Abstract
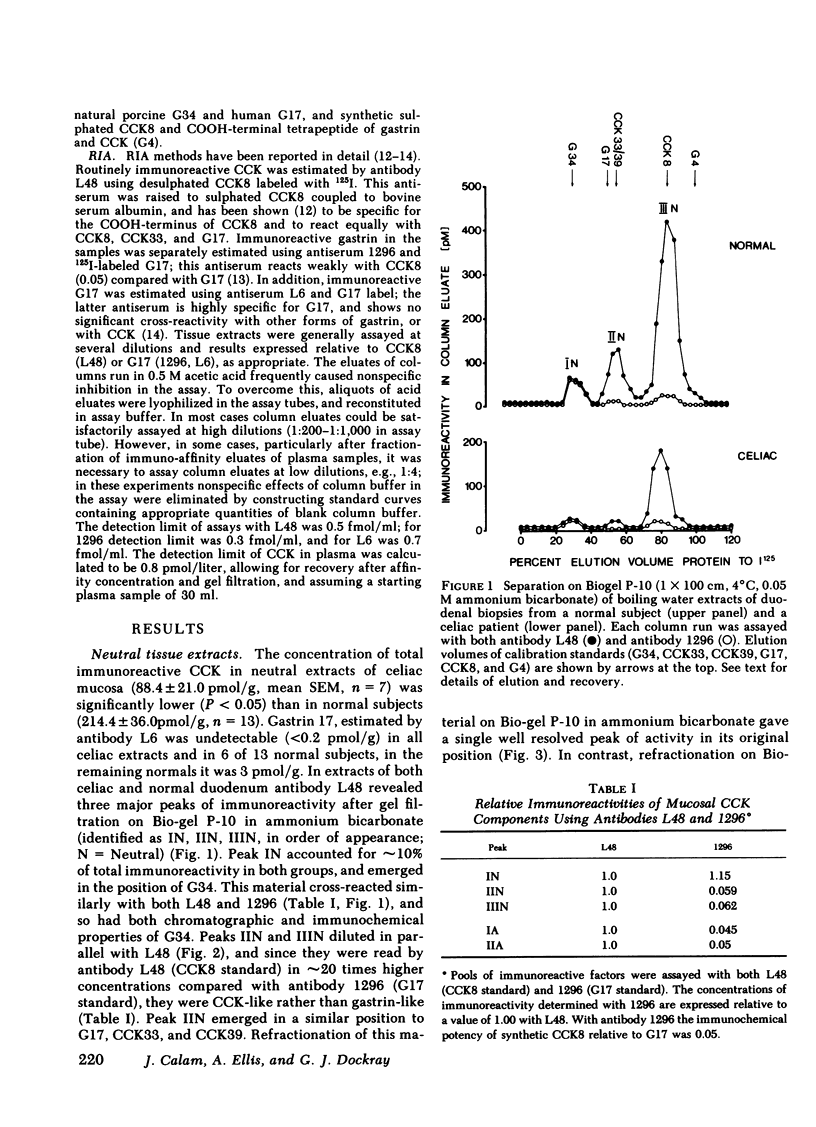
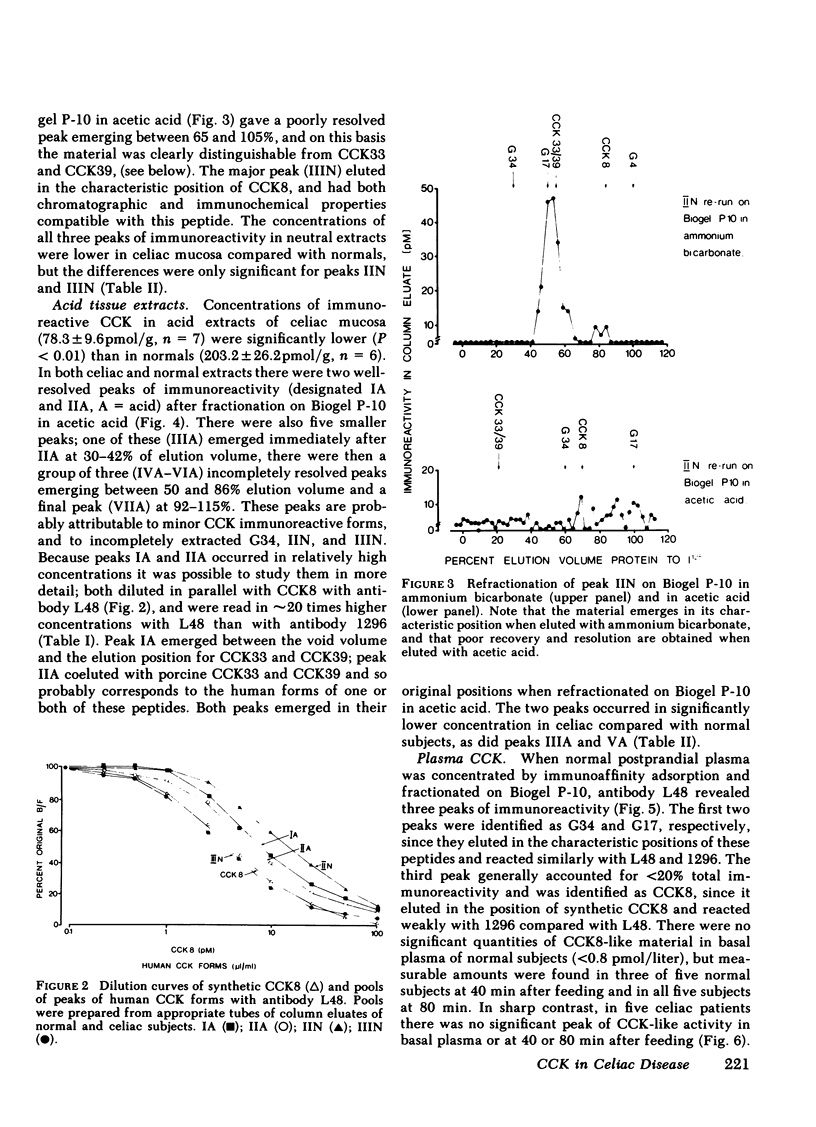
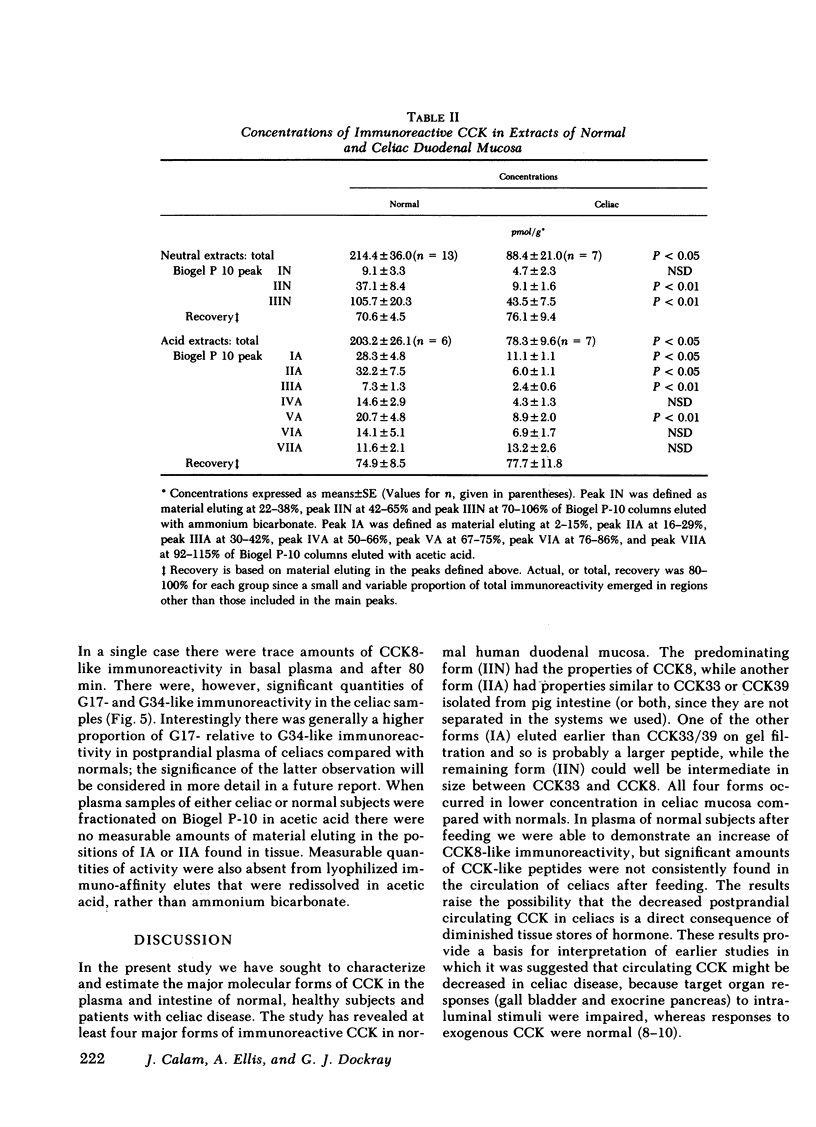
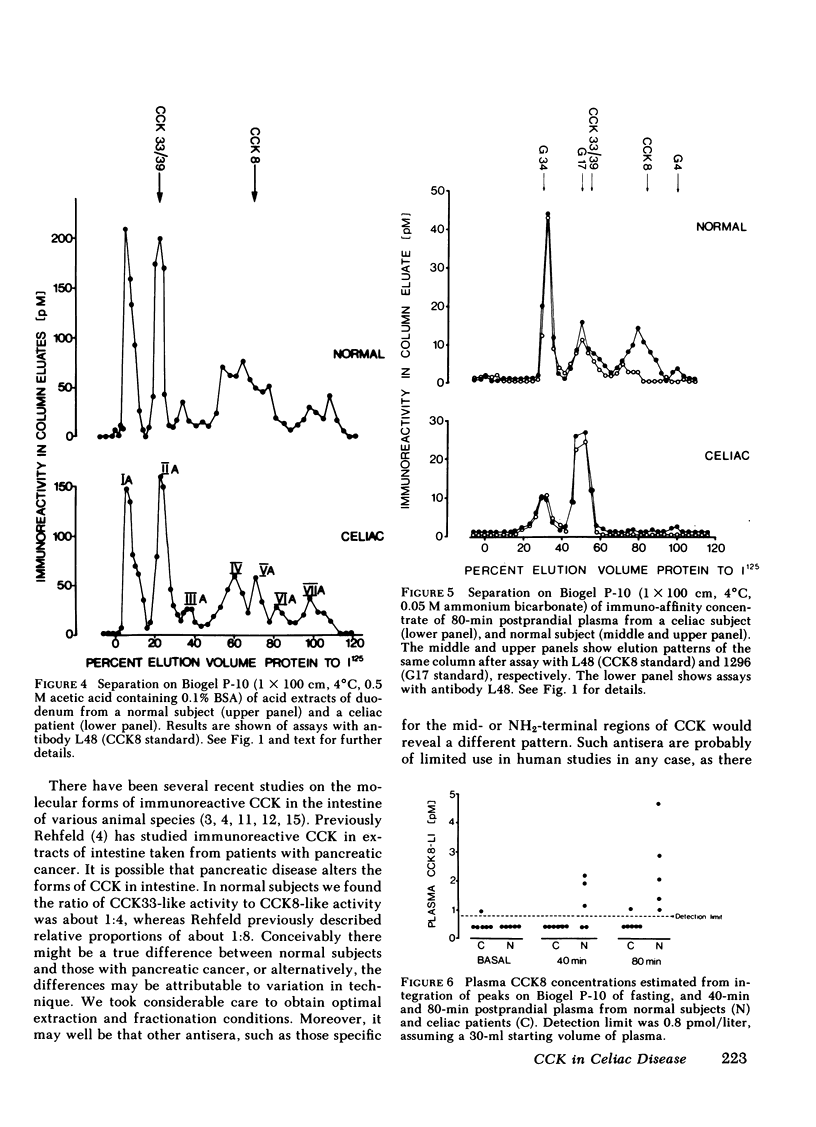
The amount and type of cholecystokinin (CCK) in duodenal extracts and plasma of celiac patients and normal subjects was studied by radioimmunoassay and gel filtration. In both groups there were similar patterns of molecular forms in extracts of duodenal biopsies, but concentrations in celiac disease were significantly depressed. In boiling water extracts of duodenal mucosa from both groups a factor with the properties of the COOH-terminal octapeptide of cholecystokinin predominated, but there were also significant amounts of a larger molecular weight form. In acid extracts of mucosa a factor with the properties of the 33 or 39 residue form was identified in amounts that were approximately 25% those of CCK8; there were also similar amounts of an acid-soluble form that had an apparent molecular weight higher than CCK39. Plasma immunoreactive cholecystokinin was studied after concentration by immunoaffinity adsorption and fractionation by gel filtration. In normal subjects fasting CCK-like immunoreactivity was less than 0.8 pmol/liter, and after a light breakfast increased to 2.0 +/- 0.7 (range 1.0 to 4.8) pmol/liter; CCK8-like activity accounted for all the increased immunoreactivity. In five of six celiac patients the concentrations of both fasting and postprandial CCK-like immunoreactivity in plasma were undetectable (less than 0.8 pmol/liter). We conclude that diminished production and release of CCK could account for the impaired pancreatic and gall bladder responses to intraluminal stimuli in celiac disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besterman H. S., Bloom S. R., Sarson D. L., Blackburn A. M., Johnston D. I., Patel H. R., Stewart J. S., Modigliani R., Guerin S., Mallinson C. N. Gut-hormone profile in coeliac disease. Lancet. 1978 Apr 15;1(8068):785–788. doi: 10.1016/s0140-6736(78)92994-x. [DOI] [PubMed] [Google Scholar]
- Debas H. T., Grossman M. I. Pure cholecystokinin: pancreatic protein and bicarbonate response. Digestion. 1973;9(6):469–481. doi: 10.1159/000197476. [DOI] [PubMed] [Google Scholar]
- DiMagno E. P., Go W. L., Summerskill W. H. Impaired cholecystokinin-pancreozymin secretion, intraluminal dilution, and maldigestion of fat in sprue. Gastroenterology. 1972 Jul;63(1):25–32. [PubMed] [Google Scholar]
- Dockray G. J. Cholecystokinins in rat cerebral cortex: identification, purification and characterization by immunochemical methods. Brain Res. 1980 Apr 21;188(1):155–165. doi: 10.1016/0006-8993(80)90564-8. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Gregory R. A., Hutchison J. B., Harris J. I., Runswick M. J. Isolation, structure and biological activity of two cholecystokinin octapeptides from sheep brain. Nature. 1978 Aug 17;274(5672):711–713. doi: 10.1038/274711a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunoreactive component resembling cholecystokinin octapeptide in intestine. Nature. 1977 Nov 24;270(5635):359–361. doi: 10.1038/270359a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Taylor I. L. Heptadecapeptide gastrin: measurement in blood by specific radioimmunoassay. Gastroenterology. 1976 Dec;71(6):971–977. [PubMed] [Google Scholar]
- Dockray G. J., Walsh J. H. Amino terminal gastrin fragment in serum of Zollinger-Ellison syndrome patients. Gastroenterology. 1975 Feb;68(2):222–230. [PubMed] [Google Scholar]
- Go V. L., Ryan R. J., Summerskill W. H. Radioimmunoassay of porcine cholecystokinin-pancreozymin. J Lab Clin Med. 1971 Apr;77(4):684–689. [PubMed] [Google Scholar]
- Harvey R. F., Dowsett L., Hartog M., Read A. E. A radioimmunoassay for cholecystokinin-pancreozymin. Lancet. 1973 Oct 13;2(7833):826–828. doi: 10.1016/s0140-6736(73)90863-5. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res. 1979 Apr 13;165(2):201–218. doi: 10.1016/0006-8993(79)90554-7. [DOI] [PubMed] [Google Scholar]
- Low-Beer T. S., Harvey R. F., Davies E. R., Read A. F. Abnormalities of serum cholecystokinin and gallbladder emptying in celiac disease. N Engl J Med. 1975 May 1;292(18):961–963. doi: 10.1056/NEJM197505012921807. [DOI] [PubMed] [Google Scholar]
- Low-Beer T. S., Heaton K. W., Heaton S. T., Read A. E. Gallbladder inertia and sluggish enterohepatic circulation of bile-salts in coeliac disease. Lancet. 1971 May 15;1(7707):991–994. doi: 10.1016/s0140-6736(71)91387-0. [DOI] [PubMed] [Google Scholar]
- Muller J. E., Straus E., Yalow R. S. Cholecystokinin and its COOH-terminal octapeptide in the pig brain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3035–3037. doi: 10.1073/pnas.74.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondetti M. A., Rubin B., Engel S. L., Pluscec J., Sheehan J. T. Cholecystokinin-pancreozymin: recent developments. Am J Dig Dis. 1970 Feb;15(2):149–156. doi: 10.1007/BF02235646. [DOI] [PubMed] [Google Scholar]
- Reeder D. D., Becker H. D., Smith N. J., Rayford P. L., Thompson J. C. Measurement of endogenous release of cholecystokinin by radioimmunoassay. Ann Surg. 1973 Sep;178(3):304–310. doi: 10.1097/00000658-197309000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978 Jun 10;253(11):4022–4030. [PubMed] [Google Scholar]
- Rubin B., Engel S. L., Drungis A. M., Dzelzkalns M., Grigas E. O., Waugh M. H., Yiacas E. Cholecystokinin-like activities in guinea pigs and in dogs of the C-terminal octapeptide (SQ 19,844) of cholecystokinin. J Pharm Sci. 1969 Aug;58(8):955–959. doi: 10.1002/jps.2600580810. [DOI] [PubMed] [Google Scholar]
- Sjölund K., Alumets J., Berg N. O., Håkanson R., Sundler F. Duodenal endocrine cells in adult coeliac disease. Gut. 1979 Jul;20(7):547–552. doi: 10.1136/gut.20.7.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T. E., Grossman M. I. Effect of atropine and vagotomy on response of transplanted pancreas. Am J Physiol. 1979 Feb;236(2):E186–E190. doi: 10.1152/ajpendo.1979.236.2.E186. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Species specificity of cholecystokinin in gut and brain of several mammalian species. Proc Natl Acad Sci U S A. 1978 Jan;75(1):486–489. doi: 10.1073/pnas.75.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]



