Abstract
Patient: Male, 28
Final Diagnosis: Posterior reversible encephalopathy syndrome
Symptoms: Headache • pain around umblical region • seizures • visual disturbances
Medication: Mycophenolate mofetil
Clinical Procedure: Treatment of parasitosis • antiepileptic treatment • control of hypertension • changing mycophenolate mofetil to everolimus
Specialty: Transplantology
Objective:
Unusual or unexpected effect of treatment
Background:
Posterior reversible encephalopathy syndrome (PRES) is characterized by reversible neurological findings with clinical hallmarks such as headache, confusion, seizures, cortical visual disturbances, and other focal neurological signs.
Case Report:
A 28-year-old male patient was hospitalized secondary to diarrhea and abdominal pain. He had renal transplantation due to renal amyloidosis secondary to familial Mediterranean fever (FMF). In his clinical follow-up, he had seizures, hemiparesis, blurred vision, and vomited an Ascaris lumbricoides. MRI results led to diagnosis of PRES. Mycophenolate mofetil was changed to everolimus, his systolic blood was pressure kept below 140 mm hg, and his intestinal parasitosis was treated. During follow-up, he had no pain and no diarrhea. His neurological symptoms turned to normal within 48 hours and neuroradiological findings returned to normal within 2 weeks.
Conclusions:
PRES is a rare disorder of unknown incidence in renal transplantation patients. Early diagnosis is very important to prevent irreversible neurological sequelae. PRES is totally reversible with cessation of the offending agent, rapid control of hypertension, and treatment of the underlying disease. For early diagnosis and to reduce morbidity and mortality, stool sample examination should be made in patients taking immunosuppressive drugs.
Keywords: posterior reversible encephalopathy syndrome, mycophenolate mofetil usage renal transplanted patient, intestinal parasitosis, familial Mediterranean fever-related amyloidosis
Background
Posterior reversible encephalopathy syndrome (PRES) was first described in 15 patients in 1996 by Hinchey et al. [1]. PRES is a cliniconeuroradiological entity characterized by several symptoms such as headache, confusion, seizures, cortical visual disturbances, and other focal neurological signs. The MRI finding of PRES is subcortical edema without infarct, especially in posterior regions of the brain and brain stem. There are many known causes, such as hypertension, pre-eclampsia/eclampsia, immunosuppressive therapies (mostly tacrolimus and cyclosporin), and cytotoxic therapies such as cisplatin, uremia, porphyria, systemic lupus erythematosus [2]; there are also many unknown causes.
We report on a male patient with PRES, who had renal transplantation secondary to FMF. He was using immunosuppressive therapies such as tacrolimus, prednisolone, and mycophenolate mofetil for 29 months. He has also abdominal pain and diarrhea secondary to parasitosis, in addition to PRES. Herein we report the first case of PRES with simultaneous intestinal parasitosis and mycophenolate mofetil usage in an FMF-related amyloidosis secondary renal transplanted patient.
Case Report
A 28-year-old male patient had preemptive renal transplantation from his mother in 2008. He had been followed-up with kidney disease secondary to FMF-related amyloidosis from 2002 to 2008. He was hospitalized because of a pain around umbilical region, with elevated liver enzymes (ALT: 156 U/l, AST: 144 U/l) and diarrhea. His possible diagnosis was colchicine toxication. He had used an increased dosage of colchicine (every 2 hours) because he thought he was having an FMF attack, but did not experience relief as previously. He had been using colchicine, deltacortyl 5 mg, tacrolimus 2×1.5 mg, mycophenolate mofetil 2×500 mg, and L-Thyroxine 100 mcg 1×1. His systolic and diastolic blood pressure was 150 and 90 mm Hg, respectively. He did not have hepatosplenomegaly or lymphadenopathy. His laboratory findings were CRP: 11.5 mg/dl, creatinine: 0.8 mg/dl (0.7–1.2), K: 4.3 meq/l (3.5–5.1), Na: 134 meq/l (136–145), WBC: 23200/mm3 (4800–10800), PLT: 482000/mm3 (15000–45000), and Hg: 9 gr/dl (12–16).
His pain was not related to an FMF attack, but his elevated liver enzymes and creatinine kinase were associated with high-dose use of colchicine. Esophagogastroduodenoscopy (EGD) was done because of abdominal pain, vomiting, and diarrhea. EGD showed antral gastritis and alkaline reflux and abdominal ultrasonography showed minimal intestinal wall edema in the left abdomen. Rectosigmoidoscopy findings were normal. Abdomen and pelvic tomography showed minimal wall edema in jejunal loops, possibly secondary to the gastroenteritis. We started to give the patient meropenem 3×1 parenterally.
He had headache at occipital and bilateral frontal regions, blurred vision, nausea, vomiting, and speech impairment during the follow-up period. Brain CT visualized a round hyperdense lesion that looked like an intracerebral hematoma or opportunistic infection, which was seen in the right occipital lobe. Later, meropenem was changed to cefepime 3×1 parenterally and 3×10 mg/kg acyclovir was added to treatment because of possible herpetic and opportunistic encephalitis as part of the differential diagnosis. He had generalized tonic-clonic seizures 1 day after these symptoms. Neurology started oxcarbazepine 2×150 mg. He vomited an Ascaris lumbricoides within an alkalic and green content on the same day (Figure 1). We treated him with mebendazole 3×100 mg for 3 days. A brain MRI was taken and inT2 FLAIR sequence showed us right occipitally, right frontally, and bilateral periventricularly cortical-subcortical edema evaluated as PRES (Figure 2). We stopped mycophenolate mofetil and started everolimus 2×0.75 mg. EEG showed postictal ground activity 3–4 HZ delta and 5–7 HZ and low-amplitude beta waves. EEG was compatible with medium and severe encephalopathy. His mean arterial pressure was tightly controlled and amlodipine 1×10 mg was started. His systolic tension was kept below 140 mmHg and his diastolic tension kept below 90 mm Hg.
Figure 1.
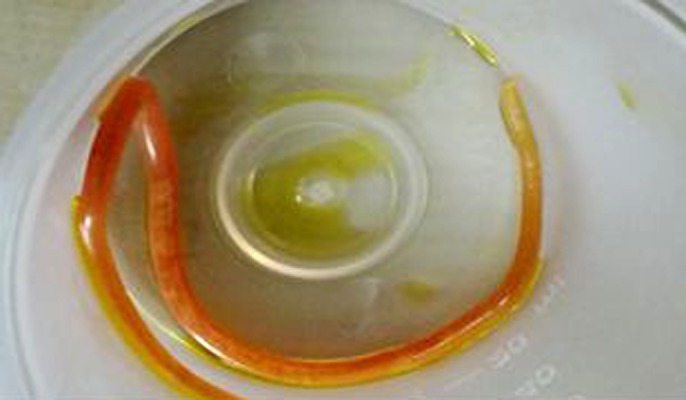
Ascaris lumbricoides vomited by the patient.
Figure 2.
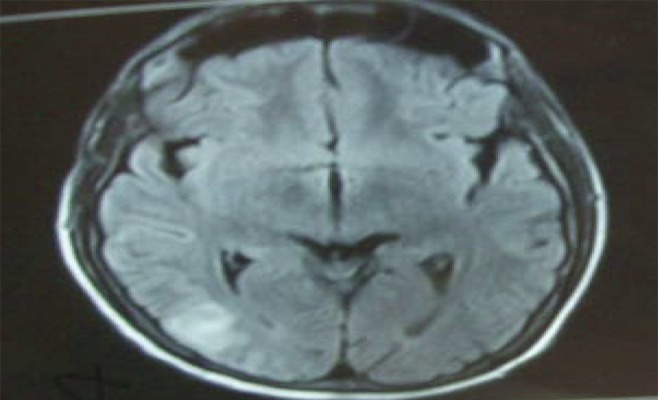
Coronal fluid attenuation inversion recovery (FLAIR) MRI showing gray area of high signal in posterior brain within the cortical area and subcortical white matter of right occipital lobe.
Brain control MRI showed nonspecific gliotic changes 2 weeks later (Figure 3). He had no pain or diarrhea. He was discharged with outpatient neurology and transplantation polyclinic visits. During follow-ups, he had no seizures or neurological symptoms. Oxcarbazepine was stopped 2 months later at neurology polyclinic visitation.
Figure 3.
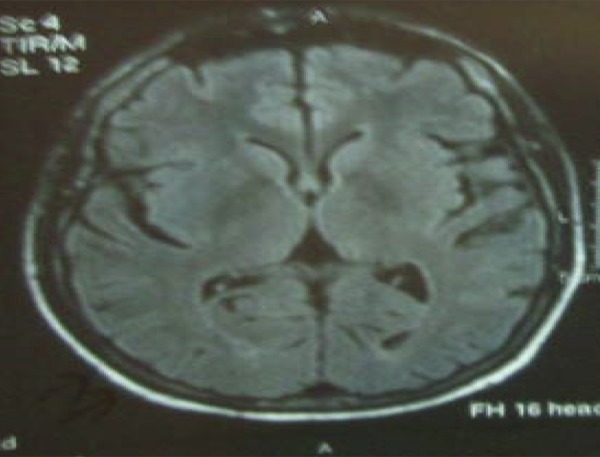
Normal brain MRI findings with non-specific gliotic changes taken after two weeks of the MRI showing PRES syndrome.
Discussion
Patients with PRES have symptoms of nausea, vomiting, altered mental function, headache, visual abnormalities, and seizures, with an increasing frequency written in the order. Seizures may be generalized tonic-clonic, but may start also focally [3]. Pathophysiology has not been clear. The most popular theory is the disruption of the blood brain barrier secondary to hypertension, but blood pressure is normal or mildly elevated in 30% of cases [1,3]. The second theory is endothelial dysfunction because of circulating toxins in septic, immune-suppressed, and autoimmune disease patients [3–5]. The third theory is ischemic edema secondary to focal vasospasm and blood flow decrement [3,6]. The cause of systemic hypertension is possibly to maintain the cerebral blood flow [3,7,8]. In our case, systemic hypertension was mildly elevated.
FLAIR MRI is more sensitive in diagnosis of PRES [3,7]. The typical lesion is vasogenic edema predominantly in the occipital lobe, which may be patchy or confluent; but almost all patients (90–98%) have edema in the parietoccipital region [3,7]. Subarachnoid hemorrhage, parenchymal hematoma, and small minute hemorrhages <5 mm have also been seen in 15% to 19% of all cases [3,9]. In our case, brain CT imaging was done first and showed lesions with an appearance similar to intracerebral hematoma or opportunistic infection, but T2 FLAİR MRI showed us cortical and subcortical patchy gray lesions in the lateral area of the right occipital lobe, compatible with PRES (Figure 3).
We believe this is the first report of PRES in an FMF-related amyloidosis secondary renal transplanted patient. This is also the first report of PRES in a renal transplanted patient taking mycophenolate mofetil while simultaneously having intestinal parasitosis.
The main step in treating PRES is withdrawal of the offending agent and controlling high blood pressure. PRES is generally seen in usage of calcineurin inhibitors like tacrolimus and cyclosporin. We changed mycophenolate mofetil to everolimus to prevent transplanted kidney rejection. We kept systemic hypertension below 140 mm Hg, although our patient’s systemic hypertension was mildly elevated. We also started antiparasitic therapy.
There is also a case report of an FMF patient’s PRES during an attack and after colchicine treatment the PRES resolved. In this case systemic blood pressure also was in normal range [10].
The prognosis in PRES is good, and symptoms generally resolve within a week [3]. Improvement of MRI-detected lesions takes 6 weeks in 72% of cases [7]. In our case, the MRI findings returned to normal within 2 weeks and the neurological symptoms resolved within 48 hours.
Differential diagnosis of PRES includes posterior circulation stroke, reversible cerebral vasoconstriction syndrome, primary CNS vasculitis, status epilepticus, and, especially, herpetic encephalitis [3]. In this case, opportunistic brain infection and encephalitis secondary to immunosuppressive agents were thought of in the differential diagnosis, so the patient was treated with acyclovir after symptoms such as speech impairment, seizures, and herpes simplex encephalitis suspected upon brain tomography visualization. When the MRI showed the PRES, acyclovir was stopped. It is also important that seizure therapy should not be given for more than 3 months without ongoing epileptic activity on EEG, or without recurrent seizures [7,8,11].
There has not been any case report associating intestinal parasitosis with PRES and mycophenolate mofetil usage. This concurrence was possibly incidental. The prevalence of parasitic infections in a study of 657 kidney recipients is 2.4% and is mostly Strongyloides stercoralis[12] and none of them used cyclosporine in the immunosuppressive protocol. Cyclosporine has a parasiticidal effect and, after the improvement of new immunosuppressive drugs, there has been an increase in parasitic infections in renal transplant patients [12]. In a study from a developing country (Iran), the prevalence of parasitosis is 33% [13]. The prevalences are 10.6% Entamoeba coli, 7.4% Giardia lamblia, 4.7% Blastocystis spp., and 0.7% Ascaris lumbricoides[13].
Conclusions
PRES should be considered when neurological symptoms occur in patients who are taking immunosuppressive drugs, have received a transplant, or who have impaired renal function or autoimmune disease [3]. In renal transplanted patients with symptoms of seizure with disturbed vision and/or headache, MRI should be performed immediately.
PRES is a reversible syndrome, especially with complete cessation of the offending agent rather than dose reduction and keeping systolic blood pressure below 140 mm Hg [3,10]. Immuno-suppressive therapy should be adjusted in PRES.
References:
- 1.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Varaprasad I, Agrawaal S, et al. Posterior reversible Encephalopathy syndrome in systemic lupus erythematosis. J Rheumatol. 2011;38(8):1607–11. doi: 10.3899/jrheum.101308. [DOI] [PubMed] [Google Scholar]
- 3.Roth C, Ferbert A. The Posterior reversible encephalopathy syndrome: what’s certain, what is new. Pract Neurology. 2011;11:136–44. doi: 10.1136/practneurol-2011-000010. [DOI] [PubMed] [Google Scholar]
- 4.Bartynski WS, Boardman JF, Zeigler ZR, et al. Posterior reversible encephalopathy syndrome in infection, sepsis and shock. AJNR Am Neuroradiol. 2006;27:2179–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Rodegers GM, Taylor RN, Roberts JM. Preeclempsia is associated with a serum factor cytotoxic to human endothelial cells. Am J Obstet Gynecol. 1988;159:908–14. doi: 10.1016/s0002-9378(88)80169-8. [DOI] [PubMed] [Google Scholar]
- 6.Lin JT, Wang SJ, Fuh JL, et al. Prolonged reversible vasospasm in cyclosporin A-induced encephalopathy. AJNR Am J Neuroradiol. 2003;24:102–4. [PMC free article] [PubMed] [Google Scholar]
- 7.Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: long term follow-up. J Neurol Neurosurg Psyhiatry. 2010;81:773–77. doi: 10.1136/jnnp.2009.189647. [DOI] [PubMed] [Google Scholar]
- 8.Lee VH, Wijdicks EF, Manno EM, et al. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205–10. doi: 10.1001/archneurol.2007.46. [DOI] [PubMed] [Google Scholar]
- 9.Hefzy HM, Bartynski WS, Boardman JF, et al. Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. AJNR Am J Neuroradiol. 2009;30:1371–79. doi: 10.3174/ajnr.A1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulaslı A, Kutlu G, Kocaturk O, et al. Posterior reversible encephalopathy during an attack of familial Mediterranean fever. Rheumatol Int. 2010;296:1388–83. doi: 10.1007/s00296-010-1388-3. [DOI] [PubMed] [Google Scholar]
- 11.Kozak OS, Wijdicks EF, Manno EM, et al. Status Epilepticus as initial manifestation of posterior reversible encephalopathy syndrome. Neurology. 2007;69:894–97. doi: 10.1212/01.wnl.0000269780.45472.16. [DOI] [PubMed] [Google Scholar]
- 12.Valar C, Keitel E, et al. Parasitic İnfections in Renal Transplant Recipients. Transplantation Preceedings. 2007;39:2–460. doi: 10.1016/j.transproceed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Azami M, Sharifi M, et al. İntestinal parasitic infections in renal transplant recipients. The Brazilian JID. 2010;14:1–15. doi: 10.1590/s1413-86702010000100004. [DOI] [PubMed] [Google Scholar]


