Abstract
Recent evidence suggests that Medicare Part D increased prescription drug use among seniors, and increased pharmaceutical firms’ revenues from sales. Previous studies also indicate that increases in market size induce pharmaceutical innovation. This paper assesses the impact of the Medicare Part D legislation on pharmaceutical research and development (R&D), using time-series data on the number of drugs entering preclinical and clinical development by therapeutic class and phase. We find that the passage and implementation of Medicare Part D is associated with significant increases in pharmaceutical R&D for therapeutic classes with higher Medicare market share.
Keywords: Research and Development, Innovation, Pharmaceutical Industry, Medicare Part D
1 Introduction
Understanding the responsiveness of innovation to expected future revenues and market expansions is central to understanding the behavior of private sector innovative firms, and is also critical for evaluating the welfare effects of public policies such as insurance expansions, price controls, and patent protection. Although previous studies have shown that increases in market size are significant drivers of pharmaceutical innovation, magnitudes of the estimates of elasticity of innovation with respect to market size vary widely (Acemoglu and Linn, 2004; Dubois et al., 2011)
This paper builds on this existing literature on the impact of market size on innovation by analyzing the effects of one of the largest expansions of prescription drug insurance on pharmaceutical research and development (R&D). Specifically, we estimate the elasticity of drug R&D efforts—as measured by preclinical testing and clinical trials—following passage of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA), and evaluate changes over time in the magnitude of this investment response.
Prior to the MMA’s implementation in 2006, with only a few exceptions1 the Medicare program covered only prescription medicines associated with physician services, i.e., drugs provided in physician offices and hospitals. Medicare Part D significantly expanded drug coverage among older individuals, and as of 2010 approximately 28 million Medicare beneficiaries were enrolled in Part D plans.2,3 Recent evidence indicates that this expanded insurance coverage increased prescription drug use by seniors (Duggan et al., 2008; Ketcham and Simon, 2008; Lichtenberg and Sun, 2007; Yin et al., 2008).
This increased use of prescription drugs due to expansion of insurance might be expected to yield increases in biopharmaceutical firms’ R&D via two mechanisms. First, Scherer (2001) previously showed firms’ R&D expenditures are approximately unit elastic with respect to increases in their revenues from sales. Duggan and Scott Morton (2010) showed that overall revenues for pharmaceutical firms increased upon implementation of Part D, despite the price decreases negotiated by private insurers. Thus, R&D might have increased after implementation of Part D simply due to established firms’ increased cash flows.
Second, economic theory and prior studies also suggest that firms’ investments in R&D should be responsive to changes in the expected profitability of candidate products in their pipelines. Consistent with this notion, Friedman (2009) observed immediate increases after passage of Part D in stock market share prices for firms launching brand-name drugs with high exposure to the Medicare market. These expectations of near-term and future revenues also likely contributed to the pharmaceutical industry’s switch from opposition towards advocacy for Medicare outpatient prescription drug legislation in 1999:
Successfully expanding prescription drug coverage for seniors and disabled persons will ensure that breakthroughs in basic scientific knowledge become safe and effective medicines for patients. If we fail, pharmaceutical innovation—especially with respect to medicines designed to treat the illnesses of aging—may suffer, thereby reducing hope for Medicare beneficiaries and their families. Modernizing Medicare is our best hope that today’s and tomorrow’s beneficiaries will reap the rewards of innovation: longer, happier, healthier, and more fulfilling lives. (Holmer, 1999)
In this paper, we identify the effects of Medicare Part D through variation across drug classes in their pre-Part D Medicare market shares, expecting larger increases in R&D for drug classes with higher pre-Part D Medicare market shares. We control for changes in demographics, as one would expect more R&D for higher-Medicare-share drug classes as the post-war Baby Boom population ages. We also control for changes in public expenditures on biomedical research, due to possible complementarities between public and private biomedical research efforts (Blume-Kohout, 2012).
To further isolate Part D’s impact, in additional specifications we investigate heterogeneous effects using variation in drug classes’ coverage status, such as whether the drug class was previously covered under Medicare Part B, whether it has “protected” status on plans’ formularies, and whether the class is highly used by Medicare-Medicaid dual eligibles. We expect smaller effects for drugs previously covered by Medicare Part B because the MMA legislation decreased physician reimbursement for cancer chemotherapy drugs covered under Part B, and so neither expected utilization nor prices for those drugs should have increased with the MMA (Shea et al., 2008). In contrast, we expect larger Part D effects for protected drug classes, as the Centers for Medicare and Medicaid Services (CMS) require most marketed drugs in certain protected drug classes to be included on Part D plans’ formularies, which precludes plans from using threat of exclusion to negotiate prices down. Similarly, we also expect larger Part D effects for drug classes heavily used by Medicare-Medicaid dual eligibles, as pharmaceutical firms are no longer required to offer steep Medicaid discounts for drugs sold to these consumers.
We find that the passage and implementation of Medicare Part D was associated with significant increases in preclinical testing and clinical trials for those drug classes most likely to be affected by Medicare Part D. These effects are robust to controls for expected demographic changes, and changes in public biomedical research funding. As expected, we also typically find smaller effects for drug classes previously covered by Medicare Part B, and larger effects for protected and dual eligible drug classes.
Our paper proceeds as follows. In the next section we describe the various datasets employed, and the construction of our panel dataset. In Sections 3 and 4, we present our empirical strategies, and summarize key results. The final section concludes, with a discussion of the implications and limitations of our analysis.
2 Data and Construction of Analytic Panels
2.1 Data on Pharmaceutical R&D Pipelines
Time-series data on the number of drugs by therapeutic class and originator firm at each stage of the pharmaceutical R&D pipeline were derived from the Pharmaprojects trend data “snapshot” published each May from 1998 through 2010. Pharmaprojects data are collected from a variety of public sources including press releases, patent filings, conference proceedings, regulatory agencies’ reports, and the medical literature, as well as through direct contacts with pharmaceutical companies and researchers. As noted by Adams and Brantner (2006), this collection process may miss some drugs in early stage development. However, commercial databases like Pharmaprojects are generally considered fairly complete for human clinical trials, as existence of a recruiting clinical trial for an already-patented molecule is more difficult to hide than proprietary investigations in a firm laboratory. Potential omissions due to underreporting are also of little concern in this analysis, as we have no reason to expect systematic bias in Pharmaprojects’ reporting across therapeutic classes that both (a) coincides with the introduction of Part D and (b) is correlated with Medicare market share. Unless both conditions (a) and (b) are met, underreporting will not bias our estimates.
The duration from entry into human clinical testing (Phase I trials) until market launch can vary widely across individual drugs and broader therapeutic classes, but averages approximately eight years (Abrantes-Metz et al., 2005; Adams and Brantner, 2006; DiMasi, 2001). Phase I trials evaluate safety of the molecule in small numbers of healthy human volunteers, and typically take several months to complete. For successful drugs (i.e., those continuing on to Phase II), duration until start of Phase II is about 20 months. Phase II trials are, in a sense, pilot studies: pharmaceutical firms evaluate efficacy of the drug in a relatively small number of patients (usually just a few hundred), with successful drugs proceeding from Phase II to the most expensive, larger-scale Phase III trials after an average of 2.5 years. Finally, Phase III trials may involve thousands of patients, with average duration of approximately 4 years. As a benchmark, then, if pharmaceutical firms had products ‘on the shelf’ they could push into clinical testing as investigational new drugs in the months after passage of the legislation, we might expect to see an increase in Phase I trials in 2004, with successful products entering Phase II in 2005–2006, and Phase III in 2008 or later. It is also possible that some molecules directly entered Phase II or Phase III clinical trials soon after passage of Part D. For example, firms could conduct Phase II or Phase III trials to investigate additional indications for drugs already on the market.
With this notional progression in mind, in Figure 1 we present graphical evidence of structural breaks in the number of drugs entering preclinical testing, and Phase I, Phase II, and Phase III clinical trials. The number of drugs entering preclinical testing was fairly steady or slightly declining until 2003, increased dramatically in 2004 after the passage of Part D, then trended upward from 2005 to its peak in 2009. The number of drugs entering Phase I trials for our panel each year was fairly steady until 2003, increased modestly in 2004 after the passage of Part D, and then remained steady through 2006 when Part D was implemented. In 2007, the number of drugs entering Phase I trials increased markedly, and continued on an upward trajectory through 2010. These aggregate trends certainly suggest that the passage and implementation of Part D was associated with increase in the flow of Phase I trials; however, these trends could also be confounded by changes in other determinants of Phase I R&D that were coincident with the passage and implementation of Part D.
Figure 1.
Number of drugs entering clinical trials by phase and year, analytic panel of 49 therapeutic classes, 1998 – 2010
Source: Authors’ calculations based on pharmaprojects data.
Note: Only includes trials for analytic panel of Pharmaprojects classes matched to Multum therapeutic categories.
Trends for Phase II and Phase III trials likewise show large increases in R&D only after Part D’s implementation. Prior to 2004, Phase II trials were only slightly increasing, and Phase III trials had a slight negative trend. In 2004, both Phase II and Phase III increased only slightly versus prior trends, and remained fairly steady at that level through 2006. Interestingly, while a pulse of drugs entered Phase III trials in 2008, that increase appears to be the peak of the Part D response for Phase III. The apparent leveling off for new Phase III trials after that point could be attributable to capital restriction in the Great Recession, or may simply reflect those ‘off the shelf’ products progressing through testing.
Two additional features of these data are worth noting. First, the average number of clinical trials in any given year varies significantly across drug classes. Our empirical models include drug class fixed effects to account for this confounding. Second, the trends in number of trials prior to implementation also differ across drug classes. Our empirical models include drug class-specific time trends to control for these pre-existing differences in R&D activity, and also control for other established determinants of R&D, such as the potential market size and previous years’ public R&D expenditures related to each drug class.
Figure 2 presents a case study to illustrate our empirical strategy. It provides a closer look at trends in clinical trials for two drug classes: treatments for Alzheimer’s disease, and hormonal contraceptives. Alzheimer’s disease is the drug class with the highest Medicare share in our panel, and (not surprisingly) contraceptives have the lowest Medicare share. The Medicare share of Alzheimer’s disease is 86% and Medicare share of contraceptives is less than 1%. The data shown in Figure 2 illustrate in the simplest form possible both our empirical strategy and our results. We see that the number of clinical trials for drugs to treat Alzheimer’s disease was declining prior to the implementation of Part D. However, this trend reversed after the passage of Part D. Implementation of Part D in 2006 is associated with further strengthening of this upward trajectory in number of clinical trials for Alzheimer’s disease treatments, with the number of trials increasing from about 10 in 2003 to roughly 50 in 2010. In contrast to Alzheimer’s disease, the trend in number of clinical trials for contraceptives seems to be uncorrelated with the passage and implementation of Part D. With the exception of sharp increase in number of trials in 2008, the number of trials has remained fairly stable since passage of Part D.
Figure 2.
Trends in the number of drugs entering clinical trials for Alzheimer’s disease versus hormonal contraceptives, 1998 – 2010
2.2 Data on Medicare Share by Therapeutic Class
We estimated the share of total prescriptions filled by Medicare-covered individuals each year for each therapeutic class using data from the 2004 and 2005 Medical Expenditure Panel Survey (MEPS), a publicly available, nationally representative survey of the U.S. civilian non-institutionalized population. Approximately 93% of the prescription fill events in the MEPS Prescribed Medicines files included the prescription drug’s classification into Multum therapeutic classes and subclasses, and all include a person-level identifier, which we used to merge the prescription filled with the person’s demographic data. These demographic data were obtained from the MEPS Full Year Consolidated Data File, which includes variables such as whether the respondent was enrolled in Medicare that year. With these data, similar to the approach used by Duggan and Scott Morton (2010), for each therapeutic subclass in MEPS we calculate the survey-weighted fraction of prescriptions filled by Medicare beneficiaries, thus generating our key explanatory variable: drug class pre-Part D Medicare market share. Because MEPS explicitly excludes drugs received while in hospital, it is particularly well-suited to estimating Medicare-eligibles’ share of outpatient prescription drugs newly covered under Part D.
Approximately 41% of outpatient prescriptions in MEPS for 2004–2005 were filled by Medicare-covered individuals. Therapeutic classes from Pharmaprojects were matched to therapeutic subclasses from the MEPS 2004 and 2005 Prescribed Medicines files (see Appendix for crosswalk). Classes with fewer than 100 prescription events observed or with standard errors of the Medicare share estimate exceeding 0.10 were either dropped from the sample, or in a few cases merged with similar larger therapeutic classes to increase precision of the classes’ respective Medicare share estimates.4 This process resulted in our panel of 49 therapeutic classes.
2.3 Population Projections to Control for Demographic Shifts
Our identification strategy exploits variation in pharmaceutical R&D activity across drug classes with varying exposure to the Medicare market, proxied by their pre-Part D shares of prescriptions filled by Medicare beneficiaries. We hypothesize that, if Medicare Part D influenced pharmaceutical R&D, drug classes with higher Medicare share markets should experience greater growth in R&D. However, because drug classes’ future Medicare market share is also strongly driven by demographic trends (specifically, population aging), failure to control for exogenous demographic changes could bias our estimates of the effect of Part D. In other words, as the Baby Boom population ages, one would expect larger increases in R&D due to demographic shifts increasing market size for drugs most used by seniors. To control for effects of these demographic changes on R&D, we predict leads of market size, , for each therapeutic class, c, in each year, t, as follows:
| (1) |
where OECDa,g,t+f is the OECD population projection for five-year age group a (e.g., ages 0–4) and gender g in year t+f, and Rxsc,a,g is the estimated annual number of prescriptions filled for class c by members of group a,g based on MEPS 2004–2005 data. The OECD population projections are derived from United Nations Population Division World Population Prospects “Medium Variant” estimates for each of the OECD member countries, which are provided at five year intervals for years 2000 through 2030.5 We first sum the population estimates for all OECD member countries for each year projected by the UN, then we linearly interpolate population estimates for the years between these intervals to derive the annual population estimates used in Equation (1). We use OECD demographic projections to estimate future market growth because, to the extent that R&D efforts of global pharmaceutical firms are driven by demographic as opposed to reimbursement-related changes, these firms should respond to demographic changes in countries representing the majority of their demand: the U.S., Canada, Europe, and Japan. IMS (2011) estimates the U.S. share of global pharmaceutical demand will fall to less than 1/3 by 2015. On the other hand, most of the “pharmerging” market spending is on generic drugs, so would likely have less impact on innovator firms’ R&D. For this reason, we use demographic changes in OECD countries as representative of demographic changes expected in brand-name innovator markets.
We employ these estimates of market size in two ways. First, we estimate all models using point estimates for log(market size) at expected year of product launch, 5 years post-launch, and 12 years post-launch. Expected time-to-market after entry into Phase I, Phase II, and Phase III trials and product launch was derived from previously published estimates (Adams and Brantner, 2006). Then, to reflect changes in market size over the entire expected exclusive market life of the drug (that is, before generic introduction), we calculate the present value of market size from the expected year of product launch through 12 years post-launch using a discount rate of 11%, corresponding to the cost of capital for pharmaceutical firms. The 12 year window loosely reflects Grabowski and Kyle’s (2007) finding that NMEs launched from 1995 through 2005 with over $100 million in sales had, on average, 11 years of market exclusivity. We find our results are robust to either choice of market size covariate.
2.4 Data on Lagged NIH R&D Funding by Disease
Blume-Kohout (2012) showed that marginal increases in National Institutes of Health (NIH) grant funding for any given disease may result in a modest increase in the number of drugs entering clinical development to treat that disease, after some lag. Because the NIH budget doubling coincides with our pharmaceutical R&D time series, and because R&D did not increase proportionally across diseases and patient age groups during the doubling period (see for example Gitterman et al., 2004), the larger increase in trials we observe for higher Medicare share drugs could theoretically be due to earlier disproportionate increases in basic research on diseases that predominantly affect older individuals. We control for this possibility by including lagged changes in NIH R&D funding for diseases treated by each therapeutic class, using data obtained via the algorithm detailed in Blume-Kohout (2012). When specific NIH R&D funding estimates were unavailable for a given therapeutic class, we substituted changes in the overall NIH Institute or Center budget most closely related to that therapeutic class. For example, in the absence of detailed annual NIH R&D funding estimates to estimate year-on-year percentage changes for Urological diseases, we substitute year-on-year percent changes in the budget for the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), obtained from the NIH website.6
3 Empirical Approach
In the analyses that follow, our primary outcome variables are the number of drugs entering each R&D stage (Preclinical, Phase I, Phase II, and Phase III), for each therapeutic class and year. If pharmaceutical companies respond to the increases in market size as predicted, then all else equal we would expect to see an increase in the flow of drugs entering preclinical and clinical development. Table 1 below provides descriptive statistics for our outcome variables and our key explanatory variable.
Table 1.
Descriptive statistics for analytic panel of 49 therapeutic classes, 1998 – 2010
| Drugs Entering Preclinical Testing, by Class | Drugs Entering Phase I Trials, by Class | Drugs Entering Phase II Trials, by Class | Drugs Entering Phase III Trials, by Class | Drug Class Medicare Market Share of Rx Fills, 2004 – 2005 | |
|---|---|---|---|---|---|
| Mean | 24.31 | 6.51 | 6.43 | 2.42 | 42% |
| Range | 0 – 240 | 0 – 62 | 0 – 39 | 0 – 19 | 0.7 – 86.5% |
| St. Dev. | 28.60 | 7.59 | 6.46 | 2.72 | 21% |
3.1 Descriptive Results for Drugs Entering Clinical Trials
We begin with descriptive analysis of changes in the total number of clinical trials by Medicare market share. For each drug class, we individually estimated:
| (2) |
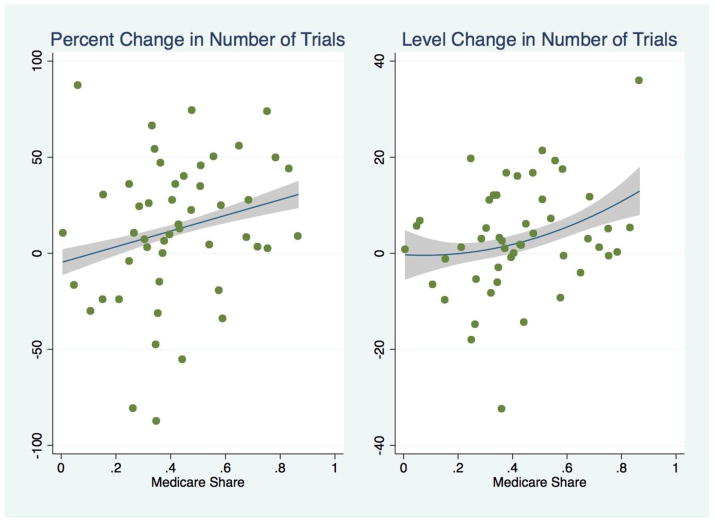
Then, we compared the coefficient estimates b across drug classes. If Part D affected drug R&D, we would expect to see larger coefficients for drug classes with higher Medicare market share. The results of this analysis are presented in Figure 3, providing some graphical evidence for our intuition. If we simply compare the number of trials predicted for each drug class based on their individual pre-Part D trends versus actual trials reported for that class post-2005, we find little change for low Medicare share classes versus prior trends, whereas the highest Medicare share classes show a more marked increase in drugs entering clinical trials after Part D implementation than would otherwise be expected. For a drug class with average Medicare market share, this corresponds to about a 12% increase over expectation in the number of trials by 2006, or about 3 additional drug trials added.
Figure 3.
Percent and Level Change in Clinical Trials versus Expectation, After Implementation of Medicare Part D, for Analytic Panel of 49 Drug Classes
Next, we estimated several interrupted time-series models exploiting variation in drug development across drug classes with differing Medicare market shares. All of our models include drug class fixed effects, to control for time-invariant differences in R&D levels across drug classes. In addition, because we found that pre-Part D time trends across drug classes differ significantly, we also include class-specific time trends, interacting year with the dummy variable for each class. In alternate specifications we investigated class-specific quadratic time trends with similar results.
Intuitively, while the numeric change in the number of drugs entering clinical development should be proportional to the size of the Medicare market for each class, the percentage change in the number of drugs entering clinical development for each class following Part D should be proportional to each market’s Medicare share. That is, all else (including total expected market size) equal, we would expect a greater percentage change versus classes’ respective R&D trends for markets with a higher percentage of prescriptions filled by Medicare beneficiaries.
Given the graphical evidence in Figure 1 and relevant legislative, R&D, and regulatory timelines, we also anticipated that Medicare Part D could have had dynamic impact on pharmaceutical R&D, corresponding to four periods: (1) pre-programmatic dip in investment due to uncertainty about the legislation in 2003; (2) anticipatory effects in 2004 and 2005, after the Part D legislation was passed but before implementation;7 (3) post-implementation effects observed in 2006 and 2007; and (4) lagged post-implementation effects in 2008 and beyond. The lagged post-implementation effects could arise, for example, if Part D caused an increase in Phase I trials in 2006–2007, but those marginal trials tested products already ‘on the shelf.’ Then, after pre-existing products were pushed into clinical trials, there could be a refractory period before the new equilibrium rate of R&D is reached. We therefore allow for changes in the magnitude of the effect of Part D over time.
Finally, as discussed above in Sections 2.3 and 2.4, we wanted to control for secular demographic shifts that may be correlated with Medicare share, and any effects of changes in levels of NIH R&D funding across diseases. The results reported in Tables 2 and 3 include these controls, which decrease the magnitude of the estimates only slightly. The reported models are thus given by:
| (3) |
and MktSizec,s is the f* to f*+12 year lead of expected market size at time of product launch, in year t + f*, calculated via Equation (1). For example, for our Phase I models, reported in Table 3, f* = 8. Finally, based on prior estimates of the relevant lags of NIH R&D funding, we also include the 4th through 15th lags of log(NIH R&D funding). Standard errors are clustered on therapeutic class, and allow for cross-model (cross-phase) correlations within classes via simultaneous post-estimation of the variance-covariance matrix.
Table 2.
Dynamic changes in number of drugs entering preclinical versus clinical-stage R&D trials due to Medicare Part D
| (1) Preclinical | (2) All Clinical Trials | |
|---|---|---|
| Medicare Share * Part D 2003 |
0.118 (0.119) | −0.146 (0.147) |
| Medicare Share * Part D 2004–2005 |
0.812*** (0.175) | 0.264 (0.173) |
| Medicare Share * Part D 2006–2007 |
0.743*** (0.253) | 0.435** (0.178) |
| Medicare Share * Part D 2008–2010 |
1.412*** (0.386) | 0.892*** (0.212) |
| Observations | 637 | 637 |
| Number of Therapeutic Classes | 49 | 49 |
| Dispersion | 1.67 | 1.37 |
significant at 10%;
significant at 5%;
significant at 1%
Notes: Authors’ calculations based on Medical Expenditure Panel Survey (MEPS) 2004–2005 and Pharmaprojects 1998–2010 trend data. The outcome variable for the Preclinical model in column (1) is the number of drugs entering preclinical testing in a given therapeutic class, in a given year. The outcome variable for the All Clinical Trials model presented in column (2) is the number of drugs entering Phase I, Phase II, or Phase III clinical testing in a given therapeutic class and year. All models are Poisson, and include therapeutic class fixed effects and class-specific time trends, 4- to 15-year lags of NIH R&D funding, and discounted lead of market size at expected time of product launch to 12 years post-launch. Standard errors are presented in parentheses below each coefficient estimate, adjusted for clustering on therapeutic class and estimated simultaneously across all models presented in Tables 2 and 3, to allow for cross-phase correlations within each class.
Table 3.
Dynamic changes in the number of drugs entering clinical testing after passage of Medicare Part D
| (1) Phase I Trials | (2) Phase II Trials | (3) Phase III Trials | |
|---|---|---|---|
| Medicare Share * Part D 2003 |
0.353 (0.232) | −0.503*** (0.190) | −0.550* (0.317) |
| Medicare Share * Part D 2004–2005 |
0.640*** (0.190) | −0.127 (0.270) | 0.331 (0.362) |
| Medicare Share * Part D 2006–2007 |
0.810*** (0.272) | −0.038 (0.307) | 0.639 (0.452) |
| Medicare Share * Part D 2008–2010 |
1.251*** (0.397) | 0.284 (0.390) | 1.408** (0.572) |
| Observations | 637 | 637 | 637 |
| Number of Therapeutic Classes | 49 | 49 | 49 |
| Dispersion | 1.21 | 1.24 | 1.10 |
significant at 10%;
significant at 5%;
significant at 1%
Notes: Authors’ calculations based on Medical Expenditure Panel Survey (MEPS) 2004–2005 and Pharmaprojects 1998–2010 trend data. The outcome variable for each model is the number of drugs entering Phase I (column 1), Phase II (column 2), or Phase III (column 3) clinical testing in a given therapeutic class and year. All models are Poisson, and include therapeutic class fixed effects and class-specific time trends, 4- to 15-year lags of NIH R&D funding, and discounted lead of market size at expected time of product launch to 12 years post-launch. Standard errors are presented in parentheses below each coefficient estimate, with the VCE matrix adjusted for clustering on therapeutic class, and estimated simultaneously across all models presented in both Tables 2 and 3, to allow for cross-phase correlations within each class.
4 Results
In this section, we present regression results from panel count data estimation for drugs entering preclinical and clinical testing. We also compare results for drug trials in classes previously covered by Medicare Part B, and protected drug classes including those most used by Medicare-Medicaid dual eligible beneficiaries. Finally, we discuss results from our analysis of worldwide drug launches and firms’ R&D expenditures, which support the trends we observe in firms’ R&D efforts.
4.1 Dynamic Changes in Drug Development
First, we evaluated whether Medicare Part D differentially affected preclinical and clinical investigations for drug classes with higher Medicare market share over four time periods: (1) pre-programmatic effects in 2003; (2) transitional effects in 2004 and 2005, after passage but before Part D was implemented; (3) post-implementation effects in 2006 and 2007, as more information became available to industry about Part D’s implementation and consequences, and revenues from sales to Medicare beneficiaries increased; and (4) lagged post-implementation effects in 2008 and beyond, as any increases in testing earlier in the R&D pipeline propagated through to clinical-stage investigations.
Although the number of drugs in preclinical development might respond rapidly to policy changes, it would take time for those new drugs to achieve investigational new drug (IND) approval, and then proceed through the R&D pipeline. So, any Part D-related increase in Phase III trials prior to, say, 2008 would likely be for products that were (a) ‘on the shelf’ and responsive at the margin to the increase in net present value presented by Part D, (b) already marketed, and in trials for supplemental indications, and/or (c) combination therapies with component active ingredients that had already been tested in humans. These products might simply bypass preclinical and Phase I testing.
Table 2 shows the effects of Medicare Part D on the number of drugs entering preclinical testing, and on new clinical trials across all R&D stages. We see no overall pre-programmatic effect in 2003 for preclinical or all clinical trials combined. However, for the average drug class, we estimate a 31–33% increase (p<.01) over expected trends in the number of drugs entering preclinical testing in 2004 through 2007, and a 58% increase (p<.01) over expected trends in the number of drugs entering preclinical testing after 2008. Effects on clinical trials are somewhat more modest, with no significant effect seen until after implementation in 2006. For the average drug class, we see an 18% increase versus expectation in drugs entering all stages of clinical testing in 2006–2007 (p<.05), and a 38% increase in 2008–2010 (p<.01).
Next, we estimated effects of Part D on each stage of clinical R&D, Phase I through Phase III. As shown in Table 3, our results for Phase I trials are similar to our results for preclinical testing. For the average drug class, including controls for expected market size and lagged NIH funding, we estimate the number of drugs entering Phase I trials in 2004–2005 increased by 27% versus expected trends. By 2006–2007, for the average drug class, the number of drugs entering Phase I trials had increased by about 34% versus expected trends, and by 2008–2010 Phase I trials had increased by a little over 50%. At the means, this translates to about 2–3 additional drugs reported as entering Phase I trials, per drug class.
Although neither preclinical nor Phase I trials evidenced any pre-programmatic effect, we do see a significant decrease in higher-Medicare share drug classes’ new Phase II and Phase III trials in 2003, perhaps reflecting uncertainty about the effects or provisions of the eventual legislation. Phase II trials appear to have been unresponsive otherwise, but Phase III trials increased significantly after a lag, with the average drug class experiencing about a 59% increase (p<.05) in Phase III trials in 2008 and beyond.
All of the results reported in Tables 2 and 3 are robust to alternative estimation procedures (e.g., Poisson versus negative binomial estimation, or inclusion of quadratic drug class specific time trends) and to casewise deletion of individual drug classes from the panel.
4.2 Differential Effects of Part D for Protected Drug Classes, Classes Previously Covered Under Medicare Part B, and Classes Used by Dual Eligibles
Prior to the passage of Medicare Part D, certain drugs (e.g., IV cancer drugs, immunosuppressants, insulin for patients using pumps, COPD medications for patients using nebulizers, etc.) were already covered under Medicare Part B. In fact, 12 of the top 20 medications covered by Part B, representing over 40% of Part B expenditures in 2001, were for cancer treatment (Medicare Payment Advisory Commission, 2003). Because a significant fraction of cancer drugs and immunosuppressants did not experience a change in insurance coverage with Part D, we would expect relatively lower effects of Part D on R&D for these classes, all else equal.
On the other hand, although in most cases Part D plans are only required to have two drugs on their formularies for each therapeutic class, plans essentially cannot exclude any marketed prescription drugs in six “protected” classes: HIV antivirals, cancer drugs, immunosuppressants, antipsychotics, antidepressants, and anticonvulsants. For these protected markets, we would expect relatively higher impact of Part D on R&D, because private Part D plans cannot use threats of formulary exclusion to negotiate lower prices. Given these competing effects (prior Part B coverage for some drugs, and protection from exclusion for nearly all drugs) in the markets for immunosuppressants and cancer treatments, it is not immediately clear which effect should dominate.
In Table 4, we present for comparison results from a single (combined) negative binomial model, allowing different dynamic effects of Part D for protected classes and classes previously covered under Medicare Part B. As before, negative binomial versus Poisson estimation has little effect on our coefficient estimates; however, due to evidence of moderate overdispersion in the preclinical data (see Table 2), the negative binomial assumption permits more precise estimation.
Table 4.
Heterogeneous effects of Medicare Part D on preclinical testing for protected drug classes, and for drug classes previously covered under Part B.
| Non-Protected Class * Medicare Share * Part D |
Protected Class * Medicare Share * Part D |
Part B Class * Medicare Share * Part D |
|
|---|---|---|---|
| Part D 2003 | 0.141 (0.121) | −0.119 (0.179) | −0.507 (−0.399) |
| Part D 2004–2005 | 0.773*** (0.175) | 1.260*** (0.461) | −0.403 (0.546) |
| Part D 2006–2007 | 0.707*** (0.245) | 1.445** (0.625) | −0.631 (0.735) |
| Part D 2008–2010 | 1.333*** (0.372) | 2.775*** (0.736) | −0.912 (1.324) |
| Observations | 637 | ||
| Number of Therapeutic Classes | 49 | ||
| Dispersion | 1.02 | ||
significant at 10%;
significant at 5%;
significant at 1%
Notes: Authors’ calculations based on Medical Expenditure Panel Survey (MEPS) 2004–2005 and Pharmaprojects 1998–2010 trend data. All results presented were estimated within a single negative binomial model, with outcome variable the number of drugs entering preclinical testing by therapeutic class and year, and including dummy variables for therapeutic class fixed effects, 4- to 15-year lags of NIH R&D funding, discounted leads of market size at expected time of product launch to 12 years post-launch, and class-specific time trends to control for pre-existing differences across classes. Standard errors are presented in parentheses below each coefficient estimate, adjusted for clustering on therapeutic class.
As one would expect, our results for non-protected drug classes are nearly identical to the overall results presented in Table 2, with significantly higher preclinical R&D versus expectation (p<.01) in all periods after passage of Medicare Part D. As before, for the average non-protected drug class we estimate about a 30% increase in preclinical trials in the period 2004 through 2007, and a significantly higher (p<.001) 56% increase in preclinical trials over expected trends in 2008 and beyond. Although the larger point estimates for protected classes are suggestive of a stronger Part D effect in all periods 2004–2010, the difference is only statistically significant in 2008 and beyond (p=.064). In contrast, as expected we find no significant effect of Medicare Part D on drug classes previously covered under Medicare Part B. Applying a similar dynamic model for Phase I trials reveals very similar results, again with larger effects for protected classes and no significant effect on classes previously covered under Medicare Part B. However, for Phase II trials, the only significant effect (p<.05) found is prior to implementation: drug trials for protected classes declined by about 60% versus expectation in the period 2003–2005.
In Table 5, we present results from negative binomial estimation for drugs entering Phase III trials. For the average non-protected drug class, Phase III trials increase by about 34% (p<.10) after implementation of Medicare Part D in 2006. Interestingly, we do not see any significant effect on drugs entering Phase III for the protected classes. Further investigation reveals this lack of effect may be attributed to protected mental health drugs, which have had high failure rates in Phase II trials in recent years due to lack of efficacy versus placebo (Arrowsmith, 2011). Part D’s effect on these drug classes—specifically, antipsychotics and antidepressants—is further complicated due to their high utilization among Medicaid and Medicare “dual eligibles” (Verdier et al., 2008). In 2006, prescription drug coverage for dual eligible beneficiaries transferred from price-regulated state Medicaid programs to private Medicare Part D plans, increasing the revenues firms received for these classes. As such, one would expect a relatively stronger effect of Part D for the dual eligibles’ most highly used, high revenue classes: antipsychotics, antidepressants, GI ulcer treatments.
Table 5.
Heterogeneous effects of Medicare Part D on Phase III trials for protected drug classes, and for drug classes previously covered under Part B.
| Non-Protected Class * Medicare Share * Part D |
Protected Class * Medicare Share * Part D |
Part B Class * Medicare Share * Part D |
|
|---|---|---|---|
| Part D 2003 | −0.403 (0.326) | −1.597** (0.733) | −1.904* (1.131) |
| Part D 2004–2005 | 0.427 (0.380) | −0.159 (1.714) | −0.787 (1.143) |
| Part D 2006–2007 | 0.821* (0.467) | −1.842 (2.698) | −0.503 (0.894) |
| Part D 2008–2010 | 1.516** (0.608) | 0.206 (3.827) | 0.903 (1.298) |
| Observations | 637 | ||
| Number of Therapeutic Classes | 49 | ||
| Dispersion | 0.92 | ||
significant at 10%;
significant at 5%;
significant at 1%
Notes: Authors’ calculations based on Medical Expenditure Panel Survey (MEPS) 2004–2005 and Pharmaprojects 1998–2010 trend data. All results presented were estimated within a single negative binomial model, with outcome variable the number of drugs entering Phase III clinical trials by therapeutic class and year, and including dummy variables for therapeutic class fixed effects, 4- to 15-year lags of NIH R&D funding, discounted leads of market size at expected time of product launch to 12 years post-launch, and class-specific time trends to control for pre-existing differences across classes. Standard errors are presented in parentheses below each coefficient estimate, adjusted for clustering on therapeutic class.
In Table 6, we present for comparison results for Phase I and Phase III trials, separating out the effect for these classes highly used by dual eligibles. We find that the number of drugs entering Phase I trials after passage of Part D more than doubled for antipsychotic, antidepressant, and GI ulcer treatments; however, the success rate for these drugs proceeding to Phase III trials appears to have been abysmally low. This trend is shown compellingly in Figure 4, below.
Table 6.
Heterogeneous effects of Medicare Part D on drugs entering Phase I and Phase III trials, for drug classes with high dual-eligible market share
| Phase I Trials | Phase III Trials | |
|---|---|---|
| Medicare Share * Part D 2003 |
0.307 (0.239) | −0.462 (0.335) |
| Medicare Share * Part D 2004–2005 |
0.621*** (0.195) | 0.448 (0.373) |
| Medicare Share * Part D 2006–2007 |
0.747*** (0.276) | 0.762 (0.464) |
| Medicare Share * Part D 2008–2010 |
1.074*** (0.398) | 1.427** (0.601) |
| Dual* Medicare Share * Part D 2003 |
0.261 (1.115) | −0.546 (1.251) |
| Dual* Medicare Share * Part D 2004–2005 |
2.350*** (0.500) | −1.718 (1.672) |
| Dual* Medicare Share * Part D 2006–2007 |
2.854*** (0.597) | −3.613 (3.196) |
| Dual* Medicare Share * Part D 2008–2010 |
4.239*** (1.315) | −3.627 (4.117) |
| Observations | 637 | 637 |
| Number of Therapeutic Classes | 49 | 49 |
| Dispersion | 1.21 | 1.10 |
significant at 10%;
significant at 5%;
significant at 1%
Notes: Authors’ calculations based on Medical Expenditure Panel Survey (MEPS) 2004–2005 and Pharmaprojects 1998–2010 trend data. Both models are Poisson, with outcome variable number of drugs entering Phase I and Phase III trials, respectively, by therapeutic class and year. Both models also include therapeutic class fixed effects and class-specific time trends, 4- to 15-year lags of NIH R&D funding, and discounted lead of market size at expected time of product launch to 12 years post-launch. Standard errors are presented in parentheses below each coefficient estimate, adjusted for clustering on therapeutic class and estimated simultaneously across both models to allow cross-phase correlation within class.
Figure 4.
Trends in Phase I and Phase III Trials for Therapeutic Classes Heavily Used by Dual Eligible Beneficiaries
Source: Authors’ calculations based on pharmaprojects data.
Note: includes antipsychotics, antidepressants, and Gl ulcer treatments entering clinical trials.
4.3 Changes in Firms’ R&D Expenditures and Worldwide Launches After Part D
In addition to our primary analyses described above, we also investigated short-run effects of Medicare Part D on firms’ R&D expenditures, and longer-run effects on worldwide drug launches.
Medicare Part D could have affected firms’ R&D expenditures both to its expansion of expected future markets for products still in pipeline, and also via two supply side mechanisms. First, after Medicare Part D was implemented in 2006, established firms selling drugs for the Medicare market would have seen an increase in current sales revenues, and prior research indicates that short-run increases in cash flow yield contemporaneous increases in R&D expenditures at pharmaceutical firms (Scherer, 2001; Vernon, 2005b). Second, per Friedman (2009), stock prices for firms introducing high Medicare share drugs increased dramatically after Part D, and increases in stock prices may decrease the cost of external capital, thereby increasing R&D expenditures (Golec et al., 2010).
Large, established firms with relatively diverse baseline R&D portfolios could have responded to Medicare Part D by changing their research priorities, for example by in-licensing higher Medicare share products. In contrast, among firms that have smaller and more focused R&D portfolios—and therefore less flexibility to adapt to changing market conditions—the effect of Medicare Part D might have been stronger. For example, all else equal, a small firm that specializes in treatments for Alzheimer’s disease should have experienced a larger increase in R&D than a firm specializing in childhood vaccines. Exploiting variation in firms’ pre-Part D research portfolios, specifically their portfolios’ average expected Medicare market share, we do find evidence of a significant increase in R&D expenditures after implementation of Medicare Part D at firms with positive (non-zero) sales, but with less than $2 billion in market capitalization. In contrast, we find no effect of Part D on R&D expenditures among firms with the greatest stock market exposure ($10 billion or more), nor at start-up firms that had no revenue from sales.
Finally, with Phase III trials significantly increasing (p<.01) in 2006 and beyond, then typically lasting three years or more, and an estimated 18 months between submission for marketing approval and product launch, we expected any long-run changes in U.S. FDA approvals due to Part D would likely not be seen until 2010 or later. As a first approximation to U.S. approvals, we assessed possible Part D effects using Pharmaprojects’ worldwide market launches as an outcome variable. In a Poisson regression with class-specific time trends, we do find a significant effect of Part D beginning in 2008, with a MedicareShare*(Year>2007) coefficient estimate of 0.74 (p=.013), corresponding to an elasticity of new drug approvals with respect to market size of about 2.8.
5 Discussion
Our results indicate that the increase in outpatient prescription drug coverage provided through Medicare Part D has had a significant impact on pharmaceutical R&D. We observe evidence of a structural break in established R&D trends after passage and implementation of Part D, with greater percentage increases in drug trials for therapeutic classes that are most used by Medicare beneficiaries. In addition, we find stronger effects of Part D for protected classes, which, following Duggan and Scott-Morton’s (2010) suggestion, could be because protected classes did not experience price decreases after introduction of Part D. Although we cannot completely rule out exogenous changes in R&D trends both coincident with Medicare Part D and correlated with Medicare market share, our inclusion of covariates controlling for prior NIH-funded research and projected future market size, our finding of no significant effect on classes already covered under Part B, and finally our ancillary results showing corresponding increases in R&D expenditures at firms most likely to be affected and in worldwide drug launches, together support causal inference.
Finkelstein (2004) and Yin (2008) previously reported rapid, dramatic increases in private-sector R&D investment following changes in federal policies that impacted market size, for vaccines and orphan drugs respectively. Finkelstein (2004) found the Centers for Disease Control and Prevention (CDC) recommendation that all infants be vaccinated against Hepatitis B, the decision by Medicare to cover the costs of influenza vaccinations for Medicare beneficiaries, and the introduction of a policy that reduced vaccine manufacturers’ liability led to about a 2.5-fold increase in new clinical trials for vaccines for affected diseases. Yin (2008) likewise found a dramatic 182% increase in the flow of new clinical trials in the three years immediately after the Orphan Drug Act passed, but the effect was transient: the increase fell to half of that in later periods.
As Finkelstein (2004) suggests, the relatively rapid response we observe in drugs entering Phase I trials between June 2004 and May 2007 may be due to “a substantial reservoir of technologically feasible products on the shelf for whom the decision to begin clinical trials is responsive, on the margin, to increases in the expected economic return to the clinical trial.” This notion of an ‘off the shelf’ push seems reasonable, given the significant increase in higher Medicare share drugs entering in 2006–2007, followed by a possible refractory period in 2008–2010. The stronger response observed in 2006–2007 could also reflect further increases in R&D due to increases in cash flow from Part D sales after 2006.
The magnitude of our estimates also appears reasonable, given the prior literature. Duggan and Scott Morton (2010) estimated that Medicare Part D increased pharmaceutical revenues by roughly 27% for drugs with Medicare market share of 100%. For a drug class with average Medicare market share (41%, in 2004–2005), Duggan and Scott Morton’s result translates to an 11% increase in revenues following Medicare Part D. Our Phase I estimates correspond, for a drug class with average Medicare market share, to a 26% increase for 2004–2005, a 33% increase post-implementation in 2006–2007, and a lagged 51% increase in 2008–2010. These estimates imply an elasticity of Phase I clinical trials with respect to market size of 2.4 to 4.7, bracketing Acemoglu and Linn’s estimated elasticity of 3.5 for approved new molecular entities (NMEs). However, when we consider all clinical trials combined—including Phase III trials for supplemental indications—our estimated elasticity of clinical trials with respect to market size is 3.3, rather lower than Acemoglu and Linn’s estimated elasticity of 6 for all new drug approvals, but certainly still larger than the Dubois et al. (2011) estimate of about 0.25. What might explain the differences we observe here? Below we discuss four possible contributors to these differences, based on differences in empirical estimation strategies across these papers.
First, in contrast with the U.S. market focus of both our paper and Acemoglu & Linn’s, Dubois et al. (2011) estimate expected future revenues due to changes in market size using brand-name drugs’ sales revenues across 14 countries. Several of these countries regulate prescription drug prices, and regulations may change rapidly over time. Thus, given the lower expected profit per consumer and greater uncertainty about future profits and prices, firms’ R&D decisions are likely to be less responsive to a unit change in expected revenues for all these countries combined versus the same unit change in the U.S. market (Sood et al., 2009). Along these lines, elasticity of innovation to global demand might be smaller than the elasticity of innovation to U.S. demand if—as Civan and Maloney (2006) assert—the pharmaceutical industry is only responsive to changes in intensity of U.S. demand. It is therefore understandable that Dubois et al. find a lower elasticity than we and Acemoglu and Linn do, focusing on the U.S..
Second, after implementation of Medicare Part D in 2006, firms with already-launched drugs experienced increased profits from sales to the Medicare market, and increases in cash flows are expected to increase firms’ R&D expenditures (Scherer, 2001). Indeed, our supplementary analysis did find stronger effects of Part D on R&D expenditures at firms with positive sales revenues. Our estimate of the elasticity of R&D efforts—with preclinical and clinical investigations as our measure—does not distinguish between these two effects, but rather includes both. As such, our elasticity estimates should be larger than either Scherer’s (2001) or Acemoglu and Linn’s (2004), taken individually.
Third, like Finkelstein (2004) and Yin (2008), we measure firms’ innovative activities via clinical trials, whereas Dubois et al. (2011) and Acemoglu and Linn (2004) evaluate responsiveness of approved and marketed drugs to changes in market size. If we assume diminishing marginal expected returns to investment in pharmaceutical R&D, then new clinical trials added should have lower expected rates of success. For example, we observe a very strong Phase I trials response for protected drug classes most used by dual eligibles (antipsychotic and antidepressant medications), but no significant increase in those drugs entering Phase III. As discussed by Arrowsmith (2011), many of these recently tested products failed to show sufficient efficacy versus placebo. Our estimated elasticities of clinical trials with respect to market size should therefore be larger than corresponding elasticities for new drug approvals.
Both our focus on elasticity of clinical trials and inclusion of both supply- and demand-side effects of Part D should contribute to higher estimated elasticity of pharmaceutical innovation with respect to market size than found by Acemoglu and Linn (2004); however, our estimates are generally comparable to theirs or somewhat lower, implying that our corresponding elasticity for new drug approvals would be even lower still. As one indication of the likely magnitude, our supplementary analysis of worldwide drug launches after Part D corresponds to an elasticity with respect to market size of 2.8, about half of that found by Acemoglu and Linn (2004) for all drug approvals. One explanation for this difference is in the data: we employ recent years’ data—1998 to 2010—whereas prior studies relied on data from 1970 to 2000. For a variety of reasons, including perhaps fewer available “low-hanging fruit,” in recent years the success rates for pharmaceutical R&D projects have declined and the average cost per trial has increased. This would likely result in a lower estimated elasticity of innovation to future market size.
These results should be viewed in light of our study’s limitations. First, analysis of the overall effect of Part D on innovation and, ultimately, social welfare is limited both by the relatively short time-series available post-2003, and by the fact that preclinical and clinical investigations are an imperfect predictor of the number and quality of new drugs ultimately introduced. Given the long lag between drug discovery and development and new drug approvals, we cannot yet conclusively determine whether Medicare Part D will result in more (or better) drugs entering the market. For example, as noted above, the drugs added to the pipeline in response to Part D could be riskier investments, with potentially lower expected benefits. Second, these short-run effects on R&D also may not result in increases in future drug introductions if the government implements aggressive price negotiations in the future. Thus far, the price reductions achieved by private plans appear to have been outweighed by increased utilization, which has increased firms’ revenues and, unsurprisingly, increased their R&D expenditures.
The increases we observe in firms’ innovative R&D activities and related expenditures to date is greater than can be explained simply by an increase in firms’ cash flows after Part D’s implementation. Likewise, we find more treatments are entering development now for diseases that disproportionately afflict older individuals than would be expected simply due to population aging. Medicare Part D thus has not only reduced financial risk for the current cohort of older individuals, but also has potential to benefit future older individuals through increased flows of new drug treatments. However, if the flow of new (and more expensive) brand-name drugs increases, or if additional drug classes become protected from exclusion on plan formularies, the public cost of the Medicare Part D program may be higher than anticipated. Further research is needed to determine whether these observed increases in R&D will ultimately affect new drug introductions and approvals for supplemental indications, patients’ utilization and adherence to their treatment regimens, and ultimately health outcomes.
Highlights.
Market expansion due to Medicare Part D could, in theory, induce innovation
We identify Part D’s effect using variation in drugs’ Medicare market shares
Preclinical and clinical testing significantly increased after Part D
Acknowledgments
We thank Darius Lakdawalla, Dana Goldman, Greg Ridgeway, Sean Nicholson, Matthew Rutledge, Mark Duggan, and two anonymous reviewers for helpful comments on this work.
Role of Funding Sources
This work was supported in part by the Roybal Center for Health Policy Simulation, funded by the National Institute on Aging grant 5P30AG024968. N. Sood also acknowledges support from National Institute on Aging grant P01AG033559. M. Blume-Kohout likewise gratefully acknowledges partial financial support from the Ewing Marion Kauffman Foundation. Finally, we thank Pfizer Inc. for providing access to the Pharmaprojects database. These sponsors have had no role in our study design, analysis, interpretation of data, report writing, or the decision to submit this paper for publication.
Footnotes
Some exceptions to this rule included oral cancer drugs with IV equivalents, oral anti-emetics used within 48 hours of chemotherapy, immunosuppressants for recipients of Medicare-covered organ transplants, erythropoietin (EPO) for end-stage renal failure, and drugs administered via covered durable medical equipment, such as albuterol sulfate or ipratropium bromide used with a nebulizer or insulin used with an insulin pump.
See MedPAC (2003) Fact sheet on MedPAC’s Report to the Congress: Medicare Payment Policy, accessed online at http://medpac.gov/documents/Mar11_FactSheet.pdf, as of March 22, 2011.
For additional discussion of the details of Part D’s implementation, see Duggan et al. (2008).
Our panel therefore excludes Antidotes, certain subclasses of Cancer medications, Cytokines, Imaging Agents, Vaccines, Miscellaneous Respiratory treatments (treatments other than those for asthma, COPD, respiratory allergies, and cough), Antianemia treatments (e.g., Epogen, Procrit), and some musculoskeletal treatments. See Appendix for detailed crosswalk.
Data available at http://esa.un.org/UNPP as of November 20, 2010.
Historical NIH budget data available at http://officeofbudget.od.nih.gov/history.html, as of November 20, 2010.
Beginning in June 2004, discount cards were issued to Medicare beneficiaries, providing a 15–20% discount on out-of-pocket costs for prescription drugs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Margaret E. Blume-Kohout, Email: megbk@unm.edu.
Neeraj Sood, Email: nsood@usc.edu.
References
- Abrantes-Metz RM, Adams CP, Metz AD. Pharmaceutical development phases: a duration analysis. Journal of Pharmaceutical Finance, Economics and Policy. 2005;14:19–41. [Google Scholar]
- Acemoglu D, Linn J. Market size in innovation: Theory and evidence from the pharmaceutical industry. Q J Econ. 2004;119:1049–1090. [Google Scholar]
- Adams CP, Brantner VV. Estimating the cost of new drug development: Is it really $802 million? Health Affairs. 2006;25:420–428. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- Arrowsmith J. Phase II failures: 2008–2010. Nature Reviews Drug Discovery. 2011;10:1–1. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- Blume-Kohout ME. Does targeted, disease-specific public research funding influence pharmaceutical innovation? Journal of Policy Analysis and Management. 2012 doi: 10.1002/pam.21640. [DOI] [PubMed] [Google Scholar]
- Civan A, Maloney MT. Determinants of pharmaceutical research and development investments. The BE Journal of Economic Analysis & Policy. 2006;5:1–36. [Google Scholar]
- DiMasi J. New drug development in the United States from 1963 to 1999. Clinical Pharmacology & Therapeutics. 2001;69:0286–0296. doi: 10.1067/mcp.2001.115132. [DOI] [PubMed] [Google Scholar]
- Dubois P, de Mouzon O, Scott-Morton F, Seabright P. Market size and pharmaceutical innovation, Institut d’Economie Industrielle Working Paper. Institut d’Economie Industrielle, University of Toulouse; Toulouse, France: 2011. [Google Scholar]
- Duggan M, Healy P, Scott Morton F. Providing prescription drug coverage to the elderly: America’s experiment with Medicare Part D. J Econ Perspect. 2008;22:69–92. doi: 10.1257/jep.22.4.69. [DOI] [PubMed] [Google Scholar]
- Duggan M, Scott Morton F. The effect of Medicare Part D on pharmaceutical prices and utilization. Am Econ Rev. 2010;100:590–607. doi: 10.1257/aer.100.1.590. [DOI] [PubMed] [Google Scholar]
- Finkelstein A. Static and dynamic effects of health policy: Evidence from the vaccine industry. Q J Econ. 2004;119:527–564. [Google Scholar]
- Friedman JN. The incidence of the Medicare prescription drug benefit: Using asset prices to assess its impact on drug makers. Harvard University; 2009. Job Market Paper. [Google Scholar]
- Giaccotto C, Santerre RE, Vernon JA. Drug prices and research and development investment behavior in the pharmaceutical industry. J Law Econ. 2005;48:195–214. [Google Scholar]
- Gitterman DP, Greenwood RS, Kocis KC, Mayes BR, McKethan AN. Did a rising tide lift all boats? The NIH budget and pediatric research portfolio. Health Affairs. 2004;23:113–124. doi: 10.1377/hlthaff.23.5.113. [DOI] [PubMed] [Google Scholar]
- Golec J, Hegde S, Vernon JA. Pharmaceutical R&D Spending and Threats of Price Regulation. Journal of Financial & Quantitative Analysis. 2010;45:239–264. [Google Scholar]
- Grabowski HG, Kyle M. Generic competition and market exclusivity periods in pharmaceuticals. Managerial & Decision Economics. 2007;28:491–502. [Google Scholar]
- Holmer AF. Covering prescription drugs under Medicare: For the good of the patients. Health Affairs. 1999;18:23. doi: 10.1377/hlthaff.18.4.23. [DOI] [PubMed] [Google Scholar]
- Ketcham JD, Simon K. Medicare Part D’s Effects on Elderly Drug Costs and Utilization. National Bureau of Economic Research, Inc; 2008. NBER Working Papers: 14326. [PubMed] [Google Scholar]
- Lakdawalla D, Sood N. Incentives to innovate. In: Danzon PM, Nicholson S, editors. The Oxford Handbook of the Economics of the Biopharmaceutical Industry. Oxford University Press; New York: 2012. pp. 143–166. [Google Scholar]
- Lichtenberg FR, Sun SX. The impact of Medicare Part D on prescription drug use by the elderly. Health Affairs. 2007;26:1735–1744. doi: 10.1377/hlthaff.26.6.1735. [DOI] [PubMed] [Google Scholar]
- Medicare Payment Advisory Commission. Report to the Congress: Variation and Innovation in Medicare. MedPAC; Washington, DC: 2003. [Google Scholar]
- Oliver TR, Lee PR, Lipton HL. A political history of Medicare and prescription drug coverage. Milbank Quarterly. 2004;82:283–354. doi: 10.1111/j.0887-378X.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer FM. The link between gross profitability and pharmaceutical R&D spending. Health affairs (Project Hope) 2001;20 doi: 10.1377/hlthaff.20.5.216. [DOI] [PubMed] [Google Scholar]
- Shea AM, Curtis LH, Hammill BG, DiMartino LD, Abernethy AP, Schulman KA. Association between the Medicare Modernization Act of 2003 and patient wait times and travel distance for chemotherapy. JAMA-Journal of the American Medical Association. 2008;300:189–196. doi: 10.1001/jama.300.2.189. [DOI] [PubMed] [Google Scholar]
- Sood N, de Vries H, Gutierrez I, Lakdawalla DN, Goldman DP. The effect of regulation on pharmaceutical revenues: experience in nineteen countries. Health Affairs. 2009;28:W125–W137. doi: 10.1377/hlthaff.28.1.w125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier J, Bagchi A, Esposito D. Medicaid prescription drug use by dual eligibles: issues for Medicare Part D. Mathematica Policy Research; Princeton, NJ: 2008. [Google Scholar]
- Vernon JA. Examining the link between price regulation and pharmaceutical R&D investment. Health Economics. 2005a;14:1–16. doi: 10.1002/hec.897. [DOI] [PubMed] [Google Scholar]
- Vernon JA. Pharmaceutical R&D investment and cash flows: an instrumental variables approach to testing for capital market imperfections. Journal of Pharmaceutical Finance, Economics, & Policy. 2005b;13:3–17. [Google Scholar]
- Yin W. Market incentives and pharmaceutical innovation. J Health Econ. 2008;27:1060–1077. doi: 10.1016/j.jhealeco.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Yin W, Basu A, Zhang JX, Rabbani A, Meltzer DO, Alexander GC. The effect of the Medicare Part D prescription benefit on drug utilization and expenditures. Annals of Internal Medicine. 2008;148:169–177. doi: 10.7326/0003-4819-148-3-200802050-00200. [DOI] [PubMed] [Google Scholar]