Abstract
PA energy expenditure (PAEE) is the most variable component of Total Energy Expenditure (TEE) and largely due to the balance of sedentary time (SedT) and low intensity physical activity (LIPA). There has been an emergence for seeking an understanding of factors which determine variations in SedT, LIPA, and PAEE. Sedentary behavior and physical activity are relatively resistant to change by experimental dietary treatments and significant body weight changes. Although caffeine (Caf) is by far the most heavily used nutritional agent ingested to promote a sense of vigor/alertness, it is still unknown if Caf is effective in increasing PAEE and physical activity. The aim of the study was to test the hypothesis that 2 daily doses of Caf (as a capsule to blind the treatment and divided equally during breakfast and lunch) increase PAEE and TEE, and it would do so through increasing the frequent and brief bouts of physical activity (~1-5 min long) through the day as measured by accelerometry. In 21 low Caf users (<100 mg day-1), we used a double-blind crossover trial (ClinicalTrials.govID;NCT01477294) with two conditions (4-day each with a 3-day washout period) randomly ordered as 5 mg kg-1 day-1 of Caf and maltodextrin as placebo (Plc). Resting energy expenditure (REE) by indirect calorimetry, total energy expenditure (TEE) from doubly labeled water, PAEE calculated as TEE-(REE+0.1TEE), and accelerometry measurements of both LIPA and MVPA were not different between conditions. However, regardless of caffeine or placebo, there were several significant relationships between brief bouts of LIPA and MVPA with PAEE. In conclusion, this double-blind study found that low and moderate-vigorous activity as well as the total volume of PAEE in free-living conditions is resistant to dietary caffeine intake that was equivalent to 5 cups of espresso or 7 cups of tea.
Trial Registration
ClinicalTrials.gov NCT01477294
Introduction
Caffeine is one of the most widely consumed food ingredients, commonly found in beverages including coffee, tea and soft drinks, as well as in products containing cocoa or chocolate, and a variety of medications and dietary supplements [1,2]. It has been considered as a thermogenic agent [3–5] that could help in preventing a positive energy balance and obesity [6,7]. Although the effect of caffeine on energy expenditure (EE) has been the subject of numerous investigations [8–10], its effects on daily free living physical activity energy expenditure (PAEE) and physical activity patterns, specifically the frequency of short bouts of low intensity physical activity (LIPA) or moderate to vigorous physical activity (MVPA) have not hitherto been studied.
Current PA guidelines for adults are focused on increasing MVPA levels. However, recent data indicate that only about 4-6% of the Portuguese adult population meet these recommendations [11] which is in accordance with the results from the US adult population (5%) [12]. A recent operational definition [13] defined sedentary behavior as any waking behavior characterized by an energy expenditure ≤1.5 METs while in a sitting or reclining posture. Sedentary behavior may be one of the causes of many modern day chronic diseases including insulin resistance, type 2 diabetes and metabolic syndrome [14–16], and occupation-related sedentary behavior may account for a significant portion of the increase in mean body weight for women and men [17]. Recommending the accumulation of enough time in multiple short bouts of PA, at a low intensity (i.e., LIPA) distributed throughout the day may be an effective alternative for the reduction of sedentary behavior [18]. It was hypothesized by Hamilton et al. in 2007 [14] “that any type of brief, yet frequent, muscular contractions throughout the day may be necessary to short-circuit unhealthy molecular signals causing metabolic diseases”. Experimental [19] and observational [20–22] studies have only begun to suggest indirectly that interrupting sedentary behavior with short bouts of LIPA or MVPA may be positively associated with relevant health outcomes such as blood lipids, glycemic levels, insulin resistance and systolic blood pressure. However, changing human behavior to become more physically active in free-living conditions is very difficult to do, and the simple fact that caffeine is so widely used in all cultures is intriguing to consider if it provides enough of a stimulus to effect a more active behavioral change. Nevertheless, research studies are still lacking regarding the effective contribution of short bouts frequency on PAEE assessed by double labeled water under free-living conditions. In fact, only one study has investigated the relationship between PA short bouts lasting less than 10-min with EE [23] in laboratorial conditions using indirect calorimetry.
Taken together, the absence of published studies concerning the role of caffeine on PA patterns and the contribution of short bouts frequency of LIPA or MVPA to free-living PAEE led us to conduct this investigation with two purposes: a) To evaluate the impact of a moderate dose of caffeine during a 4-day period on PAEE and daily number of short bouts of at least 1 up to 5-min of either LIPA or MVPA in non-obese, physically active males under free-living conditions; b) to analyze, either under caffeine or placebo treatments, the associations between short bouts lasting 1 up to 5-min of LIPA and MVPA with PAEE assessed from doubly labeled water in combination with indirect calorimetry.
Materials and Methods
Ethics Statement and Participants
This research has been approved by the ethics Committee of the Faculty of Human Kinetics, Technical University of Lisbon and were conducted in accordance with the declaration of Helsinki for human studies [24]. All participants were informed about the possible risks of the investigation before giving their written informed consent to participate.
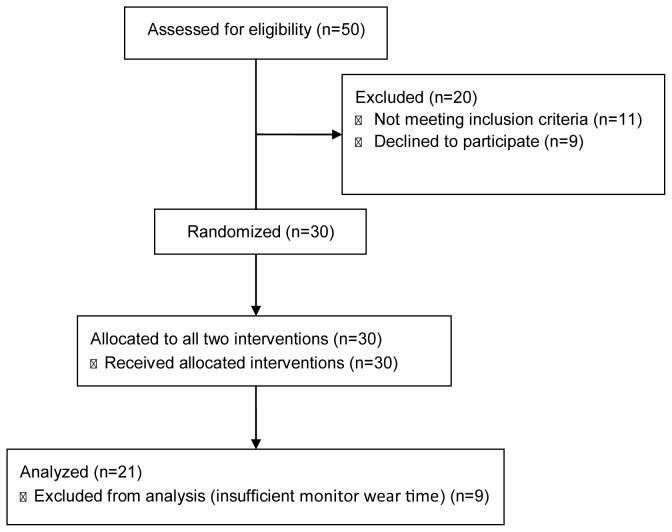
A total of 30 healthy nonsmoking males were considered for eligibility according to a screening algorithm (Figure 1). Participants were recruited through advertisements placed nearby the institution and volunteered to participate in this study. Inclusion criteria were: age between 20 and 39 years old; body mass index (BMI) between 18.5 and 29.9 kg m-2; not taking any medication or dietary supplements that may affect energy expenditure; physically active (estimated by IPAQ), which represent the accumulation of at least 30-min per day of moderate-to-vigorous physical activity, according to the recommendations of the World Health Organization [25]; and low-caffeine users (<100 mg day-1) [26]. The daily consumption of caffeine was estimated based on 7-day self-report of the daily intakes of coffee, tea, caffeinated sodas, chocolate, and other dietary sources, according to a list provided by two sources [27,28].
Figure 1. Screening, enrollment and interventions of the study participants.
Experimental design
Participants were followed in a double-blind crossover experimental design (ClinicalTrials.govID;NCT01477294) with two conditions in a random sequence (a detailed description of the methods are available as supporting information; Protocol S1, CONSORT checklist S1). Caffeine/placebo conditions were randomized by an automated computer-generated randomization scheme and assigned to specific study days. The participants as well as the study personnel, who delivered the capsules to the participants and performed all tests, were blinded to the condition allocation. A laboratorial technician, responsible for preparation of the doses, was the only person aware of the randomization code during the trial. She was not involved in other study functions. Caffeine (5 mg per kg of body mass per day) and maltodextrin as placebo, both through capsules and divided equally during breakfast and lunch. Each condition lasted for 4 days and participants were instructed to keep the same eating patterns and level of PA. There was a washout period of 3 days between each condition [29]. Moreover, to reduce the variability of individual PA patterns during the week, both conditions were performed on the same weekdays while the washout period always included the weekend days. Evaluations were performed at three time points: 1) Baseline: First visit for collecting the initial measurements; 2) Condition 1: second visit, 4 days after baseline, for collecting the final measurements of the first randomly assigned condition (placebo or caffeine); and 3) Condition 2: third visit, 7 days after the end of the first condition, including the 3-day washout period, for collecting the final measurements of the second randomly assigned condition (placebo or caffeine).
Participants were required to fast for at least 12 h prior to each visit, refrain from vigorous exercise for at least 15 h, alcohol consumption for 24 h, and consume a normal evening meal the night before the visit. All measurements were carried out in the same morning of the same week day. In brief, the procedures adopted were as follows:
Caffeine and placebo intake
After weighing the participants, the dose was individually prepared to assure that a 5 mg of caffeine per kg of body mass per day was administered. The dose of caffeine was divided into two equal parts (2.5 mg kg-1) to be orally consumed through capsules in the morning and after lunch. An equivalent dose (5 m kg-1day-1) and number of placebo capsules, of the same color as the caffeine capsules, containing maltodextrin were provided for the placebo condition.
Body composition measures
Anthropometry
Participants wearing minimal clothes and without shoes were weighed to the nearest 0.01 kg, on an electronic scale connected to a plethysmograph computer (BOD POD®, COSMED, Rome, Italy). Height was measured to the nearest 0.1 cm with a stadiometer (Seca, Hamburg, Germany) according to the standardized procedures described elsewhere [30]. BMI was calculated as body mass (kg) height-2 (m).
Fat mass (FM) and fat free mass (FFM)
Dual energy X-ray absorptiometry (DXA) (Hologic Explorer-W, fan-beam densitometer, software QDR for windows version 12.4, Waltham, Massachusetts, USA) was used to estimate FM and FFM. The equipment measures the attenuation of X-rays pulsed between 70 and 140 kV synchronously with the line frequency for each pixel of the scanned image. Following the protocol for DXA described by the manufacturer, a step phantom with six fields of acrylic and aluminium of varying thickness and known absorptive properties was scanned to serve as an external standard for the analysis of different tissue components. The same technician positioned the participants, performed the scans and executed the analysis according to the operator’s manual using the standard analysis protocol.
Resting energy expenditure
Resting energy expenditure (REE) was assessed in the morning (7.00–11.00 a.m.), 1-hour after the first half-dose of caffeine (2.5 mg kg-1) or placebo to be orally consumed through capsules. All measurements were performed in the same room at an environmental temperature and humidity of approximately 22°C and 40-50%, respectively.
The MedGraphics CPX Ultima (Medical Graphics Corp, St Paul, MN, with Breeze suite software) indirect calorimeter was used to measure breath-by-breath oxygen consumption (VVO2) and carbon dioxide production (VCO2) using a facial mask. One trained technician conducted all measurements. The oxygen and carbon dioxide analysers were calibrated in the morning before testing using known gas concentration. The flow and volume were measured using a pneumotachograph calibrated with a 3 L-syringe (Hans Rudolph, inc. TM). Device auto calibration was performed between participants. Before testing, participants were instructed about all the procedure and asked to relax, breathe normally, not to sleep, and not to talk during the evaluation. Total rest duration was 60-min, participants lied supine for 30-min covered with a blanket and the calorimeter device was then attached to the mask and breath by breath VO2 and VCO2 were measured for another 30-min period. Outputs of VO2, VCO2, respiratory exchange ratio (RQ), and ventilation were collected and averaged over 1-min intervals for data analysis. The first and the last 5-min of data collection were discarded and the mean of a 5-min steady state interval between the 5 and the 25-min with RQ between 0.7 and 1.0 was used to determine REE. Steady state was defined as a 5-min period with ≤ 10% CV for VO2 and VCO2 [31]. The mean VO2 and VCO2 of 5-min steady states were used in Weir equation [32] and the period with the lowest REE was considered.
Total and physical activity energy expenditure
Total energy expenditure (TEE) was measured by doubly labeled water (DLW) [33,34]. Deuterium oxide and 18-Oxygen were administered in the morning of the first visit (baseline). Briefly, participants were weighed in the morning and baseline urine was collected. An oral dose of 2.7 g kg-1 of TBW of a 10 atom% (AP) solution of H2 18O (Taiyo Nippon Sanso Corporation, Tokyo, Japan), assuming that TBW is 0.61xTotal body weight, and 0.24 g kg-1 of TBW of a 99.9 AP solution of 2H2O (Sigma-Aldrich, Co, St Louis, Mo, USA), diluted in 50 ml of water and administered to the participants at 7.00 a.m. Post-dose urine samples of the first visit day were taken and stored from voids at 4 and 5h. Morning urine samples and 1h after were collected on day 4 (end of first condition), after the washout period (day 7), and at the end of the second condition (last day). Urine samples were prepared and filled with the equilibration gas. The equilibration period lasted for 3 days and 8h, respectively for 2H and 18O. Samples were analyzed in duplicates and calibrated against standard mean ocean water (SMOW), using Hydra isotope ratio mass spectrometer (PDZ, Europa Scientific, UK). A two-point sample method was used to evaluate the elimination constants (kd and ko, respectively for deuterium and 18-oxygen) over the first and the second 4-day periods (condition 1 and 2, respectively). A similar procedure was used elsewhere [35]. For analyzing condition 2, urine samples collected after the washout period (day 7) and on the last day of the trial were considered to evaluate the elimination constants. Energy expenditure measured by the DLW method was calculated from a modified Weir’s equation by use from DLW and calculated from the food quotient obtained by dietary intake records [32].
Physical activity energy expenditure (PAEE) was calculated as the difference between TEE and the sum of REE with 0.1*TEE (assuming the thermic effect of food is ~10% of TEE).
Physical activity patterns and short bouts assessment
All participants were asked to wear an accelerometer (ActiGraph, GT1M model, Fort Walton Beach, Florida) on the right hip, near the iliac crest during eleven consecutive days, including two weekend days [36]. The delivery and reception of the accelerometers to the participants, as well the explanation of its use, were made personally [37]. The devices were activated on the first day at 7.00 a.m. and data were recorded in 10-sec epochs. The device activation and data download were performed using the software Actilife Lifestyle (v.3.2). Processing was performed using the software MAHUffe v.1.9.0.3 (available at www.mrc-epid.cam.ac.uk) from the original downloaded files (*. dat). For the analyses, a valid day was defined as having 600 or more min (≥10 hours) of monitor wear, corresponding to the minimum daily use of the accelerometer [37]. Apart from accelerometer non-wear time (i.e. when it was removed for sleeping or water activities), periods of at least 60 consecutive min of zero activity intensity counts were also considered as non-wear time.
For the short bouts frequencies assessment, we analyzed the number of bouts of at least 1 up to 5-min individually and for both PA of at least low and of at least moderate intensity, using the MAHUffe software. For assessing the frequency of short bouts of LIPA, we calculate the difference between the number of bouts of at least low from those of moderate intensity PA, considering the bouts durations defined (1 up to 5-min).
The amount of activity assessed by accelerometry was expressed as the min per day spent in different intensities and the frequency of short bouts of at least 1 up to 5-min of low (LIPA) and at least moderate (MVPA) intensity PA. The cutoff values used to define the intensity of PA and therefore to quantify the mean time in each intensity (sedentary, low, moderate or vigorous) were: sedentary: < 100 counts min-1; low: 100-2019 counts min-1; moderate: 2020-5998 counts min-1 (corresponding to 3-5.9 METs); vigorous: ≥ 5999 counts min-1 (corresponding to ≥ 6 METs) [12].
Energy and nutrient intake
Food intake was assessed throughout the study using 24-h diet records during the 11-day period of this trial. Participants were instructed regarding portion sizes, food preparation aspects, and others aspects pertaining to an accurate recording of their energy intake. Accuracy of the food intake recordings was ascertained by the study nutritionist at the second study visit (4 days after baseline). At the last visit, records were turned in and reviewed for water ingestion, macronutrient composition and total energy intake by the same nutritionist. Diet records were analyzed using a software package (Food Processor SQL).
Statistical analysis
Statistical analysis was performed using PASW Statistics for Windows version 18.0, 2010 (SPSS Inc., an IBM Company, Chicago IL, USA). Descriptive analysis included means ± SD for all measured variables. Normality was explored using the Shapiro-Wilk test.
Comparison of means between conditions was performed using paired sample T-Test. To compare the effects of caffeine on mean values of the main dependent variables, linear mixed models for repeated measures were used.
In addition, to evaluate the effect of caffeine on the dependent variables, fat-free mass and energy intake were used as covariates.
To evaluate the association between short bouts of at least 1 up to 5-min of either LIPA or MVPA with PAEE, multiple regression analysis was performed, adjusting for sedentary time. Unstandardized residuals were explored to check homoscedasticity and outliers.
Reliability for all the methods employed in this study was assessed using the coefficient of variation (CV). Based on test–retest using ten participants, the coefficient of variation (CV) in our laboratory for FM and FFM are 1.7% and 0.8%, respectively [38], 4.3% for TEE, and using seven participants CV for REE is 4.0%.
Statistical significance was set at p < 0.05.
Power Calculation
Considering EE, prior data indicate that the difference in the response of matched pairs is normally distributed with standard deviation of 120 Kcal [39]. Considering a true difference of 77 Kcal [39] in the mean response of matched pairs, 21 pairs of participants are necessary to reject the null hypothesis that the response difference is zero with a power of 80% and a type I error probability of 0.05.
Regarding the larger variability in EE values due to the free living settings we enrolled 30 participants.
Results
Twenty one participants were included in the study (Figure 1). The participant’s characteristics and body composition at baseline are presented in Table 1 .
Table 1. Participant’s characteristics and body composition (N=21).
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 24.3 ± 4.5 | 20–38 |
| Height (m) | 1.76 ± 0.07 | 1.64-1.87 |
| Body mass (kg) | 72.4 ± 9.4 | 51.8-90.2 |
| BMI (kg m-2) | 23.7 ± 2.4 | 19.6-27.7 |
| FM (kg) | 11.7 ± 4.3 | 5.1-20.6 |
| FFM (kg) | 59.9 ± 6.9 | 46.2-70.3 |
Abbreviations: SD, standard deviation; N, number of participants; BMI, body mass index; FM, fat
mass; FFM, fat-free mass.
The different components of energy expenditure, physical activity and dietary patterns, during treatment conditions are presented in Table 2 .
Table 2. Total, resting, and physical activity energy expenditure, habitual physical activity, and dietary intake under treatment conditions (N = 21).
| Placebo | Caffeine | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| TEE (kcal day-1) | 3107 ± 438 | 3046 ± 542 |
| REE (kcal day-1) | 1442 ± 240 | 1428 ± 258 |
| PAEE (kcal day-1) | 1354 ± 373 | 1368 ± 359 |
| Daily Steps | 8527 ± 2910 | 9374 ± 3158 |
| Sedentary (min day-1) | 703 ± 87 | 709 ± 94 |
| Low (min day-1) | 151 ± 38 | 157 ± 38 |
| Moderate (min day-1) | 45 ± 21 | 49 ± 24 |
| Vigorous (min day-1) | 5 ± 6 | 6 ± 5 |
| Energy Intake (kcal day-1) | 2682 ± 641 | 2472 ± 391 |
| Carbohydrates (g) | 309 ± 86 | 298 ± 56 |
| Fat (g) | 92 ± 24 | 79 ± 21 |
| Protein (g) | 124 ± 35 | 115 ± 21 |
Abbreviations: SD, standard deviation; N, number of participants; TEE, total energy expenditure; REE, resting energy expenditure; PAEE, physical activity energy expenditure; Min, minutes
No differences were observed between treatment conditions for PAEE (-13.7 ± 248.0 kcal day-1; p=0.803), TEE (60.8 ± 417.4 kcal day-1; p=0.512), REE (13.6 ± 153.1 kcal day-1; p=0.689), steps (-847 ± 3352 steps day-1; p=0.261), and time spent in sedentary behavior (-7 ± 108 min; p=0.783), low (-6 ± 30 min; p=0.394), moderate (-3 ± 25 min; p=0.549), and vigorous (-1 ± 6 min; p=0.430) intensities. After controlling for fat free mass and energy intake, the results remained the same (p>0.05) for all the aforementioned variables. Also, dietary intake did not differ between caffeine and placebo treatment conditions, specifically with respect to energy intake (p=0.105) and the amount of carbohydrates (p=0.553), fat (p=0.052), and protein (p=0.225) consumed. Therefore, the food quotients were not different in both conditions, which reduced the variability within the DLW method.
Table 3 shows the frequency of PA divided by the intensity and the bout duration for both conditions.
Table 3. The daily physical activity levels by bout duration on both conditions (N = 21).
| Bout duration |
Placebo
|
Caffeine
|
||
|---|---|---|---|---|
| Frequency (bouts day-1) |
||||
| LIPA | MVPA | LIPA | MVPA | |
| > 1 min | 93 ± 44 | 35 ± 22 | 99 ± 42 | 36 ± 21 |
| > 2 min | 35 ± 19 | 19 ± 15 | 39 ± 23 | 19 ± 12 |
| > 3 min | 15 ± 9 | 10 ± 9 | 17 ± 10 | 10 ± 7 |
| > 4 min | 9 ± 7 | 7 ± 7 | 10 ± 7 | 6 ± 5 |
| > 5 min | 7 ± 6 | 6 ± 6 | 7 ± 4 | 5 ± 4 |
The data are expressed as the means with standard deviations (mean ± SD)
Abbreviations: LIPA, low physical activity; MVPA, moderate-to-vigorous physical activity; Min, minutes
The results showed no significant differences (p ≥ 0.05) between placebo and caffeine conditions with mean differences for the frequencies of short bouts of at least 1 (-11 ± 41), 2 (-7 ± 20), 3 (-3 ± 10), 4 (-3 ± 7), and 5 (-1 ± 5) min of LIPA. Similarly, there were no significant differences between conditions, for the MVPA short bouts of at least 1 (-6 ± 23), 2 (-2 ± 14), 3 (-1 ± 8), 4 (0 ± 6), and 5 (0 ± 5) min. After controlling for fat free mass and energy intake, the results remained the same (p>0.05).
Concerning the second purpose of this investigation, to analyze the association between short bouts and PAEE under caffeine and placebo, Figure 2 shows the relationship between the daily frequencies of LIPA by the bout duration with PAEE, for both placebo and caffeine conditions.
Figure 2. Physical activity energy expenditure and frequency of low intensity short bouts (>4-min), under both conditions.
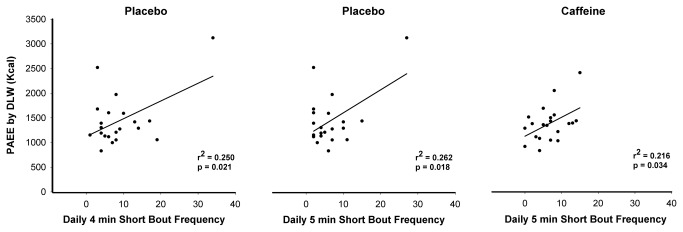
Association between physical activity energy expenditure (PAEE) from doubly labeled water (DLW) and the frequency of short bouts (>4 min) performed at a low intensity physical activity, under placebo and caffeine conditions.
As illustrated in Figure 2 , for LIPA, short bouts lasting longer than 4 (9 ± 7) and 5 (6 ± 6) min under placebo, and at least 5 (7 ± 4) min under caffeine predicted PAEE [placebo: β=36, p=0.021 (4-min); β=47, p=0.018 (5-min); caffeine: β=38, p=0.034 (5-min), respectively]. Therefore, our results demonstrated an additional 36 kcal bout-1, and 38 kcal bout-1 in PAEE for each bout of at least 4-min, under placebo and caffeine respectively. These associations remained significant when adjusted for sedentary time (<100 cpm).
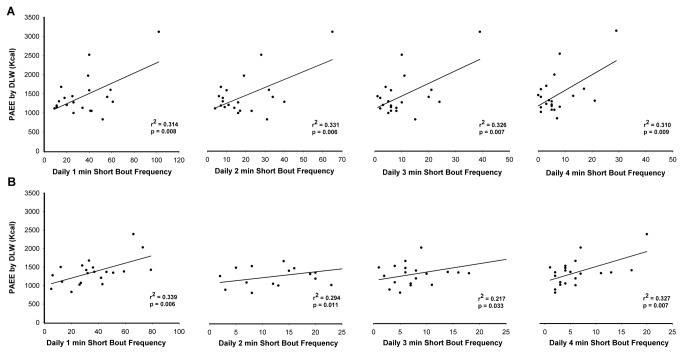
The associations between the frequencies of MVPA short bouts with PAEE are presented in Figure 3 , under placebo (A) and caffeine (B) treatment conditions, respectively.
Figure 3. Physical activity energy expenditure and frequency of moderate-to-vigorous intensity short bouts, under placebo-(A) and caffeine-(B).
Association between physical activity energy expenditure (PAEE) from doubly labeled water (DLW) and the frequency of short bouts (1-4 min) performed at a moderate-to-vigorous physical activity intensity, under placebo (panels A) and caffeine (panels B).
MVPA short bouts lasting longer than 1 (34 ± 23), 2 (19 ± 15), 3 (10 ± 9), and 4 (7 ± 7) min under placebo condition, and at least 1 (36 ± 21), 2 (19 ± 12), 3 (10 ± 7), and 4 (6 ± 5) min under caffeine were associated with PAEE, [placebo: β=13, p=0.008 (1-min); β=20, p=0.006 (2-min); β=33, p=0.007 (3-min); β=40, p=0.009 (4-min); caffeine: β=10, p=0.006 (1-min); β=16, p=0.011 (2-min); β=23, p=0.033 (3-min); β=40, p=0.007 (4-min)]. A single bout of at least 1, 2, 3, and 4-min of MVPA resulted in an additional 13, 20, 33, and 40 kcal bout-1 in PAEE under placebo, and 10, 16, 23, and 40 kcal bout-1 in PAEE in caffeine condition. These associations remained significant when adjusted for sedentary time (<100 cpm).
Discussion
The present investigation examined the effect of a moderate dose of caffeine on PAEE and the frequency of short bouts of both LIPA and MVPA, on real life settings and using objective methods such as DLW and accelerometry, respectively.
It is well known that caffeine works as an ergogenic substance for the central nervous system [10], then it would be expected an increase on daily PA, specifically the daily low intensity activities [40]. Unexpectedly, our novel finding revealed that a moderate dose of caffeine ingestion, corresponding to approximately 5 espresso cups of coffee (30 mL each) or 7 servings of tea, with each containing nearly 75 mg of caffeine had no effect on PAEE, the time spent in LIPA and MVPA, and the daily number of short bouts of both LIPA and MVPA intensities.
Previous studies indicated wide intra-individual variation in sedentary behavior and physical activity making it necessary to pay close attention to results within studies rather than comparing results between studies. Also, accelerometers in theory fail to record activity counts during certain very low intensity activities (LIPA) and thereby may underestimate the time spent in some activities [41–43]. We used a reliable type of accelerometer frequently used in the literature. Based on accelerometry data our participants performed approximately 2.5 hours of LIPA which is somewhat below the typical values presented for the Portuguese male population on this age range (3.3 hours) [11]. Furthermore, sedentary time (700 min) was also higher than the reference value (594 min). The high PAEE and other modest step count and MVPA suggest, that a large amount of energy expenditure occurs in LIPA and therefore the lack of change in PAEE (from DLW) confirms our findings that caffeine did not reduce the high amount of sedentary time in our participants because a shift toward more LIPA.
There is also some confusion concerning the best cutoff value to distinguish sedentary behavior and LIPA. The most commonly used is the 100 counts min-1, however based on a previous investigation it seems that this value is more accurate only when sedentary time is lower, while the 150 count min-1 is more accurate when sedentary time is higher. Despite participants being active, they also spent a major part of their waking day in sedentary behavior, therefore it could have been used the 150 cutoff value [44]. Two studies investigated the effects of caffeine on TEE in a metabolic chamber during 1-day [5,9]. Berube-Parent et al found an increase of 179 kcal in TEE considering a 600 mg day-1 caffeine dosage whereas the later investigation, using a lower caffeine dose (150 mg day-1) did not present significant differences between caffeine and placebo [9]. Though our mean caffeine dose administration was considerable higher than that reported by Dulloo et al [9], we also found non-significant effects of caffeine on TEE, over a 4-day period. Accordingly, the most likely explanation for these findings is probably related to the mean dosage used (~360 mg day-1), which is below the threshold for stimulating thermogenesis, i.e., 600–1000 mg caffeine day-1 [6]. In addition, a possible tolerance to caffeine effects [45] during the 4-day period may also explain these results. A previous investigation found significant effects of caffeine on increasing REE [3,46], even when participants were moderate caffeine consumers [4,47]. Although no study considered a period longer than 24 h, we were expecting that our low-caffeine users (<100 mg day-1) would increase REE under a 4-day caffeine intake. However, the results from the present investigation showed no significant increase in daily REE (13.6 kcal day-1; p=0.689).
It has been speculated that the contradictory results for all the EE components might be explained by the great variability on the individual response to caffeine due to body composition and dietary intake variables [48]. However, our findings did not confirm that fat-free mass or dietary intake mediated the effect of caffeine on PAEE, TEE, REE, and the frequencies of short bouts of both LIPA and MVPA.
There is still some uncertainty regarding the role of caffeine intake on both sedentary behavior and interruptions of sedentary time. Caffeine intake has been associated with lower sleep time and higher screen time, which are major sedentary behavior promoters [49]. Another investigation found small but statistically significant differences in spontaneous PA between catechins/caffeine treatment compared with the caffeine-only treatment, but no differences between caffeine-only and placebo conditions [50].
Recent research has focused on the detrimental effects of a sedentary lifestyle [51–53]. Researchers have hypothesized that breaking up sedentary behavior with LIPA and MVPA short bouts will improve the health of sedentary individuals, independently of PA levels [21]. However, to our knowledge only one study [23] investigated the acute contribution of PA short bouts of LIPA and MVPA to PAEE using direct calorimetry in laboratorial settings. We also found no significant contribution of short bouts of LIPA lasting less than 4-min to PAEE, which is also in agreement with previous findings [18].
The compendium of physical activities [54] documented “walking when gathering things at work ready to leave” has a metabolic equivalent of 3.0 METS or 10.5 ml kg-1 min-1, “walking less than 3.2 km on a firm surface” has 2.0 METS or 7.0 ml kg-1 min-1. Accordingly [23], observed that short bouts, with an intensity of 5.0 ml kg-1 min-1 were in the LIPA spectrum. This level of EE may possibly have an important impact on weight maintenance, or even possibly weight loss. Experimental studies should be conducted to test the effects of increasing PA short bouts on weight loss or maintenance.
It is important to mention some limitations and strengths of the current investigation. Our participants were already physically active at baseline which may have hindered or reduced the potentiating effect of caffeine on increasing PA levels. However, considering their low level of caffeine habituation we were not expecting increases in MVPA but in spontaneous LIPA. Still, these participants had about 700 min/day of sedentary time as measured with our accelerometers and thus ample time for caffeine to cause individuals to “get up and move” if caffeine were to stimulate more LIPA or some other kind of activity.
Hip-worn accelerometers have been used to estimate sedentary time from total body movement. While accelerometers function well for many purposes, most models are not designed to accurately measure postures like sitting and standing. Therefore, as presented earlier in this section, the use of an accelerometer to measure sedentary time and LIPA transitions may be less sensitive and somehow underestimated LIPA.
We did not assess hormonal changes that, in fact, could vary under caffeine intake, which would likely affect EE, specifically during rest. Studies indicated that caffeine increases catecholamine production [9,48,55] however, we did not find significant differences between REE values assessed at baseline and at the end of each condition. Our findings are only generalized to non-obese physically active males, low-caffeine users, and for a 4-day period. Further research should be conducted with a higher caffeine dose and in a population that vary in age, body mass index, gender, and activity levels. The major strengths of this study include the study design with double-blind randomized crossover trial and state-of-the-art methods for assessing PAEE, specifically the DLW technique for TEE in combination with indirect calorimetry for REE in free living conditions.
In conclusion, our findings revealed that resting, total, and physical activity EE, and accelerometry measurements of both LIPA and MVPA were not affected by the ingestion of a moderate dose of caffeine. Also, a significant contribution to PAEE was observed in males that increased short bouts frequency of at least 1 up to 4-min of MVPA and at least 4-min of LIPA. Regardless of sedentary time, shifting sedentary behavior to LIPA through bouts of at least 4-min can markedly increase PAEE in healthy males.
Supporting Information
(DOC)
(PDF)
Acknowledgments
We would like to express our gratitude to the participants for their time and effort.
Funding Statement
This work was supported by the Portuguese Institute of Hydration and Health (http://www.ihs.pt/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Barone JJ, Roberts HR (1996) Caffeine consumption. Food Chem Toxicol 34: 119-129. doi:10.1016/0278-6915(95)00093-3. PubMed: 8603790. [DOI] [PubMed] [Google Scholar]
- 2. Andrews KW, Schweitzer A, Zhao C, Holden JM, Roseland JM et al. (2007) The caffeine contents of dietary supplements commonly purchased in the US: analysis of 53 products with caffeine-containing ingredients. Anal Bioanal Chem 389: 231-239. doi:10.1007/s00216-007-1437-2. PubMed: 17676317. [DOI] [PubMed] [Google Scholar]
- 3. Arciero PJ, Gardner AW, Calles-Escandon J, Benowitz NL, Poehlman ET (1995) Effects of caffeine ingestion on NE kinetics, fat oxidation, and energy expenditure in younger and older men. Am J Physiol 268: E1192-E1198. PubMed: 7611396. [DOI] [PubMed] [Google Scholar]
- 4. Astrup A, Toubro S, Cannon S, Hein P, Breum L et al. (1990) Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. Am J Clin Nutr 51: 759-767. PubMed: 2333832. [DOI] [PubMed] [Google Scholar]
- 5. Bérubé-Parent S, Pelletier C, Doré J, Tremblay A (2005) Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in men. Br J Nutr 94: 432-436. doi:10.1079/BJN20051502. PubMed: 16176615. [DOI] [PubMed] [Google Scholar]
- 6. Dulloo AG, Geissler CA, Horton T, Collins A, Miller DS (1989) Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am J Clin Nutr 49: 44-50. PubMed: 2912010. [DOI] [PubMed] [Google Scholar]
- 7. Hursel R, Westerterp-Plantenga MS (2010) Thermogenic ingredients and body weight regulation. Int J Obes (Lond) 34: 659-669. doi:10.1038/ijo.2009.299. PubMed: 20142827. [DOI] [PubMed] [Google Scholar]
- 8. Bracco D, Ferrarra JM, Arnaud MJ, Jéquier E, Schutz Y (1995) Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am J Physiol 269: E671-E678. PubMed: 7485480. [DOI] [PubMed] [Google Scholar]
- 9. Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N et al. (1999) Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 70: 1040-1045. PubMed: 10584049. [DOI] [PubMed] [Google Scholar]
- 10. Hursel R, Viechtbauer W, Dulloo AG, Tremblay A, Tappy L et al. (2011) The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obes Rev 12: e573-e581. doi:10.1111/j.1467-789X.2011.00862.x. PubMed: 21366839. [DOI] [PubMed] [Google Scholar]
- 11. Baptista F, Santos DA, Silva AM, Mota J, Santos R et al. (2012) Prevalence of the Portuguese population attaining sufficient physical activity. Med Sci Sports Exerc 44: 466-473. doi:10.1249/MSS.0b013e318230e441. PubMed: 21844823. [DOI] [PubMed] [Google Scholar]
- 12. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T et al. (2008) Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40: 181-188. doi:10.1249/01.mss.0000322246.48549.fc. PubMed: 18091006. [DOI] [PubMed] [Google Scholar]
- 13. Sedentary Behaviour Research N (2012). Letter Editors Standardized Use Termssedentary" and "sedentary behaviours". Appl Physiol Nutr Metab 37: 540-542 [Google Scholar]
- 14. Dunstan DW, Howard B, Healy GN, Owen N (2012) Too much sitting - A health hazard. Diabetes Res Clin Pract. [DOI] [PubMed] [Google Scholar]
- 15. Hamilton MT, Hamilton DG, Zderic TW (2007) Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655-2667. doi:10.2337/db07-0882. PubMed: 17827399. [DOI] [PubMed] [Google Scholar]
- 16. Owen N, Healy GN, Matthews CE, Dunstan DW (2010) Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev 38: 105-113. doi:10.1097/JES.0b013e3181e373a2. PubMed: 20577058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP et al. (2011) Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLOS ONE 6: e19657. doi:10.1371/journal.pone.0019657. PubMed: 21647427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macfarlane DJ, Taylor LH, Cuddihy TF (2006) Very short intermittent vs continuous bouts of activity in sedentary adults. Prev Med 43: 332-336. doi:10.1016/j.ypmed.2006.06.002. PubMed: 16875724. [DOI] [PubMed] [Google Scholar]
- 19. Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E et al. (2012) Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 35: 976-983. doi:10.2337/dc11-1931. PubMed: 22374636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boreham CA, Kennedy RA, Murphy MH, Tully M, Wallace WF et al. (2005) Training effects of short bouts of stair climbing on cardiorespiratory fitness, blood lipids, and homocysteine in sedentary young women. Br J Sports Med 39: 590-593. doi:10.1136/bjsm.2002.001131. PubMed: 16118293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE et al. (2008) Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care 31: 661-666. doi:10.2337/dc07-2046. PubMed: 18252901. [DOI] [PubMed] [Google Scholar]
- 22. Miyashita M, Burns SF, Stensel DJ (2008) Accumulating short bouts of brisk walking reduces postprandial plasma triacylglycerol concentrations and resting blood pressure in healthy young men. Am J Clin Nutr 88: 1225-1231. PubMed: 18996856. [DOI] [PubMed] [Google Scholar]
- 23. Swartz AM, Squires L, Strath SJ (2011) Energy expenditure of interruptions to sedentary behavior. Int J Behav Nutr Phys Act 8: 69. doi:10.1186/1479-5868-8-69. PubMed: 21708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Medical Association (2008) Declaration of Helsinki - ethical principles for medical research involving human subjects WMJ 54: 122-125. [Google Scholar]
- 25. World Health Organization (2007) Steps to Health. A Eur Framework Promote Physical Act Health Copenhagen (Denmark) World Health Organization: 32–34. [Google Scholar]
- 26. Currie SR, Wilson KG, Gauthier ST (1995) Caffeine and chronic low back pain. Clin J Pain 11: 214-219. PubMed: 8535041. [PubMed] [Google Scholar]
- 27. Burke LM (2008) Caffeine and sports performance. Appl Physiol Nutr Metab 33: 1319-1334. doi:10.1139/H08-130. PubMed: 19088794. [DOI] [PubMed] [Google Scholar]
- 28. Heckman MA, Weil J, Gonzalez de Mejia E (2010) Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 75: R77-R87. doi:10.1111/j.1750-3841.2010.01561.x. PubMed: 20492310. [DOI] [PubMed] [Google Scholar]
- 29. Bird ET, Parker BD, Kim HS, Coffield KS (2005) Caffeine ingestion and lower urinary tract symptoms in healthy volunteers. Neurourol Urodyn 24: 611-615. doi:10.1002/nau.20179. PubMed: 16167356. [DOI] [PubMed] [Google Scholar]
- 30. Lohman TG, Roche AS, Martorell R (1988) Anthropometric standardization reference manual. Champaign, IL: Human Kinetics. 177 p. [Google Scholar]
- 31. Compher C, Frankenfield D, Keim N, Roth-Yousey L (2006) Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 106: 881-903. doi:10.1016/j.jada.2006.02.009. PubMed: 16720129. [DOI] [PubMed] [Google Scholar]
- 32. Weir JB (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1-9. PubMed: 15394301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schoeller DA, Taylor PB (1987) Precision of the doubly labelled water method using the two-point calculation. Hum Nutr Clin Nutr 41: 215-223. PubMed: 3301748. [PubMed] [Google Scholar]
- 34. Schoeller DA, van Santen E (1982) Measurement of energy expenditure in humans by doubly labeled water method. J Appl Physiol 53: 955-959. PubMed: 6759491. [DOI] [PubMed] [Google Scholar]
- 35. Martin CK, Correa JB, Han H, Allen HR, Rood JC et al. (2012) Validity of the Remote Food Photography Method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity (Silver Spring) 20: 891-899. doi:10.1038/oby.2011.344. PubMed: 22134199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trost SG, McIver KL, Pate RR (2005) Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc 37: S531-S543. doi:10.1249/01.mss.0000185657.86065.98. PubMed: 16294116. [DOI] [PubMed] [Google Scholar]
- 37. Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP (2005) Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc 37: S582-S588. doi:10.1249/01.mss.0000185292.71933.91. PubMed: 16294121. [DOI] [PubMed] [Google Scholar]
- 38. Santos DA, Silva AM, Matias CN, Fields DA, Heymsfield SB et al. (2010) Accuracy of DXA in estimating body composition changes in elite athletes using a four compartment model as the reference method. Nutr Metab (Lond) 7: 22. doi:10.1186/1743-7075-7-22. PubMed: 20307312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roberts AT, de Jonge-Levitan L, Parker CC, Greenway F (2005) The effect of an herbal supplement containing black tea and caffeine on metabolic parameters in humans. Altern Med Rev 10: 321-325. PubMed: 16366740. [PubMed] [Google Scholar]
- 40. Greenberg JA, Axen KV, Schnoll R, Boozer CN (2005) Coffee, tea and diabetes: the role of weight loss and caffeine. Int J Obes (Lond) 29: 1121-1129. doi:10.1038/sj.ijo.0802999. PubMed: 15925959. [DOI] [PubMed] [Google Scholar]
- 41. John D, Tyo B, Bassett DR (2010) Comparison of four ActiGraph accelerometers during walking and running. Med Sci Sports Exerc 42: 368-374. doi:10.1249/01.MSS.0000384654.62019.ac. PubMed: 19927022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS (2011) Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc 43: 1561-1567. doi:10.1249/MSS.0b013e31820ce174. PubMed: 21233777. [DOI] [PubMed] [Google Scholar]
- 43. Lyden K, Kozey Keadle SL, Staudenmayer JW, Freedson PS (2012) Validity of two wearable monitors to estimate breaks from sedentary time. Med Sci Sports Exerc 44: 2243-2252. doi:10.1249/MSS.0b013e318260c477. PubMed: 22648343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kozey-Keadle S, Libertine A, Staudenmayer J, Freedson P (2012) The Feasibility of Reducing and Measuring Sedentary Time among Overweight, Non-Exercising Office Workers. J Obes, 2012: 2012: 282303. PubMed: 22175004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robertson D, Wade D, Workman R, Woosley RL, Oates JA (1981) Tolerance to the humoral and hemodynamic effects of caffeine in man. J Clin Invest 67: 1111-1117. doi:10.1172/JCI110124. PubMed: 7009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koot P, Deurenberg P (1995) Comparison of changes in energy expenditure and body temperatures after caffeine consumption. Ann Nutr Metab 39: 135-142. doi:10.1159/000177854. PubMed: 7486839. [DOI] [PubMed] [Google Scholar]
- 47. Collins LC, Cornelius MF, Vogel RL, Walker JF, Stamford BA (1994) Effect of caffeine and/or cigarette smoking on resting energy expenditure. Int J Obes Relat Metab Disord 18: 551-556. PubMed: 7951476. [PubMed] [Google Scholar]
- 48. Hamada T, Kotani K, Higashi A, Ikeda J, Tagaki E et al. (2008) Lack of association of the Trp64Arg polymorphism of beta3-adrenergic receptor gene with energy expenditure in response to caffeine among young healthy women. Tohoku J Exp Med 214: 365-370. doi:10.1620/tjem.214.365. PubMed: 18441513. [DOI] [PubMed] [Google Scholar]
- 49. Drescher AA, Goodwin JL, Silva GE, Quan SF (2011) Caffeine and screen time in adolescence: associations with short sleep and obesity. J Clin Sleep Med 7: 337-342. PubMed: 21897768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gregersen NT, Bitz C, Krog-Mikkelsen I, Hels O, Kovacs EM et al. (2009) Effect of moderate intakes of different tea catechins and caffeine on acute measures of energy metabolism under sedentary conditions. Br J Nutr 102: 1187-1194. doi:10.1017/S0007114509371779. PubMed: 19445822. [DOI] [PubMed] [Google Scholar]
- 51. Katzmarzyk PT, Church TS, Craig CL, Bouchard C (2009) Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 41: 998-1005. doi:10.1249/MSS.0b013e3181930355. PubMed: 19346988. [DOI] [PubMed] [Google Scholar]
- 52. Matthews CE, George SM, Moore SC, Bowles HR, Blair A et al. (2012) Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr 95: 437-445. doi:10.3945/ajcn.111.019620. PubMed: 22218159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vandelanotte C, Sugiyama T, Gardiner P, Owen N (2009) Associations of leisure-time internet and computer use with overweight and obesity, physical activity and sedentary behaviors: cross-sectional study. J Med Internet Res 11: e28. doi:10.2196/jmir.1084. PubMed: 19666455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM et al. (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32: S498-S504. doi:10.1097/00005768-200009001-00009. PubMed: 10993420. [DOI] [PubMed] [Google Scholar]
- 55. Dulloo AG, Seydoux J, Girardier L (1992) Potentiation of the thermogenic antiobesity effects of ephedrine by dietary methylxanthines: adenosine antagonism or phosphodiesterase inhibition? Metabolism 41: 1233-1241. doi:10.1016/0026-0495(92)90015-3. PubMed: 1435297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)