Abstract
In the present study, we demonstrate that the yeast form of Blastomyces dermatitidis can proliferate for short periods of time in the absence of ferric iron but not in the absence of calcium or magnesium. The results of this study shed light on the resistance of B. dermatitidis to chelating agents, such as deferoxamine, and may explain how B. dermatitidis resists the iron-binding activity of serum transferrin.
Iron is an essential nutrient for virtually all microorganisms. Although the human body contains an abundance of iron, the majority is bound to hemoglobin, myoglobin, and cytochromes and thus is not available in a form that can be used to support the growth of microorganisms (16). The availability of ferric iron is further limited by transferrin, a high-affinity iron-binding plasma protein that contributes to innate immunity against several pathogenic microorganisms (16). Some pathogenic fungi possess mechanisms to overcome the iron-limiting conditions within the host. For example, the dimorphic fungus Histoplasma capsulatum produces siderophores, which are high-affinity iron-binding ligands that facilitate the acquisition, transport, and assimilation of ferric iron in vitro (12, 13). Whether these fungal siderophores are involved in acquisition and assimilation of iron during the course of an infection in vivo is not known. Other strategies utilized by microorganisms to acquire iron include the reduction of exogenous ferric iron by fungal cell wall ferric reductases, degradation of host proteins complexed with iron, and regulation of phagolysosome pH to promote the disassociation of ferric iron from transferrin (7, 9, 14). Some microorganisms (for instance, Borrelia burgdorferi) do not require ferric iron for growth at all (19). In a previous study, we observed that serum transferrin did not inhibit the proliferation of the yeast form of the dimorphic fungal pathogen Blastomyces dermatitidis in vitro (10). This observation was of great interest to us given that the majority of fungal pathogens that cause disease in humans and other mammals are susceptible to the iron-binding activity of serum transferrin, which is an effective innate defense mechanism against pathogens.
B. dermatitidis is a thermally dimorphic fungal pathogen with a wide geographic distribution, and it causes disease primarily in humans, dogs, and other mammals (1, 3, 4, 6, 8, 20, 21). B. dermatitidis resides in the environment as a saprophytic mold that forms infectious conidia that are aerosolized under the appropriate environmental conditions. Following inhalation, conidia transform into a pathogenic yeast form that can multiply in the lung. The resulting pulmonary infection can range in severity from asymptomatic to a severe progressive pneumonia that can disseminate to the skin, eye, and other body sites. We have a limited understanding of innate defense mechanisms that affect the multiplication of B. dermatitidis yeast during the course of disease.
Transferrin is a high-affinity iron-binding protein that contributes to innate immunity against fungal pathogens such as Cryptococcus neoformans, Candida albicans, the dermatophytes (Trichophyton, Microsporum, and Epidermophyton), and H. capsulatum by limiting the availability of ferric iron in the host environment (16, 17, 23-25). During the course of an infection, plasma transferrin concentrations increase and the expression of cell surface transferrin receptors decreases, resulting in a rapid reduction in plasma ferric iron concentrations (to as low as 10−15 M) (16). This is below the threshold level of ferric iron (10−6 M) required to support most microbial growth. In the present study, we sought to assess the ability of the yeast form of B. dermatitidis to proliferate under iron-limiting conditions in vitro.
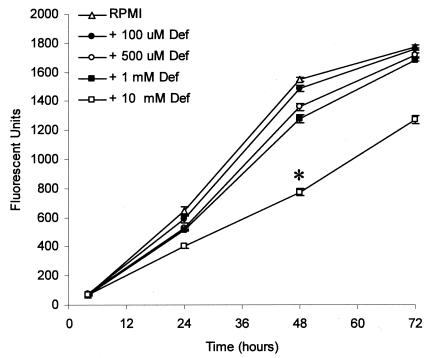
RPMI 1640 medium, which is a chemically defined medium that contains only trace concentrations of ferric and ferrous iron, meets the nutritional requirements for the proliferation of B. dermatitidis yeast in vitro (10, 11) and was used in all of the experiments described below. We have previously demonstrated that apotransferrin, the iron-depleted form of serum transferrin, lacks inhibitory activity against B. dermatitidis yeast. We hypothesized that the absence of transferrin-mediated inhibitory activity might be due to utilization of iron complexed with transferrin or that B. dermatitidis had minimal iron requirements for growth. To further investigate the effect of iron limitation on the growth of B. dermatitidis yeast, we treated B. dermatitidis yeast with deferoxamine, a high-affinity iron-chelating agent. We observed substantial inhibition (P, <0.05 at all time points) of B. dermatitidis yeast growth at the highest deferoxamine concentration tested (10 mM); somewhat delayed yeast growth was observed at deferoxamine concentrations of 0.5 to 1 mM (Fig. 1). A lesser concentration of deferoxamine (0.1 mM) had no effect on yeast growth. The concentrations of deferoxamine required to inhibit the growth of B. dermatitidis were much higher than those required to inhibit the growth of other pathogenic fungi. Newman et al. reported that the 50% effective dose of deferoxamine for the yeast form of H. capsulatum is 1 mM (18), and Clarkson et al. reported that concentrations of deferoxamine as low as 100 to 200 μM inhibit the growth of Pneumocystis carinii by 90% (5). Thus, approximately 10 to 100 times more deferoxamine is required to inhibit the growth of the yeast form of B. dermatitidis than has been previously reported to inhibit other pathogenic fungi.
FIG. 1.
Growth of B. dermatitidis yeast is inhibited by deferoxamine only at concentrations of 0.5 mM or greater. Yeast was inoculated into RPMI 1640 medium containing the indicated concentrations of deferoxamine (Def) and incubated for the indicated times at 37°C. Numbers of viable yeast cells were then determined using alamarBlue. Deferoxamine at 0.5 or 1 mM significantly inhibited yeast growth at 48 h (P < 0.05) but not at the 72-h time point, whereas deferoxamine at 10 mM significantly inhibited the proliferation of B. dermatitidis yeast at all time points (P < 0.05). Yeast incubated in untreated RPMI 1640 medium served as a control. Data presented are the means ± standard errors of the means (SEM) of results from three separate experiments. The asterisk indicates significant inhibition of growth compared to control cultures (P < 0.05).
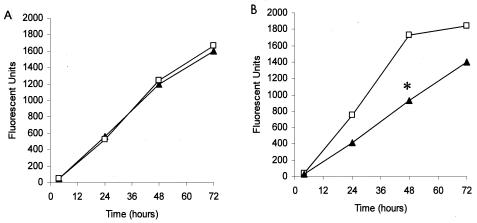
We envisioned several possibilities that would allow the yeast form of B. dermatitidis to overcome the iron-binding activity of deferoxamine. For example, B. dermatitidis might acquire iron that was complexed to deferoxamine, which has been reported for Rhizopus spp. and Aspergillus fumigatus (2), or deferoxamine might stimulate iron uptake by B. dermatitidis, as has been reported for Cryptococcus neoformans (15). To distinguish among these possibilities, we used Chelex-100 to deplete RPMI 1640 medium of iron and other cations. In initial studies, we found that B. dermatitidis yeast could grow equally well in either Chelex-100-treated RPMI 1640 medium or fresh RPMI 1640 medium (Fig. 2A). However, mid-log-phase yeast grown in Chelex-100-treated RPMI 1640 medium for 48 h, washed twice in Chelex-treated RPMI 1640 medium, and then subcultured in Chelex-100-treated RPMI 1640 medium exhibited significantly less growth (P < 0.05) than yeast in untreated RPMI 1640 medium (Fig. 2B). The ability of B. dermatitidis yeast to proliferate when inoculated directly into Chelex-100-treated RPMI 1640 medium but not when subcultured a second time in the same medium suggests that B. dermatitidis yeast may utilize intracellular cations to meet short-term metabolic needs. If true, this would explain how B. dermatitidis resists the iron-binding activity of iron-chelating agents, such as transferrin and deferoxamine.
FIG. 2.
Effects of chelation of RPMI 1640 medium with Chelex-100 on growth of B. dermatitidis yeast. (A) When B. dermatitidis yeast was grown in Chelex-100-treated RPMI 1640 medium ▴ or RPMI 1640 medium □ for up to 72 h, no difference in growth was observed. (B) When B. dermatitidis yeast was first grown in Chelex-100-treated RPMI 1640 medium for 48 h, washed, and then subcultured in fresh Chelex-100-treated RPMI 1640 medium, there was a substantial reduction in yeast growth compared with that of yeast incubated in untreated RPMI 1640 medium as a control (P, <0.05 at 24 to 72 h). Data presented are the means ± SEM of results from two separate experiments, although the error bars are obscured by the size of the symbols. The asterisk indicates significant inhibition of growth compared to control cultures (P < 0.05).
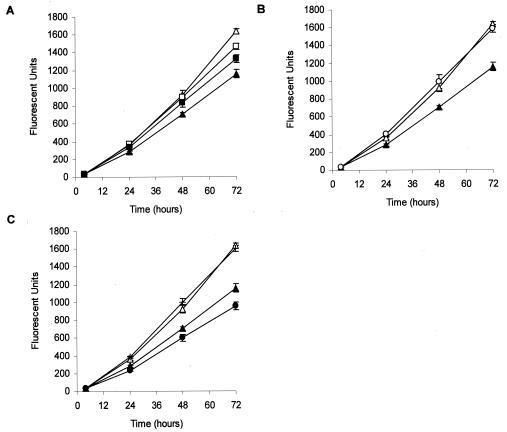
Because Chelex-100 binds cations (e.g., calcium and magnesium) other than ferric iron, we considered the possibility that the growth inhibition observed in the above-described experiments may have been due to limitation of cations other than ferric iron. In preliminary experiments, we assessed whether the addition of calcium, magnesium, ferric iron, zinc, or manganese, alone or in various combinations, could restore the ability of B. dermatitidis to multiply in Chelex-100-treated RPMI 1640 medium. We found that only calcium or magnesium, alone (Fig. 3A) or in combination (Fig. 3B), enhanced the proliferation of B. dermatitidis yeast. The addition of a combination of ferric iron, calcium, and magnesium to Chelex-100-treated RPMI 1640 medium did not further enhance yeast proliferation. In contrast, the addition of ferric iron alone to Chelex-100-treated RPMI 1640 medium resulted in a reduction in yeast proliferation (Fig. 3C). These results suggest that short-term B. dermatitidis yeast proliferation is dependent on the availability of calcium and magnesium, but not ferric iron.
FIG. 3.
Addition of calcium and magnesium, alone or in combination, restores the ability of B. dermatitidis yeast to grow in Chelex-100-treated medium. (A) The addition of calcium [Ca(NO3)2; 0.1 mg/ml (□)] or magnesium (MgSO4; 0.1 mg/ml ▪) to Chelex-100-treated RPMI 1640 medium (▴) enhanced the proliferation of B. dermatitidis yeast. (B) Calcium and magnesium in combination (both at 0.1 mg/ml ○) completely restored the proliferation of B. dermatitidis yeast compared to that of yeast incubated in Chelex-100-treated RPMI 1640 medium (▴). (C) Addition of ferric iron alone (50 mM [•]) neither restored the ability of B. dermatitidis to grow in Chelex-100-treated RPMI 1640 medium nor inhibited the ability of calcium and magnesium to completely restore yeast growth (+). In all three panels, yeast incubated in untreated RPMI 1640 medium (▵) served as a positive control for yeast growth. Data presented are the means ± SEM of results from three separate experiments.
The impaired growth of B. dermatitidis yeast at low calcium levels illustrates the importance of this cation for growth and suggests that B. dermatitidis yeast may possess specialized mechanisms to facilitate the acquisition of exogenous calcium in the host. The closely related fungal pathogen H. capsulatum has been reported to produce a calcium-binding protein (CBP1) that is important for its intracellular parasitism of macrophages (22). Deletion of CBP1 results in impaired intracellular growth of H. capsulatum in macrophages (22). Perhaps B. dermatitidis yeast possesses a similar calcium-binding protein that facilitates its multiplication in mammalian tissues.
In summary, the results of this study demonstrate that B. dermatitidis yeast can proliferate for short durations of time in the absence of extracellular ferric iron if calcium and magnesium are available. Given the results of our present and previous studies, it is unlikely that host defense mechanisms that sequester iron (e.g., transferrin) contribute to innate immunity against B. dermatitidis yeast.
Acknowledgments
This study was supported by the Barbara Rettgen Blastomycosis Fund, University of Wisconsin—Madison School of Veterinary Medicine, and a Robert D. Watkins fellowship from the American Society for Microbiology (S.S.G.).
REFERENCES
- 1.Bloom, J. D., R. E. Hamor, and P. A. Gerding. 1996. Ocular blastomycosis in dogs—73 cases, 108 eyes (1985-1993). J. Am. Vet. Med. Assoc. 209:1271-1274. [PubMed] [Google Scholar]
- 2.Boelaert, J., M. de Locht, J. Van Cutsem, V. Kerrels, B. Cantinieaux, A. Verdonck, H. Van Landuyt, and Y. Schneider. 1993. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J. Clin. Investig. 91:1979-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradsher, R., W. Ulmer, D. Marmer, J. Townsend, and R. Jacobs. 1985. Intracellular growth and phagocytosis of Blastomyces dermatitidis by monocyte-derived macrophages from previously infected and normal subjects. J. Infect. Dis. 151:57-64. [DOI] [PubMed] [Google Scholar]
- 4.Buyukmihci, N. 1982. Ocular lesions of blastomycosis in the dog. J. Am. Vet. Med. Assoc. 180:426-431. [PubMed] [Google Scholar]
- 5.Clarkson, A. J., D. Turkel-Parrella, J. Williams, L. Chen, T. Gordon, and S. Merali. 2001. Action of deferoxamine against Pneumocystis carinii. Antimicrob. Agents Chemother. 45:3560-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cote, E., S. Barr, C. Allen, and E. Eaglefeather. 1997. Blastomycosis in six dogs in New York state. J. Am. Vet. Med. Assoc. 210:502-504. [PubMed] [Google Scholar]
- 7.De Luca, N., and P. Wood. 2000. Iron uptake by fungi: contrasted mechanisms with internal or external reduction. Adv. Microb. Physiol. 43:39-74. [DOI] [PubMed] [Google Scholar]
- 8.DiSalvo, A. F. 1992. The epidemiology of blastomycosis, p. 75-104. In A. Yousef and A. F. DiSalvo (ed.), Blastomycosis. Plenum Publishing Corporation, New York, N.Y.
- 9.Elli, M., R. Zink, A. Rytz, R. Reniero, and L. Morelli. 2000. Iron requirement of Lactobacillus spp. in completely chemically defined growth media. J. Appl. Microbiol. 88:695-703. [DOI] [PubMed] [Google Scholar]
- 10.Giles, S., and C. Czuprynski. 2002. Transferrin independent serum inhibition of Blastomyces dermatitidis. Microb. Pathog. 32:87-97. [DOI] [PubMed] [Google Scholar]
- 11.Giles, S., B. Klein, and C. Czuprynski. 1999. The effect of canine macrophages on the adherence and growth of Blastomyces dermatitidis yeast: evidence of a soluble factor that enhances the growth of B. dermatitidis yeast. Microb. Pathog. 7:395-405. [DOI] [PubMed] [Google Scholar]
- 12.Holzberg, M., and W. Artis. 1983. Hydroxamate siderophore production by opportunistic and systemic fungal pathogens. Infect. Immun. 40:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard, D. 1999. Acquisition, transport, and storage of iron by pathogenic fungi. Clin. Microbiol. Rev. 12:394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imbert, M., and R. Blondeau. 1998. On the iron requirement of lactobacilli grown in chemically defined medium. Curr. Microbiol. 7:64-66. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson, E., A. Goodner, and K. Nyhus. 1998. Ferrous iron uptake in Cryptococcus neoformans. Infect. Immun. 66:4169-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurado, R. 1997. Iron, infections, and anemia of inflammation. Clin. Infect. Dis. 25:888-895. [DOI] [PubMed] [Google Scholar]
- 17.King, R., H. Khan, J. Foye, J. Greenberg, and H. Jones. 1975. Transferrin, iron, and dermatophytes. I. Serum dermatophyte inhibitory component definitively identified as unsaturated transferrin. J. Lab. Clin. Med. 86:204-212. [PubMed] [Google Scholar]
- 18.Newman, S., L. Gootee, V. Stroobant, H. Van der Goot, and J. Boelaert. 1995. Inhibition of growth of Histoplasma capsulatum yeast cells in human macrophages by the iron chelator VUF 8514 and comparison of VUF 8514 with deferoxamine. Antimicrob. Agents Chemother. 39:1824-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posey, J., and F. Gherardini. 2000. Lack of a role for iron in the Lyme disease pathogen. Science 288:1651-1653. [DOI] [PubMed] [Google Scholar]
- 20.Sarosi, G., and S. Davies. 1979. Blastomycosis review. Am. Rev. Respir. Dis. 120:911-938. [DOI] [PubMed] [Google Scholar]
- 21.Sarosi, G., M. Eckman, S. Davies, and W. Laskey. 1979. Canine blastomycosis as a harbinger of human disease. Ann. Intern. Med. 91:733-735. [DOI] [PubMed] [Google Scholar]
- 22.Sebghati, T., J. Engle, and W. Goldman. 2000. Intracellular parasitism by Histoplasma capsulatum: fungal virulence and calcium dependence. Science 290:1368-1372. [DOI] [PubMed] [Google Scholar]
- 23.Shiraishi, A., and T. Arai. 1979. Antifungal activity of transferrin. Sabouraudia 17:79-83. [DOI] [PubMed] [Google Scholar]
- 24.Sutcliffe, M., A. Savage, and R. Alford. 1980. Transferrin-dependent growth inhibition of yeast-phase Histoplasma capsulatum by human serum and lymph. J. Infect. Dis. 142:209-219. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe, T., H. Tanaka, N. Nakao, T. Mikami, M. Suzuki, and T. Matsumoto. 1997. Anti Candida activity of induced transferrin in mice immunized with inactivated Candida albicans. Biol. Pharm. Bull. 20:637-640. [DOI] [PubMed] [Google Scholar]