Abstract
Osteoarthritis (OA) is the most common form of arthritis and is a major cause of chronic pain and disability. We currently lack disease-modifying OA medical therapeutics that effectively slow or halt the progression to destruction and failure of articular cartilage. Importantly, OA is a disease of the whole joint, including not only meniscal fibrocartilage and hyaline articular cartilage, but also subchondral bone, periarticular musculature, tendons and ligaments, articular adipose tissue, synovium, and synovial fluid (SF). Clinically, varying degrees of synovitis and joint effusion in OA contribute to signs and symptoms of inflammation (1). Multiple lines of evidence suggest that OA progression is promoted by low-grade innate articular inflammation and by synovitis (1,2). “Conventional” inflammatory cytokines expressed in cartilage and synovium likely play a role, and interleukin-1β (IL-1β), tumor necrosis factor α (TNFα), IL-6, IL-8, and IL-17 are among the players in synovitis (1,2). The report by Nair et al in this issue of Arthritis & Rheumatism reveals increased levels of soluble CD14 (sCD14) in SF to be a biomarker of innate inflammation in patients undergoing arthroscopic knee meniscectomy for treatment of meniscal tears (3). Investigators in this group previously characterized this population as “enriched for patients with preradiographic disease” (4), given the associated symptoms, synovitis, and evidence of articular cartilage damage detected by arthroscopy.
Soluble CD14 as a biomarker of inflammation
CD14 is one member of a large network of receptors and danger signals that functions in primordial innate immunity not only to support host defense (via recognition and responses to pathogen-associated molecular patterns [PAMPs]), but also to modulate multiple forms of tissue injury and repair in response to endogenous damage-associated molecular patterns (DAMPs). CD14 is anchored by glycosyl phosphatidylinositol on the cell surface and is a multifunctional adaptor protein and a coreceptor with several Toll-like receptors (TLRs), including cell surface TLR-2 and TLR-4 and intracellular TLR-3, TLR-7, and TLR-9. CD14 is believed to act both as a transporter of ligands and as a signal amplifier by moving certain TLRs, including TLR-2 and TLR-4, into kinase-rich environments of lipid raft microdomains, where they associate with Src family kinases and certain G proteins (5). Such delivery of ligands to their appropriate TLRs may stabilize the TLR–CD14 complexes (5).
In human serum, sCD14 is present in substantial quantities as a soluble protein (released from its glycolipid anchorage on the plasma membrane) (5). CD14 shedding is associated with monocyte/macrophage lineage cell activation, and there is evidence for elevated serum sCD14 being both an acute-phase reactant and a serum biomarker of monocyte activation and inflammation in a variety of conditions, including rheumatoid arthritis (RA), reactive arthritis, Kawasaki disease, and pneumonia.
Nair et al discovered the concentration of sCD14 to be increased to >2 µg/ml in SF in early-stage OA (at the time of arthroscopic meniscectomy) as well as in advanced human knee OA (at the time of total joint replacement) (3). In their study, the joint fluid sCD14 levels in early and late OA were comparable to those seen in a small (n = 6) sampling of fluids from RA patients and were ~3 times the levels seen in joint fluid from asymptomatic postmortem donors (3). Another notable finding of Nair et al was that the levels of sCD14 were significantly higher in SF than in paired sera (3). It is likely that most SF sCD14 in OA was derived from activated resident and infiltrating cells of the mononuclear phagocyte lineage. However, significant contributions of shed CD14 from chondrocytes and other connective tissue cells could not be ruled out.
Can SF sCD14 promote inflammation in OA?
Nair et al demonstrated that SF from patients with early OA, although not usually proinflammatory by itself, modulated cultured synovial lining cell inflammatory responses to exogenous microbial TLR-2 and TLR-4 ligands (3). Moreover, sCD14 levels in the SF correlated with activation of cultured synovial fibroblasts. In addition, excess anti-CD14 antibody inhibited the enhancement, by SF, of cultured synovial fibroblast inflammatory responses to exogenous, bacterial-derived, PAMP TLR-2 and TLR-4 agonists (3). However, caution should be applied in interpreting this aspect of the work, since shedding of sCD14 can promote or dampen inflammatory changes in various model systems. Moreover, there are fundamental distinctions between endogenous TLR ligands in their requirements for CD14 and other proteins in signaling.
Studies on the DAMPs in the OA innate inflammatory network will be needed to understand the net effect of sCD14 in OA pathophysiology. The biologic function in OA of the DAMP tenascin-C (TN-C) illustrates this point. Specifically, injection into the knee of TN-C induces synovitis in a TLR-4–dependent manner (6). The C-terminal fibrinogen globular domain of TN-C is a TLR-4 ligand and induces IL-6 expression in synovial fibroblasts and IL-6, TNFα, and IL-8 expression in cultured macrophages. Unlike the PAMP lipopolysaccharide studied by Nair et al (3), the fibrinogen globular domain of TN-C does not require CD14 to promote inflammatory responses (6).
TN-C is a very large molecule with multiple domains that have distinctions in receptor binding and activities. In this light, TN-C binds integrins and modulates growth factor signaling. TN-C also appears at sites of chondrogenesis and mature cartilage injury. Moreover, TN-C knockout was associated with worsened knee OA in a mechanical injury model (7). This is opposite to the major roles of TN-C, both in acute paw inflammation in response to zymosan and in knee synovitis, cartilage proteoglycan loss, and bone erosion in antigen-induced knee arthritis (6). Interestingly, in TN-C–knockout mice, cartilage defects in model OA fill with fibrous tissue but not with cartilage-like tissue (7). Hence, at least 1 proinflammatory DAMP CD14 ligand appears to have beneficial effects on cartilage repair in OA.
Where does sCD14 fit into the bigger picture of innate inflammation–modulated OA progression?
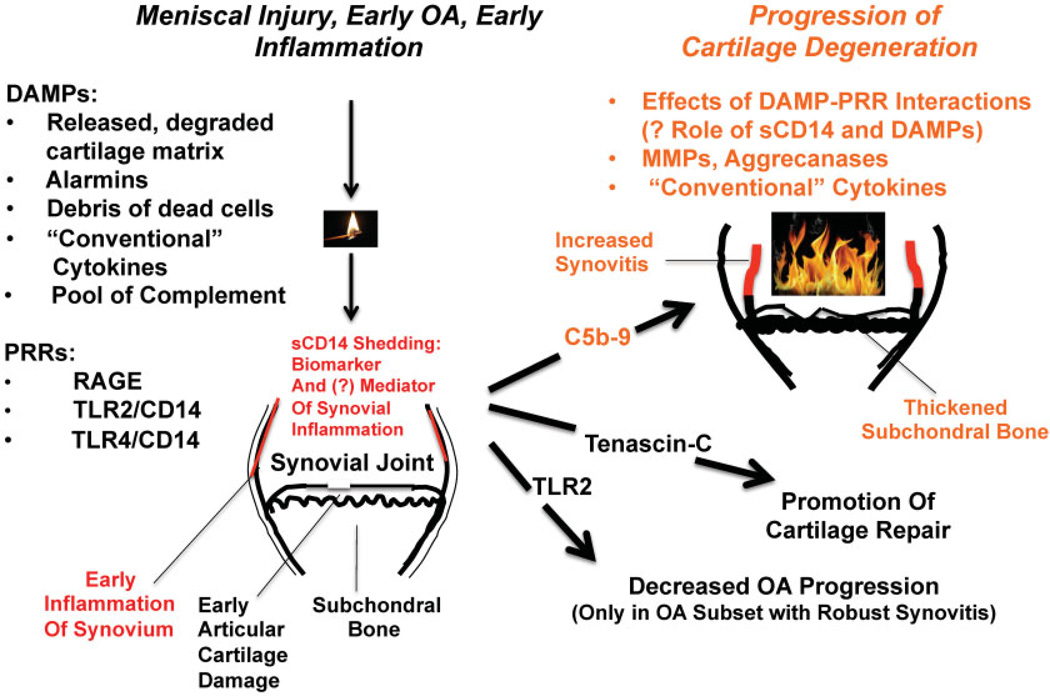
Clearly, sCD14 is only one of many innate inflammatory mediators that could modulate OA progression. Although many soluble danger signals are interwoven with “conventional” cytokines, they can be considered members of a “network within a network.” In OA, specific DAMPs include intact, posttranslationally modified, and degraded matrix proteins, apoptotic and necrotic cell debris, “alarmins” (8,9), and calcium-containing crystals (10). Recent studies schematized summarily in Figure 1 now allow more detailed modeling of how innate immune inflammatory dialogues between synovium, cartilage, and other joint tissues are carried out by DAMPs and by plasma membrane, secreted, and intracellular pattern-recognition receptors (PRRs), including sCD14, in early and progressing OA.
Figure 1.
The position of soluble CD14 (sCD14) in the larger innate inflammatory network in osteoarthritis (OA). This schematic illustrates how elevated synovial fluid sCD14 is present in the “whole joint organ” in early OA associated with meniscal injury, arthroscopically detectable synovitis, clinical symptoms, and the first onset of hyaline articular cartilage damage, prior to plain radiographic evidence of OA. Many innate inflammatory factors, including sCD14 and the others cited here, ignite or enhance noxious responses of cultured synovial fibroblasts and chondrocytes. Hence, these same factors have the potential to help switch on OA after cartilage injury (left) and affect the progression of OA (right). However, the right side of the schematic shows that experimental manipulation of certain mediators in the highlighted innate inflammatory network has yielded surprisingly mixed results to date for OA progression in animal models. In this sense, OA is another example of the “double-edged” sword of innate inflammation in tissue damage versus repair, and the innate inflammation likely embraces synovium and may involve sCD14. DAMPs = damage-associated molecular patterns; PRRs = pattern-recognition receptors; RAGE = receptor for advanced glycation end products; TLR-2 = Toll-like receptor 2; MMPs = matrix metalloproteinases.
The activation of complement, with assembly of the membrane attack complex (MAC) C5b-9, was recently elucidated to be a prime example of innate immune stimulation of OA progression (11) (Figure 1). Pulverized articular cartilage, aggrecan (11), cartilage oligomeric matrix protein, and fibromodulin, which become more abundant in OA joint fluid, are among many potential activators of complement in OA. Expression and activation of effector components of the classical, alternative, and MAC complement pathways are robustly increased in human OA synovia and SF, with conversely decreased expression of multiple inhibitors of complement in synovial membranes (11). Moreover, SF C3a and C5b-9 are increased in joints in early-stage human OA compared to healthy joints (11), providing another inflammatory biomarker of early disease.
Numerous in vitro studies establish that multiple DAMP ligands of the PRR receptor for advanced glycation end products (RAGE), and of TLR-2 and TLR-4 (and presumably CD14), are robustly expressed by synovium and/or cartilage in OA, and that they stimulate synovial cell proliferation and deleterious chondrocyte differentiation and procatabolic responses, thereby with theoretic potential to worsen OA. Specific examples include high mobility group box chromosomal protein 1, members of the S100/calgranulin family (e.g., S100A4, the S100A8/A9 heterodimer, S100A11) (8,9), matrix protein degradation products (e.g., low molecular weight hyaluronan), and modified extracellular proteins (advanced glycation end products [AGEs]).
Despite the aforementioned observations, in vivo studies to date of knockout of RAGE and TLR PRRs and their agonists in model OA have given a decidedly mixed bag of results (Figure 1). For example, repeated intraadministration of exogenous AGEs in a canine model of knee OA triggered by mild surgical damage failed to significantly enhance joint degeneration (12), and there was no significant protective effect of RAGE knockout on a late stage of blunt anterior cruciate ligament tear–induced mouse knee OA in a different study that focused primarily on the innate inflammation-modulating receptor CD36. In vivo knockout of TLR-2 appeared to increase the extent of OA in the collagenase injection–induced model of mouse knee OA, which is associated with significant synovitis (13). In contrast, knockout of TLR-2 did not appear to decrease experimental OA in a mechanical injury–induced mouse model of the disease that was not associated with robust synovitis (13).
Summary and remaining questions
The many “handles” of innate inflammation induce, interpret, transduce, and are induced by “conventional” cytokines and/or their signals in articular cells (Figure 1). It is clear that CD14 is among a multitude of innate inflammatory mediators that turn on or amplify noxious events in chondrocytes and/or synovial fibroblasts in culture. However, some of the innate inflammatory players appear to have redundant, paradoxical, or mutually antagonistic roles, or differing effects at distinct stages of OA (Figure 1). In this sense, OA represents another example of the “double-edged” sword of innate inflammation in tissue damage versus repair (14). These effects likely embrace synovium and may involve sCD14.
We speculate that the variability in the role of TLR-2 in driving OA progression (13) may be particularly relevant to the effects of CD14 in the disease, since synovitis also has been variable in human OA studies to this point in time. Physiologic functions of synovium include modulation of composition of the SF and production of the boundary lubricants lubricin and hyaluronan, which function partly by reducing cartilage friction. Lubricin also has a major physiologic role in suppressing synovial proliferation. Major questions remain concerning the potential role of sCD14 and other innate inflammatory mediators in regulating synovial homeostatic functions such as qualitative and quantitative changes in lubricin and hyaluronan that can affect OA progression.
We conclude that the relative abundance of sCD14 in SF is one of several new innate inflammatory ignition switches that leave substantial footprints in the early phase of human OA. At a minimum, the findings of Nair et al could help provide earlier and better disease phenotyping of patients and assist in the refinement of OA therapeutic trials. However, the net roles of sCD14 in OA initiation and progression remain to be determined. Disappointing results of human OA therapeutic studies that have targeted the “conventional” cytokine IL-1β are instructive. It is not yet clear which, if any, primordial innate inflammation responses, including those mediated by sCD14, could represent a potential source of transformative OA medical therapeutics.
Acknowledgments
Supported by the VA Research Service and the NIH (grants AR-1067966 and PAG-07996).
Footnotes
AUTHOR CONTRIBUTIONS
Drs. Liu-Bryan and Terkeltaub drafted the editorial, revised it critically for important intellectual content, and approved the final version to be published.
REFERENCES
- 1.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 2.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair A, Kanda V, Bush-Joseph C, Verma N, Chubinskaya S, Mikecz K, et al. Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to Toll-like receptor 4 and Toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum. 2012;64:2268–2277. doi: 10.1002/art.34495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finberg RW, Kurt-Jones EA. CD14: chaperone or matchmaker? Immunity. 2006;24:127–129. doi: 10.1016/j.immuni.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, et al. Tenascin-C is an endogenous activator of Toll-like receptor-4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 7.Okamura N, Hasegawa M, Nakoshi Y, Iino T, Sudo A, Imanaka-Yoshida K, et al. Deficiency of tenascin-C delays articular cartilage repair in mice. Osteoarthritis Cartilage. 2010;18:839–848. doi: 10.1016/j.joca.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Van Lent PL, Blom AB, Schelbergen RF, Sloetjes A, Lafeber FP, Lems WF, et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012;64:1466–1476. doi: 10.1002/art.34315. [DOI] [PubMed] [Google Scholar]
- 9.Schelbergen RF, Blom AB, van den Bosch MH, Sloetjes A, Abdollahi-Roodsaz S, Schreurs BW, et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012;64:1477–1487. doi: 10.1002/art.33495. [DOI] [PubMed] [Google Scholar]
- 10.Jin C, Frayssinet P, Pelker R, Cwirka D, Hu B, Vignery A, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci U S A. 2011;108:14867–14872. doi: 10.1073/pnas.1111101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vos PA, Degroot J, Barten-van Rijbroek AD, Zuurmond AM, Bijlsma JW, Mastbergen SC, et al. Elevation of cartilage AGEs does not accelerate initiation of canine experimental osteoarthritis upon mild surgical damage. J Orthop Res. 2012 doi: 10.1002/jor.22092. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Blom AB, van Lent PL, Abdollahi-Roodsaz S, van der Kraan P, van den Berg W. Elusive role for Toll like receptor 2 in joint pathology during experimental osteoarthritis [abstract] Osteoarthritis Cartilage. 2011;19(Suppl 1):25. [Google Scholar]
- 14.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]