Abstract
Carcinoma of the cervix is causally related to infection with the human papillomavirus (HPV), and T cells play a pivotal role in the immune response of the host to rid itself of HPV infection. Therefore, we assessed the T-cell function of women with HPV-related cervical neoplasia against a superantigen, Staphylococcus enterotoxin B (SEB). Each woman provided a cervical brush specimen for HPV DNA testing and Papanicolaou (Pap) smears for the staging of cervical lesions. They also provided a blood specimen for determination of the ability of CD4+ T and CD8+ T cells to synthesize Th1 (interleukin-2 [IL-2], gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) and Th2 (IL-10) cytokines in response to activation with SEB. Compared with control subjects with self-attested negative Pap smears, women with high-grade squamous intraepithelial lesions (HSIL) had significantly lower percentages of activated CD4+ T cells that produced IL-2 (P = 0.045), IFN-γ (P = 0.040), and TNF-α (P = 0.015) and a significantly lower percentage of activated CD8+ T cells that produced IL-2 (P < 0.01). These data indicate that women with HPV-related cervical HSIL show a decrease in Th1 cytokine production by activated CD4+ T cells and suggested that compromised T-helper functions may negatively impact the function of cytotoxic CD8+ T cells.
Infection with oncogenic human papillomavirus (HPV) can cause precancerous lesions of the cervical squamous epithelium (6). Clinical manifestation with HPV infection is dependent upon epithelial location, HPV type, and host immune responses (12). With respect to the host immune status, individuals with compromised cellular immunity are more likely to develop cervical lesions than those with intact cellular immunity (10). Moreover, a shift from a Th1 (interleukin [IL-2] and gamma interferon [IFN-γ]) to a Th2 (IL-4 and IL-10) cytokine profile was associated with poor prognosis for patients with HPV-associated cervical lesions (4). IL-10 in particular blocks cytokine synthesis by helper T cells, activated monocytes, and natural killer (NK) cells (20). A decrease in NK cell activity has been associated with reactivation of latent HPV infection (7). HPV may escape host immune surveillance by depleting intraepithelial antigen-presenting cells (21) and/or by downregulating the surface expression of major histocompatibility complex class I antigens and β2-microglobulin on antigen-presenting cells (24).
Investigators who conducted an earlier study reported that phytohemagglutinin-activated peripheral blood mononuclear cells of patients with local and invasive cervical lesions produced less IL-2 and IFN-γ than similar cell cultures of control subjects (4). In a later study (13), Lee et al. used a mechanism that bypassed the T-cell receptor (TCR) (25) to examine cytokine production by T cells that were activated by phorbol-12-myristate-13-acetate, an activator of protein kinase C. In that study, Lee et al. expanded on the observations of Clerici and coworkers (4) by demonstrating a deficiency in cytokine synthesis by CD4+ T and CD8+ T cells of HPV-infected women. As activation of T cells by HPV antigens is mediated through a mechanism that engages the TCR, the present study investigated the ability of CD4+ T and CD8+ T cells of HPV-infected women with cervical squamous intraepithelial lesions (SIL) to synthesize Th1 and Th2 cytokines following activation with Staphylococcus enterotoxin B (SEB), a microbial superantigen that activates T cells through the TCR (22).
MATERIALS AND METHODS
Subjects.
A total of 98 women (referred to the colposcopy clinic at one of three hospitals [Memorial-Hermann Hospital, Lyndon Baines Johnson Hospital, or the University of Texas M.D. Anderson Cancer Center] in Houston, Texas) were recruited over a 12-month period for this laboratory study. The Institutional Review Board at each of the individual study sites approved the study. Patients consented to provide a cervical smear for cytologic examination by Papanicolaou (Pap) staining, cervical brush specimens for HPV testing, and answers to a questionnaire of risk factors. Women also agreed to undergo a cervical biopsy if lesions were visible upon application of acetic acid. Histologically confirmed cases of cervical intraepithelial neoplasia (CIN) grades 2 to 3 were treated by a loop electrosurgical excision procedure. In addition to receiving treatment, each patient agreed to provide 5 ml of peripheral blood for investigational cytokine studies at the time of administering the questionnaire and prior to a physical examination and gynecologic procedure. Healthy control subjects were recruited for this study through a widely circulated flyer seeking nonsmoker female blood donors. Each control subject attested to having a negative Pap smear at her annual well-women gynecological examination. Questionnaires of risk factors were not administered to control subjects, and no control subject was asked to provide cervical specimens for cytologic examination and HPV testing at the time of phlebotomy.
Processing and interpretation of enrollment specimens.
Cytology studies.
Pap smears were screened by a cytotechnologist and evaluated by a cytopathologist, Anais Malpica. The Bethesda System was used to classify the cytologic findings for each woman into one of three stages of increasing disease severity: atypical squamous cells of unknown origin (ASCUS), low-grade SIL (LSIL), and high-grade SIL (HSIL) (17). In cases in which operable lesions were present, patients were referred to a colposcopy clinic for cervical biopsy and histological examination. Finally, a clinical diagnosis was rendered on the basis of the cytology, histology, and HPV test results.
Detection of HPV DNA in cervical specimens.
Exfoliated epithelial cells from all patients were obtained by cervical brushing and placed in transport medium supplied with a specimen collection kit of a Hybrid Capture 2 (HC2) assay (Digene Diagnostics, Gaithersburg, Md.) for the detection of HPV DNA. The HC2 assay is a nucleic acid hybridization assay that uses RNA probes to detect 18 HPV types, including 13 high-risk types (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and 5 low-risk types (types 6, 11, 42, 43, and 44). In some cases, a second cervical specimen was also collected to test for HPV DNA through the use of a previously described PCR method that amplified a highly conserved segment (450 bp) of the L1 gene with primers MY09 and MY11 (2, 9).
Intracellular cytokine synthesis by T-cell subsets activated by SEB.
Each study and control subject provided 5 ml of peripheral blood in heparin for an intracellular cytokine assay that assessed the ability of T cells to synthesize cytokines following activation with SEB. Briefly, 1 ml of peripheral blood was grown in a culture in the presence of 3 μg of SEB, 3 μg of anti-CD28, and 3 μg of anti-CD49d and incubated at 37°C for 5 h. At 2 h into the incubation period, 10 μg of brefeldin A, a nontoxic but potent inhibitor of intracellular transport, was added for the remaining 3 h of the incubation period. Anti-CD28 and anti-CD49d were purchased from Becton Dickinson Immunocytometry Systems (Mountain View, Calif.); other reagents were purchased from the Sigma Chemical Company (St. Louis, Mo.).
The SEB-activated cells were stained for cytokines in the cytoplasm, and the stained cells were analyzed using four-color flow cytometry and a modification of the previously described procedure of Lee et al. (14). Briefly, 10,000 CD3+ gated events were collected on the basis of the side scatter of lymphocytes and their reactivity with anti-CD3 monoclonal antibody. Anchoring on CD3+ events, a scatter plot was created on the basis of reactivity with anti-CD8 monoclonal antibody to delineate two populations of interest: CD3+ CD8+ clusters and CD3+ CD8− (or CD4+) clusters. Next, using the CD3+ CD8+ and CD3+ CD4+ clusters of T cells as anchors, two scatter plots were constructed to identify the SEB-activated T cells that coexpressed CD69 and one of the following cytokines: IL-2, IFN-γ, tumor necrosis factor alpha (TNF-α), or IL-10. Immunoglobulin isotype controls were used to verify the staining specificity of the anti-cytokine reagents and to set markers delineating positive- and negative-testing populations.
Statistical analysis.
Pearson's chi-square analysis was used to determine differences in the ethnic distribution, the number of women with cervicovaginal infections, and the proportion of smokers and oral contraceptive pill (OCP) users among patient populations. The Mann-Whitney test was performed to determine age differences between study groups. The median percentages of T-cell subsets synthesizing IL-2, IFN-γ, TNF-α, or IL-10 were obtained for patients and control subjects. The Kruskal-Wallis and Mann-Whitney tests were employed to determine the statistical differences among and between the study groups, respectively. Statistical differences with a P value of less than 0.05 were considered to be significant.
RESULTS
Patient demographics, HPV status, and clinical staging.
Only patients with HPV detectable by the HC2 assay were grouped according to pathological findings regarding their lesions. Cervical brushings from some patients were also positive by the PCR for HPV-DNA. A single pathologist reviewed all cervical biopsy specimens to provide consistent histological staging with respect to either LSIL or HSIL. In our study, women with biopsy-proven CIN were excluded from the ASCUS group for data analysis. The analysis included 64 HPV+ patients: 14 women with ASCUS, 21 women with LSIL, and 29 women with HSIL.
The 64 study subjects had a median age of 30 years (range, 20 to 63 years of age) (Table 1) and were age matched to 15 control female nonsmokers with a median age of 33 (range, 23 to 61 years). The patient population consisted of 16 African-American, 24 Hispanic, 22 Caucasian, and 2 Asian women. Among the patient groups, there were no significant differences in ethnicity (χ2 = 7.313 [P = 0.293]), proportions of smokers (χ2 = 2.787 [P = 0.248]) and OCP users (χ2 = 1.733 [P = 0.420]), and the number of women with past cervicovaginal bacterial or yeast infections and sexually transmitted diseases (χ2 = 2.046 [P = 0.359]) (Table 1). Parity among patients increased with HPV disease severity such that the average number of children per patient group was 1 for ASCUS women, 2 for LSIL women, and 3 for HSIL women, respectively. All women self attested to having no human immunodeficiency virus infection. Concomitant cervicovaginal infections in patients were rare at the time of study; one woman with LSIL had a yeast infection and another woman with HSIL had a chlamydia infection.
TABLE 1.
Ethnicity, smoking status, OCP usage, and past cervicovaginal infections in each of the patient populations
| Parameter | Value for groupa
|
||
|---|---|---|---|
| ASCUS | LSIL | HSIL | |
| Total subjects | 14 | 21 | 29 |
| Ethnicity was: | |||
| African-American | 2 | 5 | 9 |
| Hispanic | 3 | 10 | 11 |
| Caucasian | 8 | 5 | 9 |
| Asian | 1 | 1 | 0 |
| Mean age (min, max)b | 31 (21, 56) | 29 (20, 45) | 31 (20, 63) |
| Smokers | 4 | 7 | 15 |
| OCP users | 2 | 3 | 8 |
| Past infectionc | 10 | 12 | 22 |
Except where noted, values are numbers of women.
Ages are in years. Min, minimum; max, maximum.
Past cervicovaginal bacterial or yeast infections and/or sexually transmitted diseases were considered.
Syntheses of Th1 and Th2 cytokines by SEB-activated T cells. (i) Cytokine synthesis by CD4+ T cells.
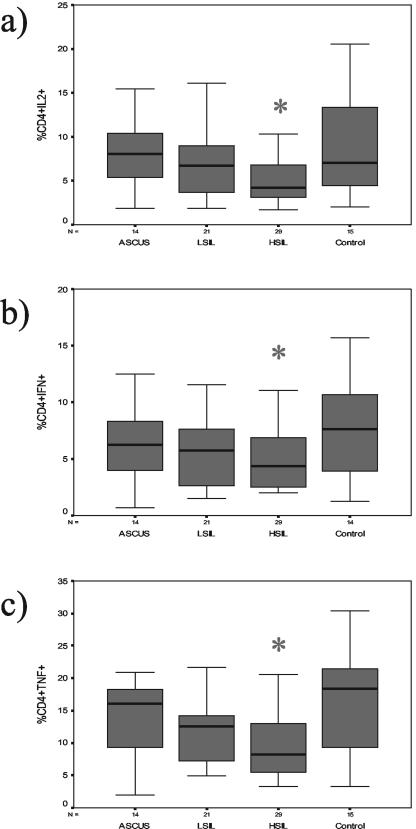
The median percentages of CD4+ and IL-2+ T cells were 8.0% (95% confidence intervals, 5.6 and 10.6%, respectively) for ASCUS, 6.7% (5.4 and 9.8%) for LSIL, 4.2% (4.1 and 6.6%) for HSIL, and 7.0% (5.9 and 12.4%) for control subjects (Fig. 1a). The median percentages of CD4+ and IFN-γ+ T cells were 6.2% (95% confidence intervals, 4.0 and 9.4%, respectively) for ASCUS, 5.7% (4.1 and 7.3%) for LSIL, 4.4% (3.8 and 5.8%) for HSIL, and 7.6% (5.3 and 10.3%) for control subjects (Fig. 1b). The median percentages of CD4+ and TNF-α+ T cells were 16.0% (95% confidence intervals, 9.9 and 17.2%, respectively) for ASCUS, 12.6% (9.2 and 14.4%) for LSIL, 8.2% (7.9 and 11.6%) for HSIL, and 18.3% (12.3 and 21.4%) for control subjects (Fig. 1c). In particular, patients with HSIL had significantly lower median percentages of CD4+ T cells that produced IL-2 (P = 0.045), IFN-γ (P = 0.040), and TNF-α (P = 0.015) than the control subjects, who had responses similar to those of patients with ASCUS and LSIL. The percentages of CD4+ T cells expressing IL-10 were similar for all groups (data not shown).
FIG. 1.
Box-and-whisker plots of the percentages of type 1 cytokine-producing CD4+ T cells for each of four study groups (14 women with ASCUS, 21 women with LSIL, 29 women with HSIL, and 15 control subjects). The box extends from the 25th percentile to the 75th percentile, with a horizontal line at the median (50th percentile). Whiskers extend down to the smallest value and up to the largest (range), excluding the outliers. Figures 1a, 1b, and 1c represent the plots of activated CD4+ T cells that expressed IL-2, IFN-γ, and TNF-α, respectively. Asterisks indicate that patients with HSIL had significantly lower median percentages of activated CD4+ T cells that expressed either IL-2 (P = 0.045), IFN-γ (P = 0.040), or TNF-α (P = 0.015) compared to the results seen with activated CD4+ T cells of control subjects, as determined by the Mann-Whitney test.
(ii) Cytokine synthesis by CD8+ T cells.
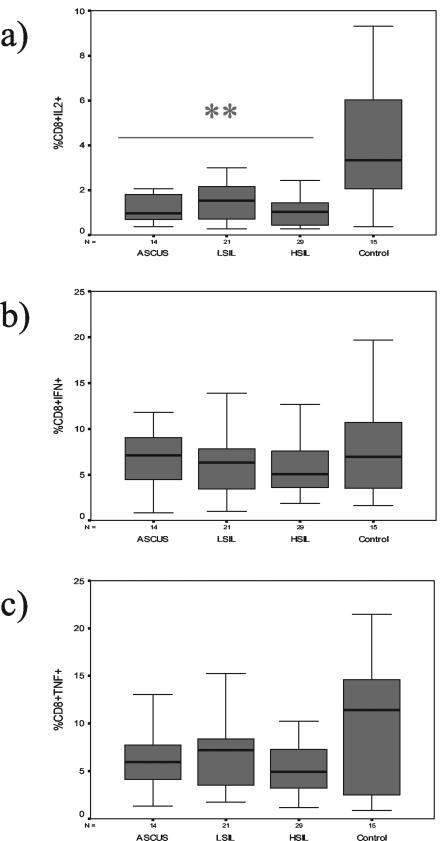
The median percentages of CD8+ and IL-2+ T cells were 1.0% (95% confidence intervals, 0.7 and 1.9%, respectively) for ASCUS, 1.5% (1.0 and 2.7%) for LSIL, 1.0% (0.8 and 1.5%) for HSIL, and 3.3% (2.5 and 5.7%) for control subjects (Fig. 2a). The median percentages of CD8+ and IFN-γ+ T cells were 7.1% (95% confidence intervals, 5.1 and 9.7%, respectively) for ASCUS, 6.3% (4.7 and 8.3%) for LSIL, 5.0% (4.7 and 7.9%) for HSIL, and 6.9% (5.0 and 11.0%) for control subjects (Fig. 2b). The median percentages of CD8+ and TNF-α+ T cells were 5.9% (95% confidence intervals, 4.8 and 8.4%, respectively) for ASCUS, 7.2% (5.1 and 8.9%) for LSIL, 4.9% (4.3 and 7.2%) for HSIL, and 11.4% (5.7 and 13.7%) for control subjects (Fig. 2c). The percentages of CD8+ T cells that produced IL-2 differed significantly among study groups (P = 0.003). Patients with ASCUS (P = 0.003), LSIL (P = 0.007), and HSIL (P = 0.001) had significantly lower median percentages of CD8+ T cells that produced IL-2 than the control subjects. All groups had similar median percentages of CD8+ T cells that produced either IFN-γ or TNF-α. The percentages of CD8+ T cells that produced IL-10 were similar for all groups (data not shown).
FIG. 2.
Box-and-whisker plots of the percentages of type 1 cytokine-producing CD8+ T cells for each of four study groups (14 women with ASCUS, 21 women with LSIL, 29 women with HSIL, and 15 control subjects). The box extends from the 25th percentile to the 75th percentile, with a horizontal line at the median (50th percentile). Whiskers extend down to the smallest value and up to the largest (range), excluding the outliers. Figures 2a, 2b, and 2c represent the plots of activated CD8+ T cells that expressed IL-2, IFN-γ, and TNF-α, respectively. Double asterisks indicate that patients with either ASCUS, LSIL, or HSIL had significantly lower median percentages of activated CD8+ T cells that expressed IL-2 (P < 0.01) compared to the results seen with activated CD8+ T cells of control subjects, as determined by the Mann-Whitney test.
DISCUSSION
Cytokine production by T cells is a reliable measure of the quality of cell-mediated immunity. In an earlier study, Lee et al. found that a significantly lower percentage of CD4+ T cells of patients with HPV-related cervical neoplasia synthesized IL-2 in response to activation with phorbol-12-myristate-13-acetate, an activator of protein kinase C that activates T cells through a mechanism that is independent of TCR-mediated signaling, compared with the results seen with CD4+ T cells of control subjects (14). In the present study we showed that the activation of T cells by a superantigen, SEB, through the TCR is compromised in patients with HSIL. In particular, patients with HSIL had significantly lower percentages of CD4+ T cells that produced Th1 cytokines (IL-2, IFN-γ, and TNF-α) than control subjects. Our data further demonstrated that impairment of IL-2 production by CD8+ T cells was detected not only in patients with HSIL but also in women who had LSIL or ASCUS. In contrast, all patient groups had similar percentages of CD8+ T cells that produced IFN-γ. These data are consistent with the results of a recent report from studies of mice; the results showed that low-avidity TCR ligand was able to drive IFN-γ production whereas IL-2 production required a strong TCR engagement (1). This impaired IL-2, and not IFN-γ, production by CD8+ T cells of all patient groups suggested a functional heterogeneity among CD8+ T cells in women at precancerous stages of HPV disease.
SEB is a microbial antigen that induces vigorous activation, proliferation, and cytokine production by T cells that express specific TCR variable beta (Vβ) chains such as Vβ3, Vβ12, Vβ14, Vβ15, Vβ17, and Vβ20 (22). In vivo administration of SEB resulted in clonal expansion and subsequent deletion of TCR Vβ-specific responding T cells; moreover, in vitro stimulation of CD4+ and CD8+ T cells with SEB caused anergy only in CD4+ T cells and not in CD8+ T cells (11). Whereas chronic and persistent antigenic stimulation may lead to the deletion of specific Vβ clones in patients with cervical cancer (19), defects in cytokine production by T cells may not be exclusively due to persistent stimulation by HPV antigen. As it is common for women with CIN to have concurrent bacterial and yeast infections (23), chronic antigen stimulation by microbial antigens could potentially eliminate certain TCR Vβ clones and, in turn, compromise the ability of T cells to respond to SEB. HPV infection usually precedes detection of bacterial vaginosis among young women (15), and cumulative insults to the host immune system by chronic and repetitive infections can lead to the development of CIN (8). While the patients of the present study had a low incidence of concurrent cervicovaginal infections, prior incidences of sexually transmitted diseases are a measure of risk behavior that increases exposure to HPV infection, which in turn increases the risk of developing CIN. As this was a cross-sectional study that surveyed for a limited number of cervicovaginal infections without the assessment of the TCR Vβ repertoire, it is difficult to determine the true impact of prior cervicovaginal infections on the T-cell repertoire and the ability of T cells to respond to antigenic stimulation.
An earlier study demonstrated that cultures of peripheral blood mononuclear cells from patients with local and invasive cervical lesions that were activated with phytohemagglutinin produced lower levels of IFN-γ than similar cultures of control subjects (4). By assessing intracellular cytokine synthesis by SEB-activated T cells, we found significantly lower percentages of CD4+ T cells (but not CD8+ T cells) that produced IFN-γ among patients with HSIL compared with the results seen with T-cell subsets of the control subjects. Others have reported that compared with the memory CD4+ T cell, the memory CD8+ T cell is more conducive to activation with SEB and more likely to secrete cytokine and proliferate (5). With respect to HPV-infected women, cervical CD8+ T cells produced IFN-γ in response to activation with HPV-L1 antigen (18). These data (in combination with findings with respect to the ability of SEB-activated CD8+ T cells of women with cervical SIL to produce TNF-α) suggest that the cytolytic capacity of CD8+ T cells might not be compromised (3). Nevertheless, the deficiency in IL-2 production by CD4+ T and CD8+ T cells of patients with HSIL may limit the expansion of HPV-specific cytotoxic T-cell clones and in turn impair the ability of patients to sustain competent cell-mediated responses.
The lack of data on the presence of HPV DNA and risk factors among control subjects is one of the limitations of this study. On the other hand, the lack of statistically significant differences in risk behaviors such as smoking, OCP use, and past history of cervicovaginal infections and sexually transmitted diseases among our patient populations suggests that the impact of these risk factors in this study was minimal. Perhaps the sample size of our study was not sufficiently large to determine epidemiological differences among cohorts. Other variables, such as the number of cigarettes smoked, the number of sex partners, the incidence and type of sexually transmitted disease, and psychosocial factors, could potentially influence the course of disease evolution (16). The results of the present study indicated the existence of a potential defect in CD4+ T-cell function that could in turn compromise the HPV-specific cellular response of HSIL patients. A prospective longitudinal study to evaluate cellular immunity and the progression of cervical lesions in women with HPV infection may provide a better understanding of the immune defects involved in the development of invasive cervical neoplasia.
Acknowledgments
We thank Karen Rabel, Joanne L. Baker, Kim Hagedorn, Christina Briggs-Amos, and Christy K. Whitmore for patient recruitment and specimen collection; Janet Bruner for HPV testing; Hui Gao for technical assistance; and Nan Earle for data management.
The study was supported in part by grants from the Texas Higher Education Coordinating Board (B.-N.L.), the National Cancer Institute (3PO1-CA82710-04) (M.F.), and the National Institute of Allergy and Infectious Diseases (AI39131 and AI36211) (W.T.S.).
REFERENCES
- 1.Auphan-Anezin, N., G. Verdeil, and A.-M. Schmitt-Verhulst. 2003. Distinct thresholds for CD8 T cells activation lead to functional heterogeneity: CD8 T cell priming can occur independently of cell division. J. Immunol. 170:2442-2448. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, H. M., Y. Ting, C. E. Greer, J. C. Chambers, C. J. Tashiro, J. Chimera, A. Reingold, and M. M. Manos. 1991. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA 265:472-477. [PubMed] [Google Scholar]
- 3.Berzofsky, J. A., J. D. Ahlers, M. A. Derby, C. D. Pendleton, T. Arichi, and I. M. Belyakov. 1999. Approaches to improve engineered vaccines for human immunodeficiency virus and other viruses that cause chronic infections. Immunol. Rev. 170:151-172. [DOI] [PubMed] [Google Scholar]
- 4.Clerici, M., M. Merola, E. Ferrario, D. Trabattoni, M. L. Villa, B. Stefanon, D. J. Venzon, G. M. Shearer, G. De Palo, and E. Clerici. 1997. Cytokine production patterns in cervical intraepithelial neoplasia: association with human papillomavirus infection. J. Natl. Cancer Inst. 89:245-250. [DOI] [PubMed] [Google Scholar]
- 5.Coppola, M. A., and M. A. Balckman. 1997. Bacterial superantigens reactivate antigen-specific CD8+ memory T cells. Int. Immunol. 9:1393-1403. [DOI] [PubMed] [Google Scholar]
- 6.Garland, S. M. 2002. Human papillomavirus update with a particular focus on cervical disease. Pathology 34:213-224. [DOI] [PubMed] [Google Scholar]
- 7.Garzetti, G. G., A. Ciavattini, G. Goteri, M. De Nicolis, S. Menso, M. Muzzioli, and N. Fabris. 1995. HPV DNA positivity and natural killer cell activity in the clinical outcome of mild cervical dysplasia: integration between virus and immune system. Gynecol. Obstet. Investig. 39:130-135. [DOI] [PubMed] [Google Scholar]
- 8.Guijon, F. B., M. Paraskevas, and R. Brunham. 1985. The association of sexually transmitted disease with cervical intraepithelial neoplasia: A case control study. Am. J. Obstet. Gynecol. 151:185-190. [DOI] [PubMed] [Google Scholar]
- 9.Hildesheim, A., M. H. Schiffman, P. E. Gravitt, A. G. Glass, C. E. Greer, T. Zhang, D. R. Scott, B. B. Rush, P. Lawler, M. E. Sherman, R. J. Kurman, and M. M. Manos. 1994. Persistence of type-specific human papillomavirus infection among cytologically normal women. J. Infect. Dis. 169:235-240. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, J. C., A. F. Burnett, G. D. Willet, M. A. Young, and J. Doniger. 1992. High frequency of latent and clinical human papillomavirus cervical infections in immunocompromised human immunodeficiency virus-infected women. Obstet. Gynecol. 79:321-327. [DOI] [PubMed] [Google Scholar]
- 11.Kawabe, Y., and A. Ochi. 1990. Selective anergy of Vβ8+, CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J. Exp. Med. 172:1065-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruger-Kjaer, S., A. J. van den Brule, E. I. Svare, G. Engholm, M. E. Sherman, P. A. Pol, J. M. Walboomers, J. E. Bock, and C. J. Meijer. 1998. Different risk factor patterns for high-grade and low-grade intraepithelial lesions on the cervix among HPV-positive and HPV-negative young women. Int. J. Cancer 76:613-619. [DOI] [PubMed] [Google Scholar]
- 13.Lee, B. N., M. Duvic, C.-K. Tang, C. Bueso-Ramos, Z. Estrov, and J. M. Reuben. 1999. Dysregulated synthesis of intracellular type 1 and type 2 cytokines by T cells of patients with cutaneous T-cell lymphoma. Clin. Diagn. Lab. Immunol. 6:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, B.-N., M. Follen, G. Tortolero-Luna, N. Eriksen, A. Helfgott, H. Hammill, W. T. Shearer, and J. M. Reuben. 1999. Synthesis of IFN-γ by CD8+ T cells is preserved in HIV-infected women with HPV-related cervical squamous intraepithelial lesions. Gynecol. Oncol. 75:379-386. [DOI] [PubMed] [Google Scholar]
- 15.Mao, C., J. P. Hughes, N. B. Kiviat, J. Kuypers, S.-K. Lee, D. E. Adam, and L. A. Kousky. 2003. Clinical findings among young women with genital human papillomavirus infection. Am. J. Obstet. Gynecol. 188:677-684. [DOI] [PubMed] [Google Scholar]
- 16.Moscicki, A.-B., N. Hills, S. Shiboski, K. Powell, J. Naomi, E. Hanson, S. Miller, L. Clayton, S. Farhat, J. Broering, T. Darragh, and J. Palefsky. 2001. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 285:2995-3002. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute Workshop. 1989. The 1988 Bethesda system for reporting cervical/vaginal cytological diagnoses. JAMA 262:931-934. [PubMed] [Google Scholar]
- 18.Passmore, J.-A. S., V. C. Burch, E. G. Shepherd, D. J. Marais, B. Allan, P. Kay, R. C. Rose, and A.-L. Williamson. 2002. Single-cell cytokine analysis allows detection of cervical T-cell responses against human papillomavirus type 16 L1 in women infected with genital HPV. J. Med. Virol. 67:234-240. [DOI] [PubMed] [Google Scholar]
- 19.Pilch, H., H. Hohn, C. Neukirch, K. Freitag, P. G. Knapstein, B. Tanner, and M. J. Maeurer. 2002. Antigen-driven T-cell selection in patients with cervical cancer as evidenced by T-cell receptor analysis and recognition of autologous tumor. Clin. Diagn. Lab. Immunol. 9:267-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rafiq, K., L. Charitidou, D. M. A. Bullens, A. Kasran, K. Lorre, J. L. Ceuppens, and S. W. Van Gool. 2001. Regulation of the IL-10 production by human T cells. Scand. J. Immunol. 53:139-147. [DOI] [PubMed] [Google Scholar]
- 21.Rosini, S., S. Caltagirone, G. Tallini, G. Lattanzio, R. Demopoulos, M. Piantelli, and P. Musiani. 1996. Depletion of stromal and intraepithelial antigen-presenting cells in cervical neoplasia in human immunodeficiency virus infection. Hum. Pathol. 27:834-838. [DOI] [PubMed] [Google Scholar]
- 22.Skov, L., J. Olsen, R. Giorno, P. Schlievert, O. Baadsgaard, and D. Leung. 2000. Application of staphylococcal enterotoxin B on normal and atopic skin induces up-regulation of T cells by a superantigen-mediated mechanism. J. Allergy Clin. Immunol. 105:820-826. [DOI] [PubMed] [Google Scholar]
- 23.Takac, I. 1998. The frequency of bacterial and yeast infection in women with different grades of cervical intraepithelial neoplasia (CIN). Eur. J. Obstet. Gynecol. Reprod. Biol. 80:231-234. [DOI] [PubMed] [Google Scholar]
- 24.Torres, L. M., T. Cabrera, A. Concha, M. R. Oliva, F. Ruiz-Cabello, and F. Carrido. 1993. HLA class I expression and HPV-16 sequences in premalignant and malignant lesions of the cervix. Tissue Antigens 41:65-71. [DOI] [PubMed] [Google Scholar]
- 25.Truneh, A., F. Albert, P. Golstein, and A. M. Schmitt-Verhulst. 1985. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature 313:318-320. [DOI] [PubMed] [Google Scholar]