Abstract
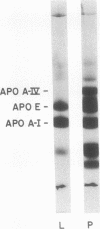
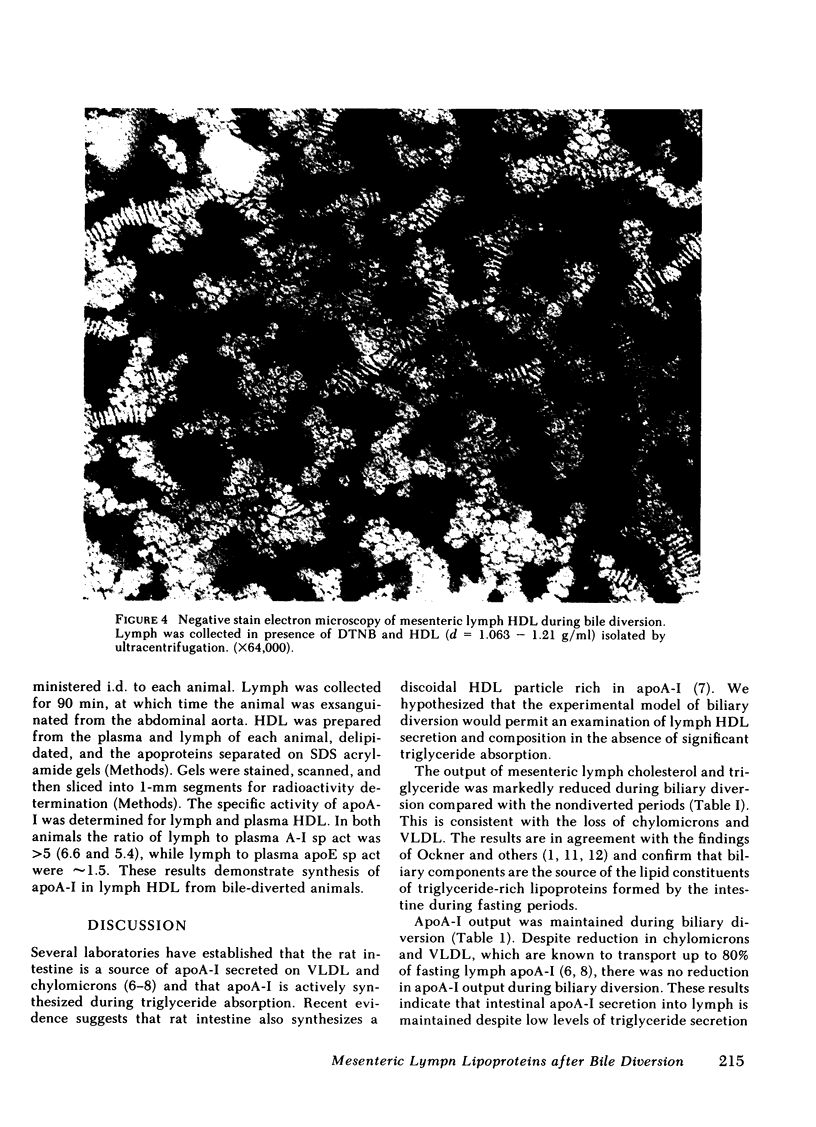
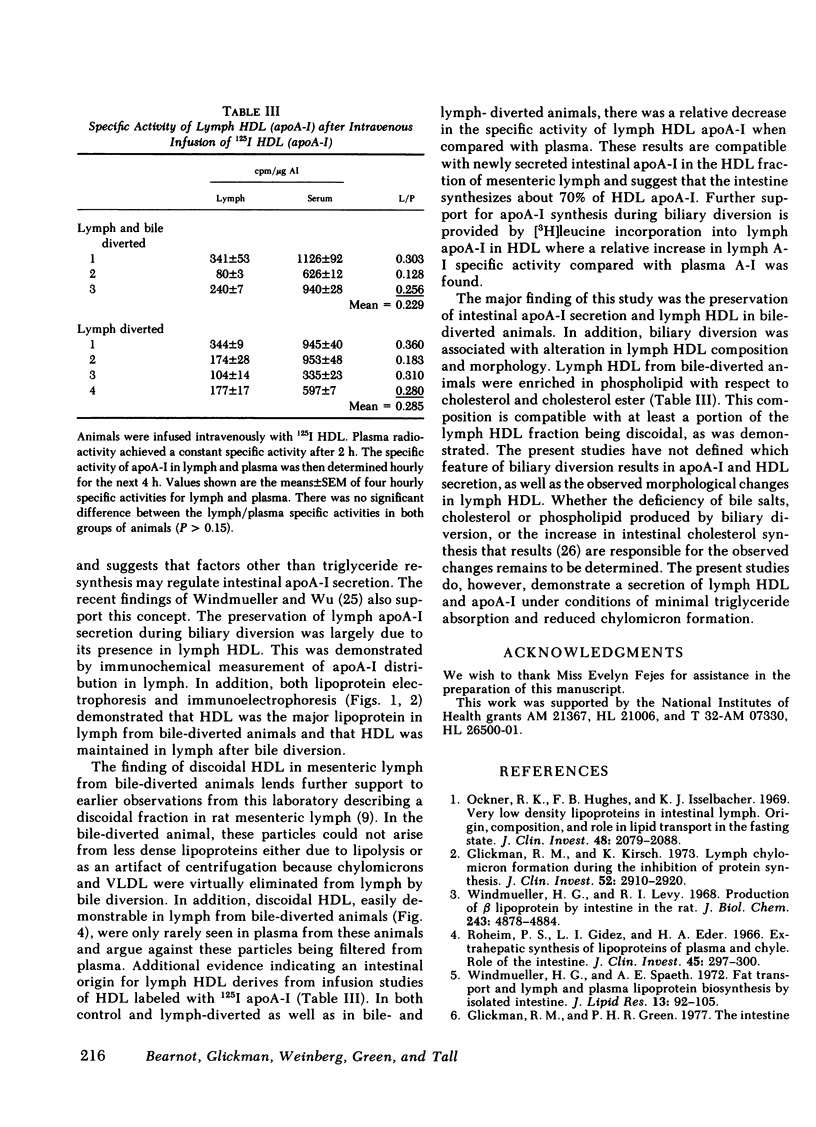
The effect of biliary diversion on intestinal apolipoprotein (apoA)-I and high density lipoprotein formation was studied in mesenteric lymph fistula rats. Bile diversion was produced by an exteriorized catheter that allowed interruption and reconstitution of the enterohepatic circulation. Bile diversion reduced lymph cholesterol output from 0.47±0.05 μmol/h to 0.17±0.03 μmol/h (P < 0.025), and lymph triglyceride output from 3.6±0.3μmol/h to 0.6±0.05 μmol/h (P < 0.025) after 24 h. This was due to depletion of lymph chylomicrons and very low density lipoprotein (VLDL). Despite the reduced lipid outputs, lymph apoA-I output was maintained during biliary diversion (basal: 119±15 μg/h; diverted 140±20 μg/h, n = 12). During biliary diversion, high density lipoprotein (HDL) were maintained in mesenteric lymph as shown by lipoprotein and immunoelectrophoresis. Bile diversion altered the lipid composition of lymph HDL. Bile-diverted lymph HDL was depleted in total cholesterol and has a greater phospholipid/cholesterol ester ratio than basal lymph HDL. Lymph HDL contained discoidal particles when examined by negative stain electron microscopy. Bile diversion was associated with a reduction in the size of discoidal HDL particles (basal, nondiverted, 165±7Å (n = 112) compared with diverted 126±5Å (n = 98, P < 0.025). Experiments were then carried out to determine the source of the apoA-I and HDL found in lymph from bile-diverted animals. The transfer of HDL from plasma into lymph was determined by the intravenous infusion of 125I-apoA-I labeled HDL into lymph fistula rats. In both nonbile-diverted and diverted rats, the specific activity of apoA-I in the HDL fraction of lymph was 23% of the specific activity of apoA-I in plasma HDL, indicating that the major portion (75%) of mesenteric lymph apoA-I did not come from plasma filtration. In other experiments the intraduodenal infusion of [3H]leucine to bile fistula, lymph fistula rats resulted in relative fivefold increase in the specific activity in apoA-I in lymph HDL when compared with the specific activity of apoA-I in plasma HDL from the same animal. We conclude that intestinal apoA-I secretion is maintained during biliary diversion and that synthesis of this apoprotein occurs in the absence of chylomicron formation. We also conclude that discoidal HDL are present in mesenteric lymph despite reduced triglyceride absorption and secretion into lymph.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. V., Levy R. I., Fredrickson D. S. Studies of the proteins in human plasma very low density lipoproteins. J Biol Chem. 1969 Oct 25;244(20):5687–5694. [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965 Aug;44(8):1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing D. T. Photodensitometry in the thin-layer chromatographic analysis of neutral lipids. J Chromatogr. 1968 Nov 5;38(1):91–99. doi: 10.1016/0021-9673(68)85011-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Green P. H. The intestine as a source of apolipoprotein A1. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2569–2573. doi: 10.1073/pnas.74.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. M., Kirsch K., Isselbacher K. J. Fat absorption during inhibition of protein synthesis: studies of lymph chylomicrons. J Clin Invest. 1972 Feb;51(2):356–363. doi: 10.1172/JCI106821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. M., Kirsch K. Lymph chylomicron formation during the inhibition of protein synthesis. Studies of chylomicron apoproteins. J Clin Invest. 1973 Nov;52(11):2910–2920. doi: 10.1172/JCI107487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. H., Tall A. R., Glickman R. M. Rat intestine secretes discoid high density lipoprotein. J Clin Invest. 1978 Feb;61(2):528–534. doi: 10.1172/JCI108963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J Clin Invest. 1976 Sep;58(3):667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi K., Havel R. J., Fainaru M., Vigne J. L. Origin and transport of the A-I and arginine-rich apolipoproteins in mesenteric lymph of rats. J Lipid Res. 1978 Nov;19(8):1038–1046. [PubMed] [Google Scholar]
- Jones A. L., Ockner R. K. An electron microscopic study of endogenous very low density lipoprotein production in the intestine of rat and man. J Lipid Res. 1971 Sep;12(5):580–589. [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J Clin Invest. 1969 Nov;48(11):2079–2088. doi: 10.1172/JCI106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Jones A. L. An electron microscopic and functional study of very low density lipoproteins in intestinal lymph. J Lipid Res. 1970 Jul;11(4):284–292. [PubMed] [Google Scholar]
- Pownall H. J., Massey J. B., Kusserow S. K., Gotto A. M., Jr Kinetics of lipid--protein interactions: interaction of apolipoprotein A-I from human plasma high density lipoproteins with phosphatidylcholines. Biochemistry. 1978 Apr 4;17(7):1183–1188. doi: 10.1021/bi00600a008. [DOI] [PubMed] [Google Scholar]
- Riley J. W., Glickman R. M., Green P. H., Tall A. R. The effect of chronic cholesterol feeding on intestinal lipoproteins in the rat. J Lipid Res. 1980 Sep;21(7):942–952. [PubMed] [Google Scholar]
- Roheim P. S., Gidez L. I., Eder H. A. Extrahepatic synthesis of lipoproteins of plasma and chyle: role of the intestine. J Clin Invest. 1966 Mar;45(3):297–300. doi: 10.1172/JCI105343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roheim P. S., Hirsch H., Edelstein D., Rachmilewitz D. Metabolism of iodinated high density lipoprotein subunits in the rat. 3. Comparison of the removal rate of different subunits from the circulation. Biochim Biophys Acta. 1972 Oct 31;278(3):517–529. doi: 10.1016/0005-2795(72)90012-8. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Gotto A. M., Jr, Taunton O. D., Caslake M. J., Farish E. The in vitro interaction of human apolipoprotein A-I and high density lipoproteins. Biochim Biophys Acta. 1977 Dec 21;489(3):486–501. doi: 10.1016/0005-2760(77)90169-2. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Levy R. I. Production of beta-lipoprotein by intestine in the rat. J Biol Chem. 1968 Sep 25;243(18):4878–4884. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Fat transport and lymph and plasma lipoprotein biosynthesis by isolated intestine. J Lipid Res. 1972 Jan;13(1):92–105. [PubMed] [Google Scholar]
- Windmueller H. G., Wu A. L. Biosynthesis of plasma apolipoproteins by rat small intestine without dietary or biliary fat. J Biol Chem. 1981 Mar 25;256(6):3012–3016. [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Relative contributions by liver and intestine to individual plasma apolipoproteins in the rat. J Biol Chem. 1979 Aug 10;254(15):7316–7322. [PubMed] [Google Scholar]