Abstract
This study constitutes a demonstration of the biological route to controlled nano-fabrication via modular multi-functional inorganic-binding peptides. Specifically, we use gold- and silica-binding peptide sequences, fused into a single molecule via a structural peptide spacer, to assemble pre-synthesized gold nanoparticles on silica surface, as well as to synthesize nanometallic particles in situ on the peptide-patterned regions. The resulting film-like gold nanoparticle arrays with controlled spatial organization are characterized by various microscopy and spectroscopy techniques. The described bio-enabled, single-step synthetic process offers many advantages over conventional approaches for surface modifications, self-assembly and device fabrication due to the peptides’ modularity, inherent biocompatibility, material specificity and catalytic activity in aqueous environments. Our results showcase the potential of artificially-derived peptides to play a key role in simplifying the assembly and synthesis of multi-material nano-systems in environmentally benign processes.
Keywords: Gold-nanoparticle arrays, inorganic-binding peptides, micro-contact printing, peptide-mediated nucleation
1. Introduction
Efficient and controllable fabrication of inorganic nano- or micro-structures on solid substrates is critical for a variety of nano- and micro-technologies (e.g. electronics, photonics, and sensing) [1-5]. Arrays of gold nanoparticles organized on solid supports as a film are particularly promising for fabrication of high-throughput and cost-effective assay systems with unique photonic properties, as well as for construction of electrochemical sensor chips and bioelectronic devices [1-3]. Currently available physical approaches to fabrication of patterned metallic nanostructured surfaces include photo-, e-beam, and nanosphere-lithographies [6-8]. Many of these processes are complex, requiring well-controlled environments, sophisticated equipment, and thus, high operating costs [7]. Chemical approaches involve complex surface functionalization with thiol- or silane-based self-assembled monolayer (SAM) molecules [9, 10], which can be patterned on solid surfaces using common soft lithography techniques [11, 12]. Patterned SAMs on gold and silica surfaces, in fact, have been used to fabricate various arrays and devices [12-14]. However, there are still considerable processing limitations and drawbacks associated with SAM technology including complexity of surface modifications that are currently undertaken under biologically unfriendly conditions, applicability to only a limited number of materials, high cost, long stacking time, low yield and stability [5, 14, 15]. In addition, since such nanosystems are produced in harsh solvents and conditions, they are often not biocompatible and are of limited practical relevance in biomedical applications. Here, we demonstrate a bio-enabled fabrication route for surface micro-patterning as an alternative to conventional techniques by utilizing hetero-functional inorganic binding peptides that offer desired versatility as well as multi-material recognition characteristics. Once properly engineered, the combined peptide building blocks can link and hold two different materials within close proximity as well as exhibit catalytic materials synthesis activity [16, 17].
The genetically engineered peptides for inorganic materials (GEPIs) are selected from combinatorial peptide phage [18] or cell surface display [19, 20] libraries, and have the ability to control assembly [21, 22] and formation [16, 23-25] of various nanostructures. In the literature, combinatorially selected peptides specific to a variety of inorganic materials, e.g. Au, Ag, Pt, Cu2O, SiO2, and TiO2,, have been used in a wide range of practical applications in nano- and medical-technologies [16, 17, 26, 27]. Most recently, the peptides have been employed, by our and other groups, as modular subunits of a hetero-functional fusion construct, in combination with other proteins and peptides for a variety of directed self-assembly applications [25, 26, 28, 29]. Previously, we reported the selection and identification of gold- [30] and silica-binding [27] peptides (AuBP and QBP) from combinatorial and de novo peptide libraries and confirmed their high-affinity and selective binding onto respective solid surfaces [15, 27, 30]. In addition to exceptional binding (at sub-micromolar levels) and self-assembly on gold surfaces, the specific AuBP sequences were also reported to catalyze and promote the formation of water dispersible gold nanostructures from aqueous HAuCl4 solutions under ambient conditions without the need for additional reducing or stabilizing agents [31], such as the case in citrate-enabled gold formation [32]. Here we combine the binding and catalytic activities of these peptides into a single multi-functional unit and demonstrate their effective implementation for an addressable bio-enabled nanofabrication route.
2. Materials and Methods
2.1. Fmoc Peptide Synthesis
The peptide sequences used in this research, including QBP, AuBP, and the multi-functional peptides, QBP-AuBP and AuBP-QBP, were synthesized via automated Fmoc peptide synthesis using CSBio 336s peptide synthesizer (CSBio Inc, USA). The obtained crude peptides were then purified by C-18 reversed-phase high-performance liquid chromatography (HPLC system, Waters, USA). The mass of purified peptides were confirmed by mass spectroscopy using matrix-assisted laser desorption/ionization mass spectrometer linked with time-of-flight detector (MALDI-TOF) MS (Bruker AutoFlex II, Bruker Daltonics, USA). See Supplementary Material for more detailed protocol and for MS data of the pure QBP-AuBP, AuBP-QBP, QBP and AuBP peptide sequences.
2.2. Micro-contact Printing
The PDMS stamps were fabricated by molding a mixture of polydimethyl siloxane and curing agent (10:1, Sylgard 184, Dow Corning, USA) on the surface of a patterned silanized master for 2 days at ambient condition. The stamps were then washed several times with ethanol, heptane, and again with ethanol, and dried with inert gas before being used. Patterned side of PDMS stamp was incubated with 100 μL of 100 μM or 200 μM peptide solutions for 5 min. The peptide solution was removed from the stamp surface by careful pipetting. The stamps were then dried with inert nitrogen gas. The silica surfaces were cleaned by ethanol and sonication, and then applied to the surface of the stamp and pressed using force for 10 sec and left on stamp for 1 min. The surfaces were then washed with DI water for 2 min and dried with nitrogen.
2.3. Peptide-mediated Immobilization of Gold Nanoparticles onto Silica Surface
Peptide-patterned silica surfaces were incubated with 100 μL of 50 nm pre-made gold nanoparticles (Ted Pella, USA) for 15 min. Then silica surfaces were washed with DI water for 2 min and dried with nitrogen.
2.4. Peptide-mediated Formation of Gold Nanoparticle Film onto Silica Surface
Peptide-patterned silica surfaces were incubated with 200 μL of 10 mM aqueous solution of HAuCl4 for 48 hr in moisture chamber to eliminate evaporation. The silica surfaces were then washed with DI water for 2 min and dried with nitrogen. In our combined approach nanoparticle-patterned silica surface were incubated with 200 μL of 10 mM aqueous solution of HAuCl4 for 48 hr as described above.
2.5. Dark Field Optical Microscopy
Silica surfaces with patterned gold nanoparticle arrays produced by peptide-mediated assembly of pre-made gold nanoparticle as well as by peptide-mediated gold nanoparticle formation were characterized using dark field optical microscopy on Nikon Eclipse TE-2000U Optical Microscope (Nikon, Japan). The dark field images, which are obtained from at least five different locations on each sample, were recorded through Metamorph imaging software (Universal Imaging, USA). Analyses were repeated in three independent experiments.
2.6. Molecular Dynamics
To model the two different permutations of hetero-functional peptide, we built linear forms using the HyperChem’s molecular modeling software (Hyperchem 7.5, USA). The energy minimization of these peptides was carried out under implicit solvent conditions using the conformational analysis program. By randomly changing predefined dihedral angles we created numerous initial configurations in order to increase the sampling space of the potential energy surface. Using the conformational search module, we found 1000 different local minima on the potential energy surface and chose the lowest one as the global minimum or the lowest-energy conformation. Then, the lowest energy conformations were solvated with TIP3P water explicitly; and finally the overall system was energy minimized using the Polak-Ribiere conjugate gradient method until convergence of the gradient (0.01 kJ/mol) was reached using the CHARMM 27 force field. The final configurations were generated using the VMD (Visual Molecular Dynamics) software.
2.7. Surface characterization
Produced gold nanoparticle arrays on silica surfaces were characterized using various surface characterization techniques, including atomic force microscopy (AFM), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) as follows.
2.8. Atomic Force Microscopy
AFM images were obtained in tapping mode on a Dimension 3100 SPM, (Veeco, USA), in air, using silicon tips. The scans were obtained at a maximum of 30 μm with the maximum possible scan range of 90 μm. At least three different locations were observed on each sample.
2.9. Scanning Electron Microscopy
The samples of peptide-formed gold nanoparticle film were coated with platinum using an SPI sputter coater (SPI supplies, USA) and observed using a JSM 7000F SEM (JEOL Ltd., Japan) at 10 kV beam voltage.
2.10. X-ray photoelectron spectroscopy
The non-patterned PDMS stamps were used as described above to create maximum possible coverage of gold nanoparticle film on-silica surfaces so as to increase the photoelectron yield. Experiments were carried out in an UHV system (P ≤ 3.0 × 10−9 Torr) equipped with a monochoromatized Al Kα X-ray source and an X-ray photoelectron detector (SSL 300 from Surface Science Inc.). High resolution spectra were collected for 14 hours from a 100 μm radius spot. The data was analyzed by peak shape fitting method.
3. Results and Discussion
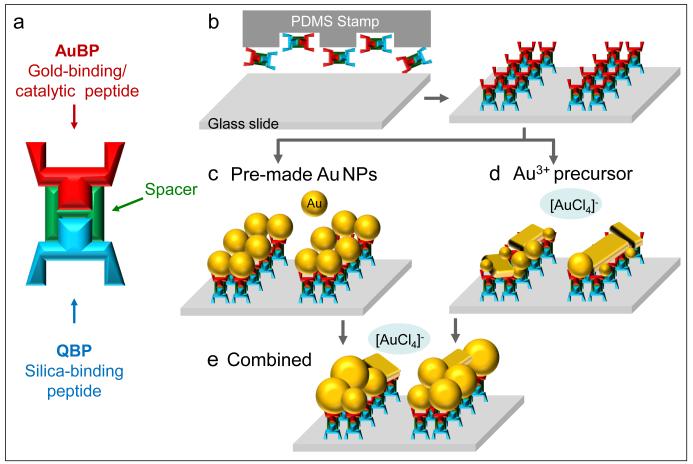
We designed a novel hetero-functional peptide by linking silica- and gold-binding peptide sequences (QBP and AuBP), through a flexible poly-glycine linker [33, 34]. QBP and AuBP components were selected in our laboratories using optimized combinatorial selection or de novo approaches adapted by our group [27, 30]. Both peptide sequences were carefully chosen for best binding and synthesis functionalities [15, 27, 30, 31]. Additionally we were careful to avoid sequences dominated by amino acids known to interact with gold, such as cysteine and histidine [35-39], and which might, in turn, promote the gold reduction and crystal growth. The peptide sequences were synthesized via automated Fmoc peptide synthesis and subsequently purified by reversed-phase high-performance liquid chromatography (corresponding molecular masses of pure peptides are shown in Figure S1 in Supplementary Material). The patterned QBP-AuBP multi-functional peptide (Figure 1a-b) was then utilized as a molecular linker for direct immobilization of gold nanoparticles onto the silica surface (Figure 1c) as well as to control the formation of a gold film by a peptide-mediated reduction process (Figure 1d). Finally, we demonstrated the robustness of our approach by combining these two different types of functionality, i.e. assembly and catalytic activity, and produced densely packed and thick gold films by inducing the growth of pre-immobilized “ seed ” nanoparticles using the engineered multi-functional peptide (Figure 1e).
Figure 1.
Fabrication Routes of Gold Nanoparticle Film Array. Multi-functional peptide design including gold – and silica-binding termini combined by a flexible spacer (GGG) (a); Schematics of the procedure of producing peptide microarray using soft lithography (b); Peptide-mediated immobilization of pre-made gold nanoparticles (c); Peptide-mediated in situ synthesis of gold nanoparticle film (d); Peptide-mediated growth of pre-immobilized gold nanoparticles (e).
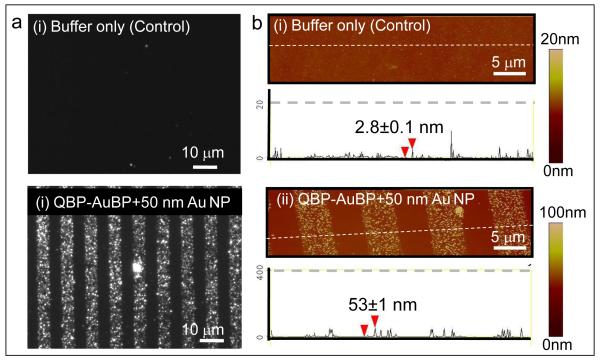
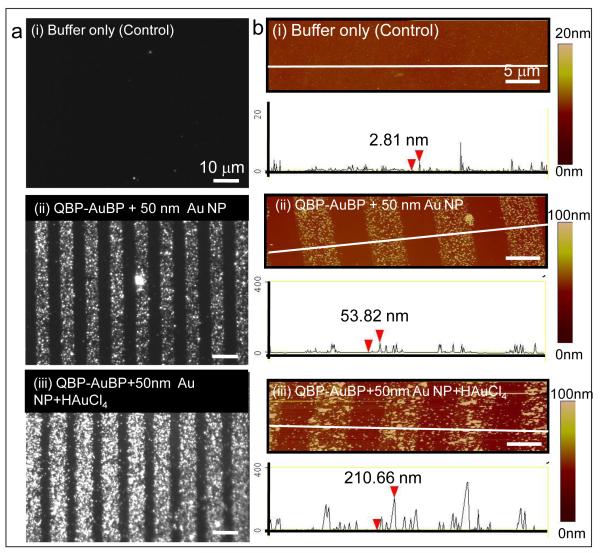
We first demonstrate the ability of QBP-AuBP peptide to link two different inorganic materials, by specifically immobilizing gold nanoparticles onto silica surfaces (Figure 1c). Well-defined peptide patterns of controllable size and shape were produced on silica surfaces using micro-contact printing technique [11, 12, 15]. We next incubated the peptide patterned surfaces with pre-made gold nanoparticles of 50 nm size (Ted Pella, USA). The resulting gold nanostructure arrays were characterized using dark field (DF) optical microscopy as well as atomic force microscopy (AFM) in tapping mode (Figure 2). The dark field optical and the atomic force micrographs revealed preferential assembly of the pre-made gold nanoparticles on to the patterned peptide templates. The height of the features observed under AFM was consistent with the expected height of the construct: 50 nm particle plus a 1-2 nm thick peptide monolayer [40].
Figure 2.
Fabrication of Gold Nanoparticle Array via Peptide-mediated Immobilization Approach. Optical dark field images of gold nanoparticle array via patterned QBP-AuBP peptide and its control sample (a); Corresponding atomic force microscopy images and the height profiles across the array with and without peptide-patterning (b).
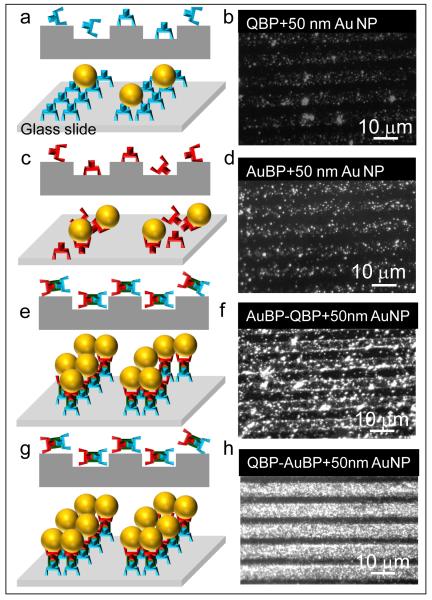
Employing QBP-AuBP peptide that combines both silica- and gold-binding motifs resulted in homogenous and continuous arrays of gold nanoparticle film when compared to randomly organized and discrete low density nanoparticles immobilized by either single QBP or AuBP peptide sequences as evidenced by dark field optical microscopy images (Figure 3). It is well known phenomenon in biology, that protein or peptide molecular architecture affects its function in essential biological interactions. However, despite the recent extensive reports of hetero-functional inorganic-binding peptide utility as functional synthesizers and molecular linkers, there is still only a limited understanding of the effects of peptide structure on their molecular binding mechanisms [29, 30, 41]. Thus, in order to test the effect of the peptide molecular fusion on the structure and therefore function of the modular components, we reversed the order of single peptide domains to AuBP-QBP. We found that the QBP-AuBP sequence performed significantly better than the reversed permutation (Figure 3). Such a difference implies that in addition to primary sequence the adapted molecular conformation of hetero-functional peptide triggered by the order of gold- and silica-binding domain conjugation is also likely to play an important role in inorganic binding functionality.
Figure 3.
Efficiency of Peptide-mediated Gold Nanoparticle Immobilization. Schematics of peptide-mediated immobilization of gold nanoparticles using single or multi-functional peptides (a, c, e and g); Respective dark field microscopy images of immobilized gold nanoparticles via QBP (b), AuBP (d), multi-functional AuBP-QBP (f) and QBP-AuBP permutations (h).
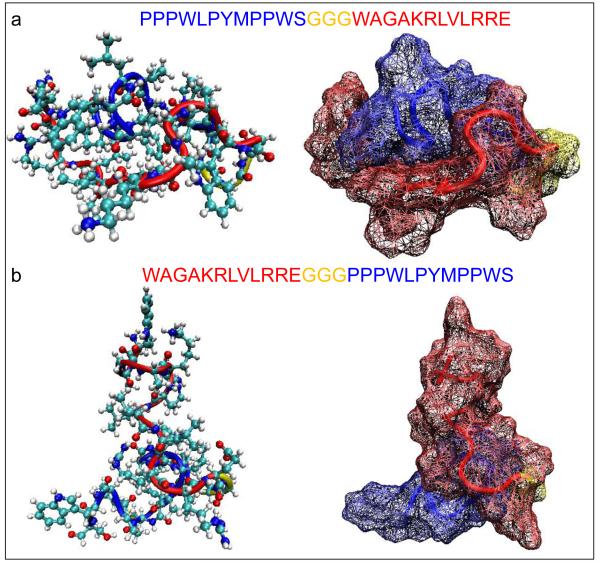
In fact our molecular structure findings (Figure 4) indicate that bifunctional QBP-AuBP and AuBP-QBP permutations adapt substantially different structures. QBP-AuBP adapts a globular conformation in which the two binding motifs (i.e., AuBP and QBP) interact with each other in a parallel-like fashion exposing gold- and silica-binding peptide domains in opposite sites (Fig. 4a). On the other hand, AuBP-QBP conformation has less interaction between the two different binding domains exposing gold- and silica-binding regions perpendicularly to each other (Fig. 4b). These structural differences may be the reason for the discrepancy observed between the gold nanopartile immobilization efficiency of QBP-AuBP and AuBP-QBP. We think that various functionalities of hetero-functional peptide permutations may be imposed by the favorable conformation of gold-binding region adapted upon assembly of QBP-AuBP on silica surface yielding an increased binding to gold nanoparticles. Whereas, in the case of AuBP-QBP either peptide assembly on the surface may hinder the gold-binding region resulting in a low yield of nanoparticle attachment or placing the gold-binding region at the N-terminus may decrease the peptide’s affinity towards silica.
Figure 4.
Predicted Structures of Hetero-functional Peptide Permutations. Molecular conformations of QBP-AuBP (a) and AuBP-QBP (b) hetero-functional peptides genereted by energy minimization calculations using Hyperchem 7.5 software.
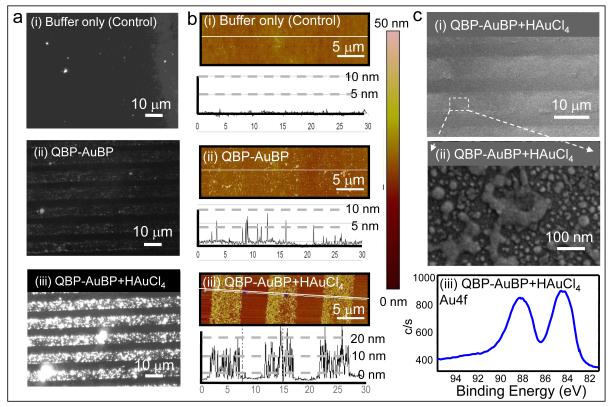
In the next set of experiments, taking advantage of the capability of the gold-binding sequence to reduce gold ions from gold precursor solution [31], we synthesized the gold nanoparticles in situ on the patterned QBP-AuBP arrays assembled on silica surfaces (Figure 1d). Peptide-mediated gold nanoparticle synthesis was achieved using an aqueous solution of 10 mM gold chloride at room temperature without additional reducing agents. All in situ synthetic experiments were conducted in the absence of any salt other than gold chloride in order to limit the effect of salt residues on gold reduction, nucleation and crystal growth processes [42]. Synthesized gold nanostructured arrays were imaged using dark field optical microscopy and AFM in tapping mode (Figure 5). The set of dark field and AFM micrographs in Figure 5a-b (ii) shows the silica substrate patterned with QBP-AuBP peptide prior to gold reduction. The AFM images confirmed that the peptide formed a homogeneous monolayer with negligible amount of agglomeration. The second set of dark field optical and AFM micrographs on Figure 5a-b (i and iii) shows a distinctive difference regarding to the gold formation on peptide-patterned surface compared to control ones after incubation with HAuCl4, the gold precursor. The results confirmed the formation of 10-20 nm gold particles on top of the QBP-AuBP patterns (for detailed AFM micrographs, see also Supplementary Material – Figure S3-S4).
Figure 5.
Fabrication of Gold Film Array via Peptide-mediated Gold Formation. Dark field images of the formed gold nanoparticle film array via the patterned QBP-AuBP peptide and its control sample (a); Corresponding AFM images of the samples along with their height profiles taken across the array (b); Scanning electron microscopy images (low and high magnification) and X-ray photoelectron spectroscopy spectrum obtained from gold nanoparticle film arrays (c).
The results in Figure 5c (i-iii) show that the patterned QBP-AuBP peptide mediates and templates the synthesis of fairly uniform porous gold film confirmed via scanning electron microscopy (SEM) and high resolution X-ray photoelectron spectroscopy (XPS) (for details see Supplementary Material – Figure S5-S6). The XPS spectrum from the same sample exhibits a significant presence of gold Au4f peaks, indicative of the formed gold film. The XPS spectra also display nitrogen-bonded carbon at 286.1 eV, as well as the N-C=O bonds at 288.0 eV, indicative of the presence of the intermediate peptide layer. In addition, we observed a decrease in the signals from the underlying elements, such as silicon and oxygen, indicating a partial obstruction of the surface by the newly formed gold. On the other hand, the spectrum at the Au4f position, in the absence of peptide, exhibits smaller peaks of the shape consistent with the unreduced gold salt which may have been left on the surface as a residue (Supplementary Material – Figure S5).
Finally, we demonstrated that these two different types of functionality, i.e. assembly and catalytic activity, can be combined to produce densely packed and thick gold films by inducing the growth of pre-immobilized “ seed ” nanoparticles using the engineered multi-functional peptide (Figure 1e). As evidenced by dark field and atomic force microscopy results shown in Figure 6 after 48 hours of incubation in aqueous gold chloride solution at room temperature, the pre-immobilized 50 nm “ seed ” particles increased in size to up to 200 nm due to the inherent catalytic activity of the multi-functional peptide.
Figure 6.
Fabrication of Gold Film Array via Peptide-mediated Immobilization and Gold Formation. Dark field images of the formed gold nanoparticle film array via QBP-AuBP-mediated gold nanoparticle immobilization and formation with control sample (a); Corresponding AFM images of the samples along with their height profiles taken across the array (b).
4. Conclusion
We demonstrate that designed and engineered multi-functional QBP-AuBP peptide can control both the assembly and the synthesis of gold nanostructures on silica surfaces and, thus, can be used successfully to fabricate gold nanoparticle arrays via a simple and robust protocol in aqueous solutions. Designed multi-functional QBP-AuBP peptide sequences reveal two promising research paths. Firstly, QBP-AuBP peptide-based linkers can be used in surface functionalization, creating hierarchical assemblies and co-assemblies of various nanostructures to be used in fabricating functional devices for biosensing and other relevant applications. The second path involves the potential utility of peptide sequences in the formation of hybrid multi-material nanostructures with controlled optical properties, e.g. gold-silica shell material, having direct applications in nanomedicine, biomolecular processes and nanophotonics [43, 44]. By combining the material linking capabilities of peptides with their catalytic activities within a single molecular framework, resulting multi-functional peptide building blocks may present a universal platform for wide range of nano- and micro-technologies where multiple materials and nano-entities may be required. In contrast to conventional chemical and physical methods, the approach described herein is highly practical while remaining biologically and environmentally friendly, and circumventing the limitations of stringent chemical or physical conditions that often prevent safe implementation of conventional methods for biomedical applications. The peptide-assisted biological fabrication route, therefore, offers modular design and versatile applicability with potential impact on diverse fields ranging from nanophotonics to medicine.
Supplementary Material
Acknowledgements
This work was supported by grants from National Science Foundation (DMR-0520567) through the Genetically Engineered Materials Science & Engineering Center (GEMSEC), an MRSEC and NIH T32 program from NCI, both at the University of Washington. The research was carried out at GEMSEC facilities, a part of MRSEC-Materials Research Facilities Network. Authors would like to thank Christopher So for technical help in obtaining AFM data.
Footnotes
Appendix A. Supplementary material Supplementary data associated with this article can be found, in the online version, at
References
- [1].Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP. Nat. Mater. 2008;7:442. doi: 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- [2].Leong K, Chen YC, Masiello DJ, Zin MT, Hnilova M, Ma H, Tamerler C, Sarikaya M, Ginger DS, Jen AKY. Adv. Funct. Mater. 2010;20:2675. [Google Scholar]
- [3].Wang CH, Yang C, Song YY, Gao W, Xia XH. Adv. Funct. Mater. 2005;15:1267. [Google Scholar]
- [4].Willner I, Baron R, Willner B. Biosens. Bioelectron. 2007;22:1841. doi: 10.1016/j.bios.2006.09.018. [DOI] [PubMed] [Google Scholar]
- [5].Tang ZY, Wang Y, Podsiadlo P, Kotov NA. Adv. Mater. 2006;18:3203. [Google Scholar]
- [6].Haynes CL, Van Duyne RP. J. Phys. Chem. B. 2001;105:5599. [Google Scholar]
- [7].Hammond PT. Adv. Mater. 2004;16:1271. [Google Scholar]
- [8].Golzhauser A, Eck W, Geyer W, Stadler V, Weimann T, Hinze P, Grunze M. Adv. Mater. 2001;13:806. [Google Scholar]
- [9].Porter MD, Bright TB, Allara DL, Chidsey CED. J.Am. Chem. Soc. 1987;109:3559. [Google Scholar]
- [10].Schreiber F. Prog. Surf. Sci. 2000;65:151. [Google Scholar]
- [11].Xia YN, Whitesides GM. Annu. Rev. Mater. Sci. 1998;28:153. [Google Scholar]
- [12].Qin D, Xia YN, Whitesides GM. Nat. Protoc. 2010;5:491. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- [13].Nath N, Chilkoti A. Anal. Chem. 2004;76:5370. doi: 10.1021/ac049741z. [DOI] [PubMed] [Google Scholar]
- [14].Seo HS, Kim SE, Park JS, Lee JH, Yang KY, Lee H, Lee KE, Han SS, Lee J. Adv. Funct. Mater. 2010;20:4055. [Google Scholar]
- [15].Kacar T, Ray J, Gungormus M, Oren EE, Tamerler C, Sarikaya M. Adv. Mater. 2009;21:295. [Google Scholar]
- [16].Brown S, Sarikaya M, Johnson E. J. Mol. Biol. 2000;299:725. doi: 10.1006/jmbi.2000.3682. [DOI] [PubMed] [Google Scholar]
- [17].Sarikaya M, Tamerler C, Jen AKY, Schulten K, Baneyx F. Nat. Mater. 2003;2:577. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- [18].Smith GP. Science. 1985;228:1315. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- [19].Boder ET, Wittrup KD. Nat. Biotechnol. 1997;15:553. doi: 10.1038/nbt0697-553. [DOI] [PubMed] [Google Scholar]
- [20].Brown S. Nat. Biotechnol. 1997;15:269. doi: 10.1038/nbt0397-269. [DOI] [PubMed] [Google Scholar]
- [21].Lee SW, Mao CB, Flynn CE, Belcher AM. Science. 2002;296:892. doi: 10.1126/science.1068054. [DOI] [PubMed] [Google Scholar]
- [22].Whaley SR, English DS, Hu EL, Barbara PF, Belcher AM. Nature. 2000;405:665. doi: 10.1038/35015043. [DOI] [PubMed] [Google Scholar]
- [23].Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO. Nat. Mater. 2002;1:169. doi: 10.1038/nmat758. [DOI] [PubMed] [Google Scholar]
- [24].Gungormus M, Fong H, Kim IW, Evans JS, Tamerler C, Sarikaya M. Biomacromolecules. 2008;9:966. doi: 10.1021/bm701037x. [DOI] [PubMed] [Google Scholar]
- [25].Matmor M, Ashkenasy N. J. Mater. Chem. 2011;21:968. [Google Scholar]
- [26].Dai HX, Choe WS, Thai CK, Sarikaya M, Traxler BA, Baneyx F, Schwartz DT. J. Am. Chem. Soc. 2005;127:15637. doi: 10.1021/ja055499h. [DOI] [PubMed] [Google Scholar]
- [27].Oren EE, Tamerler C, Sahin D, Hnilova M, Seker UOS, Sarikaya M, Samudrala R. Bioinformatics. 2007;23:2816. doi: 10.1093/bioinformatics/btm436. [DOI] [PubMed] [Google Scholar]
- [28].Kacar T, Zin MT, So C, Wilson B, Ma H, Gul-Karaguler N, Jen AKY, Sarikaya M, Tamerler C. Biotechnol. Bioeng. 2009;103:696. doi: 10.1002/bit.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tamerler C, Khatayevich D, Gungormus M, Kacar T, Oren EE, Hnilova M, Sarikaya M. Biopolymers. 2010;94:78. doi: 10.1002/bip.21368. [DOI] [PubMed] [Google Scholar]
- [30].Hnilova M, Oren EE, Seker UOS, Wilson BR, Collino S, Evans JS, Tamerler C, Sarikaya M. Langmuir. 2008;24:12440. doi: 10.1021/la801468c. [DOI] [PubMed] [Google Scholar]
- [31].Tamerler C, Sarikaya M. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2009;367:1705. doi: 10.1098/rsta.2009.0018. [DOI] [PubMed] [Google Scholar]
- [32].Turkevich J, Stevenson PC, Hillier J. Discuss. Faraday Soc. 1951:55. [Google Scholar]
- [33].Robinson CR, Sauer RT. Natl Acad Sciences. 1998:5929. doi: 10.1073/pnas.95.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wriggers W, Chakravarty S, Jennings PA. Biopolymers. 2005;80:736. doi: 10.1002/bip.20291. [DOI] [PubMed] [Google Scholar]
- [35].Slocik JM, Naik RR, Stone MO, Wright DW. J. Mater. Chem. 2005;15:749. [Google Scholar]
- [36].Slocik JM, Naik RR. Adv. Mater. 2006;18:1988. [Google Scholar]
- [37].Slocik JM, Stone MO, Naik RR. Small. 2005;1:1048. doi: 10.1002/smll.200500172. [DOI] [PubMed] [Google Scholar]
- [38].Levy R, Thanh NTK, Doty RC, Hussain I, Nichols RJ, Schiffrin DJ, Brust M, Fernig DG. J. Am. Chem. Soc. 2004;126:10076. doi: 10.1021/ja0487269. [DOI] [PubMed] [Google Scholar]
- [39].Wang Z, Levy R, Fernig DG, Brust M. Bioconj. Chem. 2005;16:497. doi: 10.1021/bc050047f. [DOI] [PubMed] [Google Scholar]
- [40].So CR, Kulp JL, Oren EE, Zareie H, Tamerler C, Evans JS, Sarikaya M. Acs Nano. 2009;3:1525. doi: 10.1021/nn900171s. [DOI] [PubMed] [Google Scholar]
- [41].Seker UOS, Wilson B, Dincer S, Kim IW, Oren EE, Evans JS, Tamerler C, Sarikaya M. Langmuir. 2007;23:7895. doi: 10.1021/la700446g. [DOI] [PubMed] [Google Scholar]
- [42].Diamanti S, Elsen A, Naik R, Vaia R. J. Phys. Chem. C. 2009;113:9993. [Google Scholar]
- [43].Bardhan R, Mukherjee S, Mirin NA, Levit SD, Nordlander P, Halas NJ. J. Phys. Chem. C. 2010;114:7378. [Google Scholar]
- [44].Lowery AR, Gobin AM, Day ES, Halas NJ, West JL. Int. J. Nanomedicine. 2006;1:149. doi: 10.2147/nano.2006.1.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.