ineffective, and it would not be approved, even though it is the better drug for the minority of patients who have the less common biological subtype of depression.
Informed consent with placebos
It is widely accepted that the well-informed consent of participants remains a bedrock ethical principle for clinical trial participation. Participants choosing to enrol in placebo-controlled trials must fully understand the nature and purpose of the treatments used in the study. Most importantly, they need to understand that the type of pill they receive in the study will be selected randomly, without consideration of any unique aspect of their situation. Participants need also to understand what a placebo is and why it is being used in the study. They must understand that they are free to withdraw at any time from the study.
Securing a robust informed consent from participants who receive placebo notwithstanding, it remains debatable whether a placebo control should be used among patients who have severe MDD, in which the quality of their lives is seriously at risk, given their demonstrated lower response to placebo. Indeed, patients with high levels of suicidality, or whose functioning is so disrupted that they are unable to complete the trial procedures, are routinely excluded from placebo-controlled trials.
In patients with severe MDD, we might instead consider using double-blind discontinuation trials, in which all subjects initially receive open-label treatment, with responders to the medication being randomly assigned at some later time to either switch to placebo or continue on the study medication. Because the potential harm consists in taking away an effective treatment, participants in this kind of trial would need to be exquisitely informed and to indicate, perhaps through a teach-back intervention, that they had a good level of comprehension of the risks inherent in replacing an active drug with placebo.
CONCLUSIONS
Psychiatry's gains in treating patients with mood and anxiety disorders can distract our attention from the fact that our knowledge remains undeveloped, if not quite poor, when it comes to understanding the basic underlying pathology of those disorders. We do not know whether our existing treatments are correcting a disrupted core biological process or are inducing modifications in other biological systems that in some way compensate for that core disruption. Given our general state of ignorance, placebos make vital contributions to understanding the biology of illnesses, the biology of treatment response and the naturalistic course of these illnesses. These understandings obviously have moral ramifications in improved treatments, better informed health professionals and their patients and more rational consumption decisions among drug purchasers.
The questions raised by the new findings regarding the safety and efficacy of existing antidepressant or anti-anxiety medications ethically justify the continued use of placebo controls. We contend that moral worries about physical suffering and other serious harms are exaggerated relative to the likelihood that such harms might materialise in trials for MDD and anxiety. For example, the largest clinical trial of MDD treatments yet performed demonstrated that the average duration of the participants' current depressive episode before entering the study was more than 2 years.32 It is highly unlikely that an additional 6 to 8 weeks on a placebo treatment will produce new, significant social harms via forces that have long been active in the person's life, even assuming the placebo is completely ineffective. In contrast, the risk to the public health from marketing ineffective or deleterious treatments that resulted from non-placebo-controlled trials is much more morally concerning and warrants greater protection.
Our valorising placebo-controlled trials hinges on a trade-off between (1) the research participants' forgoing treatment in a placebo arm of the trial and possibly experiencing discomfort and (2) putting a new drug on the market that may be inferior or perhaps carry greater risk than conventional treatment. Throughout this essay, our perspective is very much along the lines of Brendel and Miller's recent plea for pragmatism in clinical research ethics, in which they assert that inferior data resulting from an excessively earnest attempt to protect the welfare of research subjects is itself an ethically relevant and questionable choice. They remark that
A pragmatic perspective on clinical research ethics counteracts a one-sided tendency to see ethics in this domain as only a matter of protecting human subjects, without giving due consideration to the ethical significance of scientific investigation … Since both promoting science and protecting research subjects have ethical significance, it is misleading to describe ethically controversial research as a case of science versus ethics. To be sure, valuable research may exploit subjects or expose them to undue risks. Nevertheless, determining whether a clinical study is ethical requires a careful weighing and balancing of the two, potentially competing objectives of clinical research ethics. (Brendel and Miller,33 p30)
One of the greatest ethical transgressions in science is to perform substandard research that is not adequately designed to answer the research question under study. Exposing participants in trials to uncertain risks (as occurs in trials of new medications), without the ability to provide a clear answer to whether the medication is safe and effective, dishonours the contribution of the volunteer. For MDD and anxiety disorders, placebo-controlled studies provide the best protection against this kind of error. Abandoning the use of placebo controls for these conditions would result in less certainty about the safety, efficacy and validity of results derived from phase II and III studies. Given the current state of our scientific understanding, the use of placebo controls in these trials is ethically justified for illnesses such as MDD and anxiety disorders that do not pose a high risk of mortality or permanent morbidity.
Acknowledgements
The authors would like to thank the two reviewers, one of whom was Paul Biegler, for their insightful commentary on the manuscript
Funding: Dr Dunlop is supported (in part) by a K12 grant from the National Institutes of Health National Center for Research Resources, K12 RR 017643 and 1KL2RR025009. Dr Banja is supported in part by the Atlanta Clinical and Translational Science Institute award UL1RR025008 from the National Institutes of Health.
Footnotes
Competing interests: Dr Dunlop receives research support from AstraZeneca, GSK, Novartis, ONO Pharmaceuticals and Takeda. He has served as a consultant to BMS and Wyeth and has received honoraria from BMS. Dr Banja reports no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed
Studies using an active comparator with which physicians are well experienced will often yield adverse-event profiles among participants that are characteristic of the drug and easily recognised. For example, in the case of selective serotonin reuptake inhibitors (SSRIs), the standard response profile of early reduction in irritability is often accompanied by nausea, headache or sexual dysfunction—thus providing information that can easily unblind the researcher. Therefore, for an experimental medication for which the adverse-event data are relatively sparse, and with which the investigator has no previous experience, blinding is stronger when the comparison is with placebo than with an active comparator.
REFERENCES
- 1.National Center for Health Statistics . Health, United States, 2007. Public Health Service; Hyattsville, Maryland: 2007. p. 335. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Birmaher B, Ryan ND, Williamson DE, et al. Childhood and adolescent depression: a review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry. 1996;35:1427–39. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Rothman KJ, Michels KB. The continuing unethical use of placebo controls. N Engl J Med. 1994;331:394–8. doi: 10.1056/NEJM199408113310611. [DOI] [PubMed] [Google Scholar]
- 5.Charney DS, Nemeroff CB, Lewis L, et al. National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch Gen Psychiatry. 2002;59:262–70. doi: 10.1001/archpsyc.59.3.262. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin D, Broich K, Fritze J, et al. Placebo-controlled studies in depression: necessary, ethical and feasible. Eur Arch Psychiatry Clin Neurosci. 2003;253:22–8. doi: 10.1007/s00406-003-0400-2. [DOI] [PubMed] [Google Scholar]
- 7.Laughren TP. The scientifc and ethical basis for placebo-controlled trials in depression and schizophrenia: an FDA perspective. Eur Psychiatry. 2001;16:418–23. doi: 10.1016/s0924-9338(01)00600-9. [DOI] [PubMed] [Google Scholar]
- 8.Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part I: Ethical and scientific issues. Annals Intern Med. 2000;133:455–63. doi: 10.7326/0003-4819-133-6-200009190-00014. [DOI] [PubMed] [Google Scholar]
- 9.Miller FG, Brody H. What makes placebo-controlled trials unethical? Am J Bioeth. 2002;2(2):3–9. doi: 10.1162/152651602317533523. [DOI] [PubMed] [Google Scholar]
- 10.Freedman B, Glass KC, Weijer C. Placebo orthodoxy in clinical research I: Empirical and methodological myths. J Law Med Ethics. 1996;24:243–51. doi: 10.1111/j.1748-720x.1996.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 11.Freedman B, Glass KC, Weijer C. Placebo orthodoxy in clinical research II: Ethical, legal and regulatory myths. J Law Med Ethics. 1996;24:252–9. doi: 10.1111/j.1748-720x.1996.tb01860.x. [DOI] [PubMed] [Google Scholar]
- 12.Michels KB, Rothman KJ. Update on unethical use of placebos in randomized trials. Bioethics. 2003;17:188–204. doi: 10.1111/1467-8519.00332. [DOI] [PubMed] [Google Scholar]
- 13.Friend WC, Weijer C. Re: CCNP position on the use of placebos in psychiatry [comment] J Psychiatry Neurosci. 1996;21:354–6. Comment on: J Psychiatry Neurosci 1996;21:354-9. [PMC free article] [PubMed] [Google Scholar]
- 14.World Medical Organization Declaration of Helsinki. BMJ. 1996;313:1448–9. [Google Scholar]
- 15.Emanuel EJ, Miller FG. The ethics of placebo-controlled trials—a middle ground. N Engl J Med. 2001;345:915–9. doi: 10.1056/NEJM200109203451211. [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki. Adopted June 1964 and last amended by the WMA General Assembly, Seoul, South Korea, October 2008. [(accessed 13 Apr 2009)];World Med J. 2008 54:122–5. http://www.wma.net/e/policy/pdf/17c.pdf. [Google Scholar]
- 17.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edn American Psychiatric Association; Washington, DC: 1994. pp. 339–45. [Google Scholar]
- 18.US Food and Drug Administration [(accessed 20 Apr 2009)];Worsening depression and suicidality in patients being treated with antidepressant. 2004 Mar 22; http://www.fda.gov/cder/drug/antidepressants/SSRIPHA200410.htm.
- 19.US Food and Drug Administration [(accessed 13 Apr 2009)];FDA proposes new warnings about suicidal thinking, behavior in young adults who take antidepressant medications. 2007 http://www.fda.gov/bbs/topics/NEWS/2007/NEW01624.html.
- 20.Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depres Anxiety. 2001;14:19–28. doi: 10.1002/da.1043. [DOI] [PubMed] [Google Scholar]
- 21.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348:645–50. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 22.Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:260–8. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan A, Leventhal R, Khan S, et al. Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol. 2002;22:40–5. doi: 10.1097/00004714-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Angst J. Severity of depression and benzodiazepine comedication in relationship to efficacy of antidepressants in acute trials: a meta-analysis of moclobemide trials. Hum Psychopharmacol. 1993;8:401–7. [Google Scholar]
- 25.Stein DJ, Baldwin DS, Dolberg OT, et al. Which factors predict placebo response in anxiety disorders and major depression? An analysis of placebo-controlled studies of escitalopram. J Clin Psychiatry. 2006;67:1741–6. doi: 10.4088/jcp.v67n1111. [DOI] [PubMed] [Google Scholar]
- 26.Andrews G. Placebo response in depression: bane of research, boon to therapy. Br J Psychiatry. 2001;178:192–4. doi: 10.1192/bjp.178.3.192. [DOI] [PubMed] [Google Scholar]
- 27.Khan A, Khan SR, Walens G, et al. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the food and drug administration summary basis of approval reports. Neuropsychopharmacology. 2003;28:552–7. doi: 10.1038/sj.npp.1300059. [DOI] [PubMed] [Google Scholar]
- 28.Walsh B, Seidman SN, Sysko R, et al. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–7. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 29.Hasler G, Drevets WC, Manji HK, et al. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–81. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association Practice guidelines for major depressive disorder in adults. Am J Psychiatry. 2000;157(Suppl 4):1–45. [PubMed] [Google Scholar]
- 31.Bandelow B, Zohar J, Hollander E, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders—first revision. World J Biol Psychiatry. 2008;9:248–312. doi: 10.1080/15622970802465807. [DOI] [PubMed] [Google Scholar]
- 32.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 33.Brendel DH, Miller FG. A plea for pragmatism in clinical research ethics. Am J Bioeth. 2008;8:24–31. doi: 10.1080/15265160802166025. [DOI] [PubMed] [Google Scholar]