Abstract
Asian elephant spermatozoa are sensitive to chilling and do not respond well to cryopreservation. The objectives of the present study were to: (1) determine whether cholesterol content can be modified by preincubation of Asian elephant spermatozoa with cholesterol-loaded cyclodextrin (CLC); and (2) assess the effects of CLC concentration(s), temperature at time of glycerol addition (22°C vs 4°C) and dilution medium on post-thaw sperm survival. Spermatozoa incubated with ≥1.5 mg CLC exhibited increased (P < 0.05) cholesterol concentrations. Pretreatment of spermatozoa with 1.5 mg CLC resulted in improvements (P < 0.05) in all post-thaw parameters. Glycerol addition at 4°C also improved all post-thaw parameters compared with 22°C. Dilution of thawed spermatozoa in an egg yolk-based medium improved (P < 0.05) motility compared with Ham's F-10 culture medium. In summary, our findings indicate that modifying cholesterol content within the plasma membrane improves the cryosurvival of Asian elephant spermatozoa. The development of an improved cryopreservation method that includes modification of membrane cholesterol and the addition of glycerol at 4°C, as reported in the present study, is an important step towards utilisation of cryopreserved spermatozoa in captive management of this species.
Keywords: gamete, genome resource bank, storage
Introduction
Elephants are a charismatic megavertebrate with a long history of management in captivity. Although reproduction through natural breeding is desired within the North American elephant population, it is often not possible. Few breeding centres, a limited number of bulls, increased public objection to moving live elephants for breeding, logistics and cost associated with transport, as well as possible social incompatibility of paired individuals, reduce the opportunities for natural breeding in North America (Brown et al. 2004). As a result, artificial insemination (AI) with fresh or chilled semen has been used extensively to supplement natural breeding within the North American captive elephant population. However, fewer than 13% of all attempts to collect semen in Asian elephants result in ejaculates containing >60% motile spermatozoa (Kiso et al. 2011). The unavailability and lack of consistency in obtaining good-quality ejaculates at the time of an AI have become significant impediments towards improving AI efficacy in the Asian elephant. Therefore, the development of a genome resource bank (GRB) for Asian elephants through the establishment of sperm cryopreservation techniques will ensure semen is consistently available for AI and will also provide an ‘insurance policy’ for preserving genetic diversity in this endangered species.
Several studies have attempted to enhance the cryosurvival of Asian elephant spermatozoa by adding various cryoprotectants (i.e. dimethylsulfoxide, glycerol, ethylene glycol and propylene glycol) or sugars (i.e. trehalose) before cooling and freezing (Kiso and Schmitt 2004; Thongtip et al. 2004; Saragusty et al. 2009). However, the use of frozen spermatozoa for AI has not been adopted for practical application owing to inconsistent or suboptimal post-thaw sperm survival. This may be due, in part, to the cryosensitivity of Asian elephant spermatozoa (Saragusty et al. 2005) and high variation in ejaculate quality (Kiso et al. 2011), which render it difficult to develop a cryopreservation protocol.
It has been demonstrated previously that treating spermatozoa with membrane stabilisers before cryopreservation can improve success by enhancing membrane stability, modifying phase transition characteristics and increasing the tolerance of spermatozoa to freeze–thawing (Amann and Pickett 1987; Crockett 1998; Holt 2000; Medeiros et al. 2002; Purdy and Graham 2004b). Membranes with higher cholesterol or greater cholesterol : phospholipid molar ratios are also more tolerant to temperature changes during cryopreservation compared with membranes with lower cholesterol levels (Darin-Bennett and White 1977; Amann and Pickett 1987). For example, human, fowl and rabbit spermatozoa exhibit greater tolerance during cryopreservation compared with boar, ram, bull and stallion spermatozoa as a result of higher membrane cholesterol : phospholipid ratios (Darin-Bennett and White 1977; Watson 1981; White 1993). Therefore, the modification of membrane attributes via changes in the cholesterol : phospholipid ratio before the addition of extender and before cooling may help improve the cryotolerance of Asian elephant spermatozoa.
There are several possible approaches to modifying sperm membranes. Because of their ability to bind and enhance the solubility of hydrophobic compounds in aqueous solutions, cyclodextrins have been used extensively as vehicles for inserting hydrophobic compounds, such as sterols, into biological systems (Saenger 1980; Pitha et al. 1988). Modification of sperm membrane cholesterol levels through the use of cholesterol-loaded cyclodextrins (CLC; Moore et al. 2005) has resulted in enhanced tolerance to cryopreservation and improved cryosurvival in several species, including the ram (Mocé et al. 2010), bull (Purdy and Graham 2004a, 2004b; Mocé and Graham 2006; Amorim et al. 2009; Moraes et al. 2010), buck (Amidi et al. 2010) and stallion (Combes et al. 2000; Moore et al. 2005; Spizziri et al. 2010). However, to the authors’ knowledge, the efficacy of CLC treatment on the cryopreservation of elephant spermatozoa has not been investigated.
During cryopreservation, cryoprotectants are often added to protect the spermatozoa during cooling and freezing (Holt 2000). Since its first use as a cryoprotectant in 1949 (Polge et al. 1949), glycerol is probably the most widely used cryoprotectant to freeze various tissues and cells, including spermatozoa (Woods et al. 2004). Previous studies have demonstrated that low concentrations of glycerol (2.5%–16%) improve post-thaw sperm survival in Asian elephants compared with other cryoprotectants (Thongtip et al. 2004; Saragusty et al. 2009). However, there have been no systematic studies examining the relationship between the temperature at the time of glycerol addition (i.e. 22°C vs 4°C) and post-thaw sperm survival in Asian elephants.
Finally, although cryoprotectants protect spermatozoa during cryopreservation, it is important to return spermatozoa to isotonic conditions after thawing. We recently reported that components of egg yolk or skim milk, such as lipoproteins, exert a beneficial effect on Asian elephant spermatozoa during liquid storage (Kiso et al. 2011). However, components within these complex, undefined reagents have also been reported to interfere with sperm sex-sorting procedures (Johnson and Welch 1999), which is a high priority research need for the North American elephant population. Thus, an alternative post-thaw diluent void of lipoprotein content and compatibility with sperm sex-sorting technology is highly desirable.
The specific objectives of the present study were to: (1) determine whether cholesterol content can be modified by preincubation of Asian elephant spermatozoa with CLC; and (2) assess the effects of CLC concentration(s), temperature at the time of glycerol addition (22°C vs 4°C) and dilution medium on post-thaw sperm survival.
Materials and methods
Chemicals
Unless stated otherwise, all chemicals were purchased through Sigma Chemical (St Louis, MO, USA).
Preparation of CLC
Methyl-β-cyclodextrin was loaded with cholesterol as described by Purdy and Graham (2004b). Briefly, 1 g methyl-β-cyclodextrin was dissolved in 2 mL methanol in a glass tube. In a separate glass tube, 200 mg cholesterol was dissolved in 1 mL chloroform and a 450-μL aliquot of this solution was added to the methyl-β-cyclodextrin solution. The combined cholesterol/cyclodextrin solution (i.e. CLC) was mixed thoroughly until the solution was clear and then poured immediately onto a sterile glass Petri dish. The solution was allowed to evaporate for 24–36 h at ambient temperature and the resulting crystals were harvested into a glass tube and stored in the dark at 22°C until use. All experiments were performed using the same batch of CLC. For addition to spermatozoa, working solutions of fresh CLC were prepared by adding 50 mg CLC crystals to 1 mL modified Ham's F-10 culture medium (Ham's F-10; Irvine Scientific, Santa Ana, CA, USA), mixed vigorously by vortexing and maintained at 37°C until use.
Animals, semen collection and semen evaluation
Ejaculates (n = 104) were collected from captive Asian elephant bulls (n = 12 bulls; age range 7–37 years) managed in captivity in North America. All but two bulls had previously sired one or more calves. From these ejaculates, seven (n = 4 bulls) and nine (n = 4 bulls) ejaculates met the minimum criteria set for the study (exhibiting ≥60% total sperm motility) and were thus included in the sperm cholesterol analysis (Experiment 1) and sperm cryopreservation studies (Experiment 2), respectively. From these individuals, all but one bull had previously sired one or more calves. The bulls were managed under a protected contact training regimen and housed in individual enclosures with visual, olfactory and/or controlled access to females. The bulls had free access to water and regular access to feed. Animal research protocols were approved by the Smithsonian Conservation Biology Institute's Institutional Animal Care and Use Committee.
Semen was collected using the rectal massage technique, as described previously (Schmitt and Hildebrandt 1998; Kiso et al. 2011). Immediately after collection, ejaculates were evaluated for volume, sperm motility (total and forward progressive motility) and sperm concentration (× 106; Kiso et al. 2011). For acrosomal integrity and morphological examinations, an aliquot (25–40 μL) was fixed in 500 μL of 4% paraformaldehyde and stored at 4°C until further processing. Ejaculates exhibiting signs of urine contamination (i.e. colour, odour) were excluded from the study.
Spermatozoa fixed previously in 4% paraformaldehyde for evaluation of sperm morphology and acrosomal integrity were stained using a modified Coomassie blue technique, as described by Larson and Miller (1999). Briefly, spermatozoa were centrifuged (2000g) and washed twice with 0.5 mL of 0.1 M ammonium acetate (pH 9.0). The final sperm pellet was resuspended in approximately 50 μL of 0.1 M ammonium acetate, and 20 μL of this cell suspension was placed onto a glass slide, smeared and air-dried. Sperm smears were immersed in Coomassie stain (0.22% (w/v) Coomassie blue G-250, 50% (v/v) methanol, 10% (v/v) acetic acid, 40% (v/v) water) for 2 min at room temperature. Stained slides were rinsed thoroughly with water to remove excess stain, air dried and coverslips were permanently sealed with mounting medium (Harleco Krystalon Mounting Medium; EMD Chemicals, Gibbstown, NJ, USA). Stained spermatozoa were evaluated for morphology (200 spermatozoa per sample) and acrosomal integrity (200 spermatozoa per sample) using brightfield microscopy under oil immersion at ×1000 magnification. Spermatozoa exhibiting normal morphology were categorised as ‘normal’, whereas spermatozoa exhibiting morphological abnormalities in the head (i.e. microcephalic, macrocephalic, bicephalic, misshaped, detached), mid-piece (i.e. bent necks, abnormal, bent, absent, proximal or distal cytoplasmic droplet) or flagellum (i.e. double, coiled, bent) were categorised as ‘abnormal’. For evaluation of acrosomal integrity, spermatozoa with uniform staining over the acrosomal region were categorised as ‘intact acrosomes’, whereas those that exhibited non-uniform staining, vesiculation or lack of staining altogether in the region of the acrosomes were categorised as ‘non-intact acrosomes’. The number of spermatozoa with intact membranes was converted to a percentage (% INT).
Addition of CLC to spermatozoa
Ejaculates exhibiting ≥60% total sperm motility were treated with various concentrations of CLC before cryopreservation. Briefly, raw ejaculates were diluted to 120 × 106 cells mL–1 with Ham's F-10 immediately after collection and divided into seven aliquots. Each aliquot (1 mL) was treated with a different concentration of CLC (0, 0.5, 1.5, 3.0, 4.5, 6.0 or 7.5 mg CLC per 120 × 106 cells mL–1), mixed well and incubated for 15 min at 37°C in the dark.
Experiment 1: cholesterol content of CLC-treated Asian elephant spermatozoa
Seven samples from four bulls were included in this experiment. Ejaculates were treated with various concentrations of CLC, as described above. After CLC addition and incubation, samples were washed twice by centrifugation at 500g for 5 min at ambient room temperature and resuspension with Ham's F-10. The resulting sperm pellet was snap-frozen in liquid nitrogen and stored until analysis.
For preparation of samples for cholesterol analysis, sperm pellets were resuspended in 4.5–5.0 mL Milli-Q water, homogenised and lysed by sonication. Sonication was performed using a Branson Sonifier 450 (Branson Ultrasonics, Danbury, CT, USA) by exposing samples to three sequential sets of sonication at output 1, 2 and 3 on duty cycle 20 for 10 replications for 10 s. Care was taken to avoid frothing and samples were kept on ice to avoid heating.
Lipid extraction was performed using the method of Bligh and Dyer (1959) with slight modifications. Briefly, chloroform : methanol (1 : 2) was added to the homogenised suspension and placed on an orbital shaker for 15 min at 22°C. After agitation, 1 volume each of chloroform and water was added in turn. The sample was mixed after every addition for 1 min. Phase separations were accomplished by centrifuging samples at 500g for 2 min at 22°C. The lower phase was carefully aspirated and placed into another glass tube using a glass pipette. The contents of this tube were evaporated under nitrogen gas and cholesterol concentrations quantified using the Amplex Red Cholesterol Assay Kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer's instructions.
Experiment 2: influence of CLC concentration, temperature of glycerol addition and post-thaw diluent on post-thaw sperm survival and longevity
Nine ejaculates from four bulls were included in this experiment. Ejaculates were treated with various concentrations of CLC, as described above. After CLC addition and incubation, samples were centrifuged at 500g for 5 min. The supernatant was carefully removed and the sperm pellet was resuspended to half the original volume with Biladyl extender (BIL; Minitube of America, Verona, WI, USA) containing 20% (v/v) egg yolk.
Glycerol addition at 22°C or 4°C
Each aliquot of CLC-treated spermatozoa was further divided into two aliquots (500 μL each) and subjected to glycerol addition at either 22°C or 4°C. For glycerol addition at 22°C, aliquots were cooled to 22°C (~20 min) and subsequently diluted 1 : 1 with BIL extender containing 8% (v/v) glycerol in three increments (adding 1/4 volume, waiting for 15 min, adding 1/4 volume, waiting 15 min and then adding the remaining 1/2 volume). The samples then were loaded into 0.5-mL cryostraws (two straws per treatment; AgTech, Manhattan, KS, USA), heat sealed and placed in an insulated water bath (350 mL at 22°C). Straws were allowed to cool to 4°C for 2.5–3 h and cryopreserved over liquid nitrogen vapour. Briefly, straws were placed approximately 2.54 cm above liquid nitrogen for 10 min and then plunged directly into the liquid nitrogen.
For glycerol addition at 4°C, sperm aliquots (500 μL; in Eppendorf centrifuge tubes) were placed in an insulated water bath (350 mL at 22°C) and cooled to 4°C for 2.5–3 h. After reaching this temperature, an equal volume of precooled BIL containing 8% (v/v) glycerol was added in three increments (as described above) and samples were loaded into 0.5-mL straws (two straws per treatment) and cryopreserved (as described above). All samples were cryopreserved with a final sperm concentration of 120 × 106 cells mL–1 and a glycerol concentration of 4% (v/v).
Post-thaw evaluation
Post-thaw evaluations were performed after ≥2 weeks storage in liquid nitrogen. Individual straws were thawed for 40 s in a 37°C water bath and the contents emptied into a sterile 1.5-mL Eppendorf tube. An 8-μL aliquot of each sample was placed on a prewarmed slide and evaluated for percentage sperm total motility (% tMOT) and progressive sperm motility (% pMOT). A 25-μL aliquot was processed to assess acrosomal integrity (as described above). Post-thaw evaluations of % tMOT, % pMOT and acrosome intact status (% INT) were performed immediately after thawing before dilution (i.e. post-thaw) and at hourly intervals for 4 h after dilution.
Comparison of two diluents on post-thaw sperm longevity
After initial post-thaw evaluations, samples (n = 9 ejaculates from four bulls) were further divided into two aliquots and diluted in either BIL (without glycerol) or Ham's F-10 supplemented with 22 mM HEPES, 2 mM l-glutamine, 4 mM pyruvate, 2% (w/v) bovine serum albumin, 100 IU mL–1 penicillin and 100 μg mL–1 streptomycin (Ham's complete; HMC). After dilution, treated samples were kept at 22°C in a covered, insulated box for the remainder of the study. The efficacy of each diluent in preserving post-thaw sperm longevity was evaluated by measuring % tMOT, % pMOT and % INT from the time of dilution (i.e. Dilution 0 h) and at hourly intervals for 4 h (i.e. Dilution 1 h, Dilution 2 h, Dilution 3 h and Dilution 4 h).
Statistical analysis
Membrane cholesterol differences (Experiment 1) between CLC treatments and untreated sample (i.e. control; 0 mg CLC) were analysed by analysis of variance (ANOVA) followed by a post hoc Student–Newman–Keuls’ (SNK) multiple range test to separate differences among treatment means (P < 0.05). The main effects of CLC concentration, glycerol addition and post-thaw diluent, in addition to their interactions at each time point, were analysed by ANOVA (Experiment 2) followed by the SNK multiple range test. With the exception of a significant interaction between CLC concentration and glycerol treatment for % pMOT, there were no other significant interactions observed among the main treatments (i.e. CLC concentration, glycerol treatment and post-thaw diluent) for the variables % tMOT, % pMOT and % INT at all time points. As a result, the data for % pMOT for CLC concentration and glycerol treatment were compared using individual treatment means. The remaining data (i.e. CLC concentration: % tMOT and % INT; glycerol treatment: % tMOT and % INT; and post-thaw diluent: % tMOT, % pMOT and % INT) were pooled to increase the power of comparison among treatments. All statistical analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC, USA). Treatment differences were considered significant at P < 0.05. Unless indicated otherwise, results are presented as the mean s.e.m.
Results
Semen traits
Semen was collected from 12 Asian elephant bulls (7–37 years old) from seven institutions throughout the US. The semen characteristics of the ejaculates that met the minimum criterion for the present study (≥60% tMOT; n = 9 ejaculates; n = four bulls) are summarised in Table 1.
Table 1.
Semen characteristics of Asian elephant ejaculates included in the present study
| Semen characteristic | |
|---|---|
| Total volume (mL) | 42 ± 35 |
| Total sperm motility (%) | 72 ± 9 |
| Progressive forward motility (%) | 65 ± 15 |
| % Morphologically normal | 72 ± 13 |
| % Normal acrosome | 66 ± 12 |
| Sperm concentration (× 106 cells mL–1) | 682 ± 820 |
| Total no. spermatozoa (× 109 cells) | 22.2 ± 32.7 |
Data are the mean ± s.d. (n = 4 bulls; n = 9 ejaculates)
Experiment 1: cholesterol content of CLC-treated Asian elephant spermatozoa
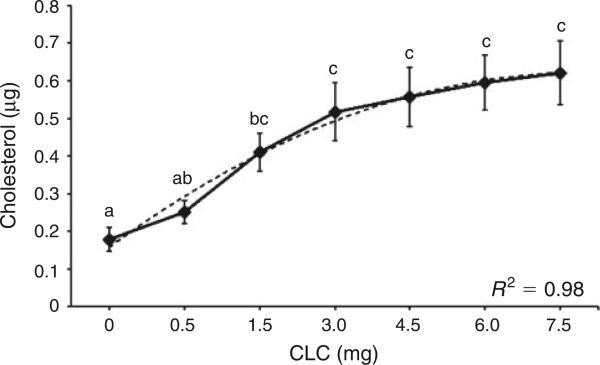
Exposing fresh Asian elephant spermatozoa to increasing concentrations of CLC (mg per 120 × 106 spermatozoa mL–1) resulted in a polynomial increase in total cholesterol (Fig. 1; μg cholesterol = –0.0116 (mg CLC)2 + 0.1698 (mg CLC) – 0.0008; R2 = 0.9843). The total amount of cholesterol in the spermatozoa after the addition of 0.0, 0.5, 1.5, 3.0, 4.5, 6.0 or 7.5 mg CLC was 0.18, 0.25, 0.41, 0.52, 0.56, 0.60 and 0.62 μg, respectively. Spermatozoa treated with ≥1.5 mg CLC contained significantly higher (P < 0.05) cholesterol compared with control spermatozoa without CLC treatment (Fig. 1). The amount of cholesterol that was incorporated into the spermatozoa exhibited an increase between 0.0 and 1.5 mg CLC, but reached a plateau at CLC concentrations ≥1.5 mg (Fig. 1; P > 0.05).
Fig. 1.
Mean (± s.e.m.) concentrations of cholesterol (μg per 106 spermatozoa) in Asian elephant spermatozoa when fresh spermatozoa (n = 7 ejaculates) were exposed to increasing amounts of cholesterol-loaded cyclodextrin (CLC; in mg per 120 × 106 spermatozoa mL–1). The dotted line depicts the line of best fit. CLC treatments with different letters differ significantly (P < 0.05).
Experiment 2: influence of CLC concentration, glycerol addition and post-thaw diluent on post-thaw sperm survival and longevity
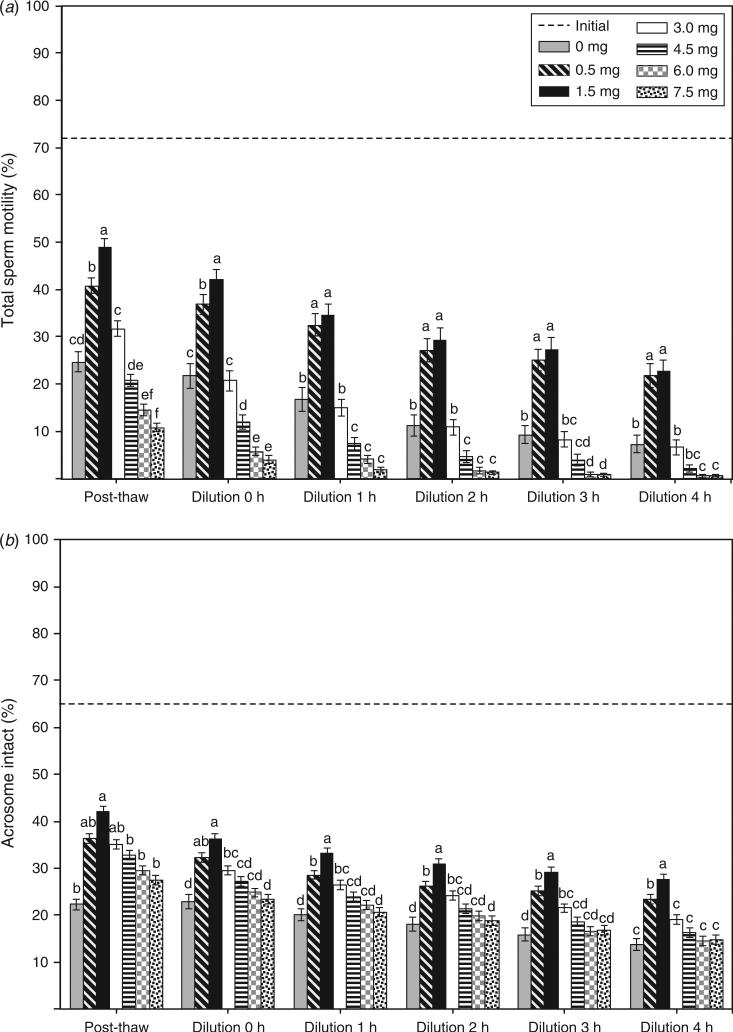
Significant differences (P < 0.05) were observed in % tMOT (Fig. 2a), % INT (Fig. 2b) and % pMOT (Table 2) among various CLC concentrations. Preincubation with 0.5 or 1.5 mg CLC consistently resulted in higher (P < 0.05) post-thaw % tMOT immediately after thawing, as well as in vitro incubation for up to 4 h, compared with aliquots cryopreserved in the absence (i.e. 0.0 mg) or presence of higher concentrations of CLC (3.0, 4.5, 6.0, or 7.5 mg; Fig. 2a). Immediately after thawing as well as dilution, aliquots cryopreserved in the presence of 1.5 mg CLC exhibited higher % tMOT (post-thaw: 48.9 ± 2.0%; Dilution 0 h: 42.1 ± 2.1%; P < 0.05) compared with aliquots cryopreserved in 0.5 mg CLC (post-thaw: 40.8 ± 1.7%; Dilution 0 h: 36.8 ± 2.1%). In contrast, sperm % tMOT declined consistently at all other concentrations of CLC tested. Furthermore, cryopreservation in the presence of 6.0 or 7.5 mg CLC had a detrimental effect on % tMOT, with a sharp decline over time (6.0 mg CLC range: 14.5% to 0.6%; 7.5 mg CLC range: 10.8% to 0.6%). Regardless of glycerol addition at either 22°C or 4°C (Table 2), 1.5 mg CLC exhibited superior % pMOT (P < 0.05) compared with the addition of 0.0, 3.0 (except at post-thaw at 22°C), 4.5, 6.0 or 7.5 mg CLC. The addition of CLC to spermatozoa prior to cryopreservation also improved post-thaw acrosomal integrity (Fig. 2b; P < 0.05). Spermatozoa cryopreserved in 1.5 mg CLC retained a higher proportion of intact acrosomal membranes compared with all other CLC concentrations after 1 h dilution. Aliquots cryopreserved in 3.0 mg CLC also consistently exhibited higher (P < 0.05) % INT compared with control (0.0 mg CLC) at 0–3 h after dilution (Fig. 2b). There was no significant difference (P > 0.05) in % INT among the control (0.0 mg), 4.5, 6.0 and 7.5 mg CLC groups after dilution.
Fig. 2.
Post-thaw (a) total sperm motility (% tMOT) and (b) acrosome intact status (% INT) of Asian elephant spermatozoa treated with different concentrations of cholesterol-loaded cyclodextrin (CLC; 0, 0.5, 1.5, 3.0, 4.5, 6.0 and 7.5 mg CLC per 120 × 106 spermatozoa mL–1) before cryopreservation. Values are the mean±s.e.m. The dotted line represents the mean motility of the raw ejaculate before CLC exposure and cryopreservation. Within each time point, CLC treatments with different letters differ significantly (P < 0.05).
Table 2.
Comparison of post-thaw progressive sperm motility of spermatozoa treated with different concentrations of cholesterol-loaded cyclodextrin and exposed to glycerol addition at either 22°C or 4°C before cryopreservation
| Glycerol addition | Time | CLC (mg per 120 × 106 spermatozoa mL–1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.5 | 3.0 | 4.5 | 6.0 | 7.5 | ||
| 22°C | Post-thaw | 8.3 ± 4.6b | 20.0 ± 7.5a2 | 21.1 ± 8.8a2 | 8.9 ± 5.0a2 | 6.1 ± 3.9b | 3.3 ± 1.7b | 1.7 ± 1.2b |
| Dilution 0 h | 1.9 ± 0.8c | 12.1 ± 3.5b2 | 20.6 ± 4.0a2 | 3.9 ± 1.4c2 | 1.7 ± 1.1c | 0.6 ± 0.4c | 0.6 ± 0.6c | |
| Dilution 1 h | 1.1 ± 0.6b2 | 10.0 ± 2.9a2 | 15.0 ± 3.3a2 | 2.2 ± 1.2b2 | 0.8 ± 0.6b | 0.3 ± 0.2b | 0.3 ± 0.3b | |
| Dilution 2 h | 0 ± 0b2 | 8.1 ± 2.4a2 | 9.4 ± 3.2a2 | 1.4 ± 1.1b2 | 0.7 ± 0.6b | 0.2 ± 0.1b | 0.3 ± 0.3b | |
| Dilution 3 h | 0 ± 0b | 6.4 ± 2.5a2 | 7.2 ± 2.6a2 | 0.8 ± 0.8b2 | 0.3 ± 0.3b | 0.1 ± 0.0b | 0.0 ± 0.0b | |
| Dilution 4 h | 0 ± 0b | 5.3 ± 2.3a2 | 6.9 ± 2.5a2 | 0.8 ± 0.8b | 0.3 ± 0.3b | 0.0 ± 0.0b | 0.0 ± 0.0b | |
| 4°C | Post-thaw | 16.1 ± 6.6c | 35.0 ± 5.5ab1 | 45.0 ± 6.0a1 | 28.9 ± 4.1bc1 | 15.6 ± 3.7c | 10.6 ± 2.4c | 9.2 ± 1.4c |
| Dilution 0 h | 12.4 ± 4.7cd | 29.4 ± 3.9b1 | 38.1 ± 3.5a1 | 14.7 ± 2.1c1 | 5.8 ± 2.1de | 3.8 ± 0.9e | 1.3 ± 0.6e | |
| Dilution 1 h | 9.3 ± 3.7b1 | 22.2 ± 4.4a1 | 28.6 ± 3.7a1 | 9.0 ± 2.1b1 | 3.3 ± 1.3bc | 1.3 ± 0.6c | 0.6 ± 0.4c | |
| Dilution 2 h | 6.9 ± 2.9c1 | 17.5 ± 3.7b1 | 23.1 ± 3.4a1 | 7.2 ± 1.6c1 | 1.8 ± 1.0cd | 0.4 ± 0.3d | 0.3 ± 0.3d | |
| Dilution 3 h | 4.7 ± 2.0cd | 15.6 ± 3.5b1 | 20.8 ± 3.3a1 | 6.3 ± 1.5c1 | 1.1 ± 0.8de | 0.0 ± 0.0e | 0.0 ± 0.0e | |
| Dilution 4 h | 3.2 ± 1.8b | 10.6 ± 2.7a1 | 14.4 ± 3.1a1 | 4.0 ± 1.4b | 0.6 ± 0.6b | 0.0 ± 0.0b | 0.0 ± 0.0b | |
Values are the mean ± s.e.m. Values with different superscript letters among cholesterol-loaded cyclodextrin (CLC) treatments within the same time point and glycerol treatment differ significantly (P < 0.05). Values with different superscript numbers between glycerol addition temperatures, within the same time point and CLC level, differ significantly (P < 0.05). % pMOT, progressive sperm motility
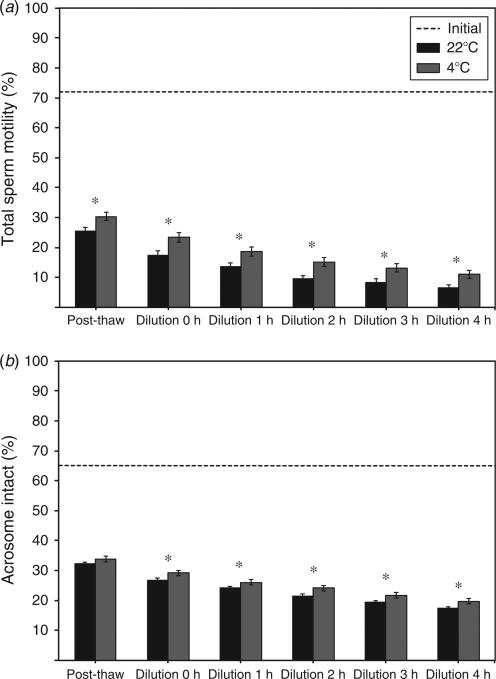
The temperature at which glycerol addition occurred had a strong influence on % tMOT (Fig. 3a), % pMOT (Table 2) and % INT (Fig. 3b). Significantly higher (P < 0.05) % tMOT (Fig. 3a) and % INT (except at post-thaw; Fig. 3b) were observed when glycerol addition occurred at 4°C. Glycerol addition at 4°C resulted in up to threefold greater % pMOT values compared with glycerol addition at 22°C (Table 2). Although % pMOT exhibited generally higher values for all CLC concentrations when glycerol addition occurred at 4°C, values were significantly higher (P < 0.05) compared with 22°C when spermatozoa were treated with 0.5, 1.5 or 3.0 mg CLC. Furthermore, untreated cells (i.e. 0 mg CLC) also exhibited higher % pMOT values (P < 0.05) at 1 and 2 h after post-thaw dilution when glycerol addition occurred at 4°C.
Fig. 3.
Comparison of post-thaw (a) total sperm motility (% tMOT) and (b) acrosome intact status (% INT) of Asian elephant spermatozoa exposed to glycerol at either 22°C or 4°C before cryopreservation. Values are the mean±s.e.m. The dotted line represents the mean motility of the raw ejaculate before CLC exposure and cryopreservation. Within each time point, bars with asterisks differ significantly (P < 0.05).
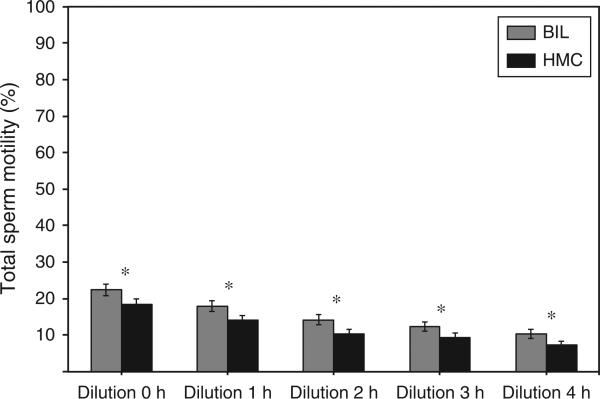
The % tMOT (Fig. 4) was consistently higher (P < 0.05) when spermatozoa were diluted and maintained in BIL compared with HMC for all post-thaw time points. However, there were no significant differences (P > 0.05) between BIL and HMC in % pMOT or % INT (data not shown).
Fig. 4.
Post-thaw total sperm motility (% tMOT) of Asian elephant spermatozoa diluted with two post-thaw diluents. BIL, Biladyl +20% egg-yolk; HMC, Ham's complete. Values are the mean±s.e.m. Within each time point, bars with asterisks differ significantly (P < 0.05).
Discussion
The present study represents the first systematic effort to modify elephant sperm membrane properties to improve cryosurvival. In the present study, we found that: (1) spermatozoa treated with CLC concentrations ≥1.5 mg exhibited increased membrane cholesterol concentrations; (2) preincubation of Asian elephant spermatozoa with 1.5 mg CLC resulted in a significant improvement in all post-thaw sperm parameters; (3) post-thaw sperm motility (% tMOT and % pMOT) was further enhanced when spermatozoa were exposed to glycerol at 4°C; and (4) post-thaw % tMOT was improved when thawed aliquots were diluted in an egg yolk-based medium (BIL) compared with standard culture medium (HMC).
The concentration of CLC that provided the best protection was similar to that reported for stallion (1.5 mg CLC; Moore et al. 2005) and ram (2.0 mg CLC; Mocé et al. 2010) spermatozoa. The amount of cholesterol that was incorporated into elephant spermatozoa reached saturation at CLC concentrations ≥3.0 mg, with higher concentrations failing to increase membrane cholesterol content. A similar phenomenon has been reported previously in stallion (Moore et al. 2005) and ram (Mocé et al. 2010) spermatozoa. These results suggest that the amount of cholesterol that can be loaded into elephant spermatozoa is limited and may be species specific.
Although the exact mechanism by which cholesterol provides protection is not fully understood, the protective nature of cholesterol is probably due to its ability to stabilise membranes (Crockett 1998; Mocé and Graham 2006). Cholesterol has a role in the organisation and packing order of other membrane components, and thus the amount of cholesterol present in the membrane has an influence on the permeability, thickness and rigidity of the membrane (Darin-Bennett and White 1977; Amann and Pickett 1987; Crockett 1998). During cooling, membrane damage typically occurs when the membrane transitions from a liquid to a more gel-crystalline phase state (Parks and Graham 1992), and results in a highly rigid and excessively permeable membrane (Amann and Pickett 1987; Crockett 1998; Holt 2000). Increased cholesterol has been suggested to stabilise membranes during these membrane phase transitions and counteracts the detrimental effect of cooling on membranes (Crockett 1998; Holt 2000). Thus, because Asian elephant spermatozoa are particularly sensitive to membrane phase transitions that occur during cooling (Saragusty et al. 2005), enhancing membrane cholesterol levels in Asian elephant spermatozoa may have reduced membrane damage arising from these membrane phase transitions during cryopreservation. Furthermore, previous studies have demonstrated that loading additional cholesterol into membranes (using CLCs) can enhance osmotic tolerance of spermatozoa (Aksoy et al. 2010; Moraes et al. 2010). Thus, preloading Asian elephant spermatozoa with additional cholesterol may have mitigated the membrane or osmotic damage that would otherwise occur during cooling and thawing.
During cryopreservation, spermatozoa can lose up to 50% of cholesterol in their membranes (Cerolini et al. 2001), which often results in premature capacitation and reduced viability and/or fertilisation competence. Pretreating spermatozoa with CLCs may load additional cholesterol into membranes and prevent excessive loss of cholesterol during cryopreservation, thus preserving sperm viability and fertilising capacity. However, modifying membrane cholesterol levels to enhance sperm ‘durability’ during cryopreservation can also result in excessive loading of cholesterol and alter the adequate amount of cholesterol efflux that is required for spermatozoa to undergo normal capacitation and acrosomal exocytosis in a timely fashion (Cross 1996; Aksoy et al. 2010). Although recent studies have demonstrated that CLC treatment does not compromise fertility (Spizziri et al. 2010) or reduce the ability of the spermatozoa to undergo capacitation or acrosomal exocytosis (Purdy and Graham 2004a), additional studies are warranted to examine the impacts of CLCs on elephant sperm function and fertilising capacity.
In the present study, glycerol addition at 4°C enhanced the cryosurvival of Asian elephant spermatozoa compared with glycerol addition at 22°C. Differences in membrane structure, duration of glycerol exposure and reduced sperm metabolism could account, in part, for the observed differences in cryosurvival between the two temperatures. When cooled, spermatozoa undergo membrane transitions from a liquid to a more crystallinegel phase (Amann and Pickett 1987; Parks and Graham 1992), resulting in changes in membrane structure and permeability. Thus, the effectiveness and protective (or toxic) quality of glycerol may depend on the temperature and/or sperm membrane properties of the target species. Furthermore, the addition of glycerol at 4°C resulted in shorter duration of sperm exposure to glycerol and when sperm metabolism was reduced (Blackshaw et al. 1957; Bartlett and Van Demark 1962), thus possibly minimising glycerol toxicity. A significant interaction between CLC concentration and glycerol exposure on post-thaw progressive sperm motility also was observed. Perhaps the effectiveness of CLC treatment on sperm forward motility is only optimised if the membrane is allowed to stabilise from cholesterol addition and cooled slowly before spermatozoa are exposed to glycerol. Nonetheless, the ability to process and treat spermatozoa with cryoprotectant at 22°C as opposed to 4°C has practical consequences, because it allows elephant semen to be collected and preserved under field conditions, where electricity, refrigeration or liquid nitrogen may not be immediately available for cooling or freezing. Thus, the treatment of spermatozoa with lower concentrations of glycerol or alternative types of cryoprotectants (or combinations) that can be used at 22°C and under field conditions warrants further investigation.
Identifying an effective post-thaw diluent is also critical to mitigate the deleterious effects of cryoprotectants and to prolong sperm longevity and survival after cryopreservation. This is necessary to ensure that a large percentage of spermatozoa remain viable until the time of insemination and/or to help facilitate additional manipulations, such as sperm sex sorting. Both BIL and HMC were just as effective in maintaining post-thaw progressive sperm motility and acrosomal integrity, but dilution in BIL consistently resulted in higher total sperm motility for the duration of the study (4 h). The enhanced effectiveness of BIL to maintain sperm motility may be attributed to the presence of egg yolk (lipoprotein) in the medium. It has been demonstrated that lipoprotein is necessary to enhance sperm survival in Asian elephants, particularly during cooled storage (Saragusty et al. 2005; Kiso et al. 2011). However, a major research objective for the North American elephant population is to incorporate sperm sex-sorting technology to increase the number of females via AI to stabilise the captive elephant population. Unfortunately, egg yolk is known to interfere with sperm sex sorting (Johnson and Welch 1999). Thus, identifying a post-thaw diluent that will enhance sperm longevity and be simultaneously compatible with sperm sexing technology is highly desirable. However, it is promising that HMC, which has no lipoprotein, was comparable to BIL in maintaining progressive sperm motility and acrosomal integrity. Further investigations are necessary to determine whether a lower egg yolk concentration or modification of HMC media composition could enhance sperm longevity and permit sperm sex sorting.
To the authors’ knowledge, the present study is the first to test whether exposing Asian elephant spermatozoa to CLC before freezing enhanced post-thaw sperm survival. Loading cholesterol into Asian elephant sperm membranes using CLCs before cryopreservation effectively improved post-thaw sperm survival. Glycerol addition at 4°C exhibited higher and superior post-thaw results compared with addition at 22°C, and enhanced the efficacy of CLC on increasing post-thaw sperm survival. These advances should help make possible the establishment of a sperm GRB (Wildt et al. 1997) for Asian elephants and facilitate better genetic management of animals maintained in captivity.
Acknowledgements
The authors express their great thanks for all the support and enthusiasm received from the veterinary and elephant management staff from the following institutions that participated in this study: African Lion Safari (Ontario, Canada), Albuquerque BioPark Zoo (Albuquerque, NM, USA), Columbus Zoo and Aquarium (Columbus, OH, USA), Fort Worth Zoo (Fort Worth, TX, USA), Riddle's Elephant Sanctuary (Greenbrier, AR, USA), Ringling Brothers Center for Elephant Conservation (Polk City, FL, USA), Rosamond Gifford Zoo (Syracuse, NY, USA), and Tulsa Zoo and Living Museum (Tulsa, OK, USA). The authors also thank Dr Amanda Moore for sharing technical information on the preparation of CLC. Funding for this study was generously provided by Feld Entertainment, Inc. and the International Elephant Foundation. WK was supported by a grant from Feld Entertainment, Inc.
References
- Aksoy M, Akman O, Lehimcioglu NC, Erdem H. Cholesterol-loaded cyclodextrin enhances osmotic tolerance and inhibits the acrosome reaction in rabbit spermatozoa. Anim. Reprod. Sci. 2010;120:166–172. doi: 10.1016/j.anireprosci.2010.02.014. doi:10.1016/J.ANIREPROSCI.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Amann RP, Pickett BW. Principles of cryopreservation and a review of cryopreservation of stallion spermatozoa. J. Equine Vet. Sci. 1987;7:145–173. doi:10.1016/S0737-0806(87)80025-4. [Google Scholar]
- Amidi F, Farshad A, Khor AK. Effects of cholesterol-loaded cyclodextrin during freezing step of cryopreservation with TCGY extender containing bovine serum albumin on quality of goat spermatozoa. Cryobiology. 2010;61:94–99. doi: 10.1016/j.cryobiol.2010.05.006. doi:10.1016/J.CRYOBIOL.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Amorim EAM, Graham JK, Spizziri B, Meyers M, Torres CAA. Effect of cholesterol or cholesteryl conjugates on the cryosurvival of bull sperm. Cryobiology. 2009;58:210–214. doi: 10.1016/j.cryobiol.2008.12.007. doi:10.1016/J.CRYOBIOL.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Bartlett FD, Van Demark NL. Effect of temperature on the survival of bovine spermatozoa stored in carbonated diluents. J. Dairy Sci. 1962;45:368–374. doi:10.3168/JDS.S0022-0302(62)89400-4. [Google Scholar]
- Blackshaw AW, Salisbury GW, Van Demark NL. Factors influencing metabolic activity of bull spermatozoa. I. 37, 21, and 5°C. J. Dairy Sci. 1957;40:1093–1098. doi:10.3168/JDS.S0022-0302(57)94600-3. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Physiol. Pharmacol. 1959;37:911–917. doi: 10.1139/o59-099. doi:10.1139/Y59-099. [DOI] [PubMed] [Google Scholar]
- Brown JL, Goritz F, Pratt-Hawkes N, Hermes R, Galloway M, Graham LH, Gray C, Walker SL, Gomez A, Moreland R, Murray S, Schmitt DL, Howard JG, Lehnhardt J, Beck B, Bellem A, Montali R, Hildebrandt TB. Successful artificial insemination of an Asian elephant at the National Zoological Park. Zoo Biol. 2004;23:45–63. doi:10.1002/ZOO.10116. [Google Scholar]
- Cerolini S, Maldjian A, Pizzi F, Gliozzi T. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Reproduction. 2001;121:395–401. doi:10.1530/REP.0.1210395. [PubMed] [Google Scholar]
- Combes GB, Varner DD, Schroeder F, Burghardt RC, Blanchard TL. Effect of cholesterol on the motility and plasma membrane integrity of frozen equine spermatozoa after thawing. J. Reprod. Fertil. Suppl. 2000;56:127–132. [PubMed] [Google Scholar]
- Crockett EL. Cholesterol function in plasma membranes from ectotherms: membrane-specific roles in adaptation to temperature. Am. Zool. 1998;38:291–304. [Google Scholar]
- Cross NL. Effect of cholesterol and other sterols on human sperm acrosomal responsiveness. Mol. Reprod. Dev. 1996;45:212–217. doi: 10.1002/(SICI)1098-2795(199610)45:2<212::AID-MRD14>3.0.CO;2-2. doi:10.1002/(SICI)1098-2795(199610)45:2<212::AID-MRD14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Darin-Bennett A, White IG. Influence of cholesterol content of mammalian spermatozoa on susceptibility to cold-shock. Cryobiology. 1977;14:466–470. doi: 10.1016/0011-2240(77)90008-6. doi:10.1016/0011-2240(77)90008-6. [DOI] [PubMed] [Google Scholar]
- Holt WV. Basic aspects of frozen storage of semen. Anim. Reprod. Sci. 2000;62:3–22. doi: 10.1016/s0378-4320(00)00152-4. doi:10.1016/S0378-4320(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Welch GR. Sex preselection: high-speed flow cytometric sorting of X and Y sperm for maximum efficiency. Theriogenology. 1999;52:1323–1341. doi: 10.1016/s0093-691x(99)00220-4. doi:10.1016/S0093-691X(99)00220-4. [DOI] [PubMed] [Google Scholar]
- Kiso WK, Schmitt DL. Masters Thesis. Missouri State University; USA: 2004. Cryopreservation of Asian elephant (Elephas maximus) semen in conjunction with the use of Spermac® stain to evaluate acrosome status in both Asian and African (Loxodonta africana) elephants. [Google Scholar]
- Kiso WK, Brown JL, Siewerdt F, Schmitt DL, Olson D, Crichton EG, Pukazhenthi BS. Liquid semen storage in elephants (Elephas maximus and Loxodonta africana): species differences and storage optimization. J. Androl. 2011;32:420–431. doi: 10.2164/jandrol.110.011460. doi:10.2164/JANDROL.110.011460. [DOI] [PubMed] [Google Scholar]
- Larson JL, Miller DJ. Simple histochemical stain for acrosome on sperm from several species. Mol. Reprod. Dev. 1999;52:445–449. doi: 10.1002/(SICI)1098-2795(199904)52:4<445::AID-MRD14>3.0.CO;2-6. doi:10.1002/(SICI)1098-2795(199904)52:4<445::AID-MRD14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Medeiros CMO, Forell F, Oliveira ATD, Rodrigues JL. Current status of sperm cryopreservation: why isn't it better? Theriogenology. 2002;57:327–344. doi: 10.1016/s0093-691x(01)00674-4. doi:10.1016/S0093-691X(01)00674-4. [DOI] [PubMed] [Google Scholar]
- Mocé E, Graham JK. Cholesterol-loaded cyclodextrins added to fresh bull ejaculates improve sperm cryosurvival. J. Anim. Sci. 2006;84:826–833. doi: 10.2527/2006.844826x. [DOI] [PubMed] [Google Scholar]
- Mocé E, Purdy PH, Graham JK. Treating ram sperm with cholesterol-loaded cyclodextrins improves cryosurvival. Anim. Reprod. Sci. 2010;118:236–247. doi: 10.1016/j.anireprosci.2009.06.013. doi:10.1016/J.ANIREPROSCI.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Moore AI, Squires EL, Graham JK. Adding cholesterol to the stallion sperm plasma membrane improves cryosurvival. Cryobiology. 2005;51:241–249. doi: 10.1016/j.cryobiol.2005.07.004. doi:10.1016/J.CRYOBIOL.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Moraes EA, Graham JK, Torres CAA, Meyers M, Spizziri B. Delivering cholesterol or cholestanol to bull sperm membranes improves cryosurvival. Anim. Reprod. Sci. 2010;118:148–154. doi: 10.1016/j.anireprosci.2009.08.002. doi:10.1016/J.ANIREPROSCI.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Parks JE, Graham JK. Effects of cryopreservation procedures on sperm membrane. Theriogenology. 1992;38:209–222. doi: 10.1016/0093-691x(92)90231-f. doi:10.1016/0093-691X(92)90231-F. [DOI] [PubMed] [Google Scholar]
- Pitha J, Irie T, Sklar PB, Nye JS. Drug solubilizers to aid pharmacologists: amorphous cyclodextrin derivatives. Life Sci. 1988;43:493–502. doi: 10.1016/0024-3205(88)90150-6. doi:10.1016/0024-3205(88)90150-6. [DOI] [PubMed] [Google Scholar]
- Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperature. Nature. 1949;164:666. doi: 10.1038/164666a0. doi:10.1038/164666A0. [DOI] [PubMed] [Google Scholar]
- Purdy PH, Graham JK. Effect of adding cholesterol to bull sperm membranes on sperm capacitation, the acrosome reaction, and fertility. Biol. Reprod. 2004a;71:522–527. doi: 10.1095/biolreprod.103.025577. doi:10.1095/BIOLREPROD.103.025577. [DOI] [PubMed] [Google Scholar]
- Purdy PH, Graham JK. Effect of cholesterol-loaded cyclodextrin on the cryosurvival of bull sperm. Cryobiology. 2004b;48:36–45. doi: 10.1016/j.cryobiol.2003.12.001. doi:10.1016/J.CRYOBIOL.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Saenger W. Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. 1980;19:344–362. doi:10.1002/ANIE.198003441. [Google Scholar]
- Saragusty J, Hildebrandt TB, Natan Y, Hermes R, Yavin S, Goeritz F, Arav A. Effect of egg-phosphatidylcholine on the chilling sensitivity and lipid phase transition of Asian elephant (Elephas maximus) spermatozoa. Zoo Biol. 2005;24:233–245. doi:10.1002/ZOO.20045. [Google Scholar]
- Saragusty J, Hildebrandt TB, Behr B, Knieriem A, Kruse J, Hermes R. Successful cryopreservation of Asian elephant (Elephas maximus) spermatozoa. Anim. Reprod. Sci. 2009;115:255–266. doi: 10.1016/j.anireprosci.2008.11.010. doi:10.1016/J.ANIREPROSCI.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Schmitt DL, Hildebrandt TB. Manual collection and characterization of semen from Asian elephants (Elephas maximus). Anim. Reprod. Sci. 1998;53:309–314. doi: 10.1016/s0378-4320(98)00120-1. doi:10.1016/S0378-4320(98)00120-1. [DOI] [PubMed] [Google Scholar]
- Spizziri BE, Fox MH, Bruemmer JE, Squires EL, Graham JK. Cholesterol-loaded-cyclodextrins and fertility potential of stallions spermatozoa. Anim. Reprod. Sci. 2010;118:255–264. doi: 10.1016/j.anireprosci.2009.08.001. doi:10.1016/J.ANIREPROSCI.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Thongtip N, Saikhun J, Damyang M, Mahasawangkul S, Suthunmapinata P, Yindee M, Kongsila A, Angkawanish T, Jansittiwate S, Wongkalasin W, Wajjwalkul W, Kitiyanant Y, Pavasuthipaisit K, Pinyopummin A. Evaluation of post-thaw Asian elephant (Elephas maximus) spermatozoa of extender and cryoprotectant. Theriogenology. 2004;62:748–760. doi: 10.1016/j.theriogenology.2003.11.021. doi:10.1016/J.THERIOGENOLOGY.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Watson PF. The effects of cold shock on sperm cell membranes. In: Morris GJ, Clarke A, editors. Effects of Low Temperatures on Biological Membranes. Academic Press; New York, NY: 1981. pp. 189–218. [Google Scholar]
- White IG. Lipids and calcium uptake of sperm in relation to cold shock and preservation: a review. Reprod. Fertil. Dev. 1993;5:639–658. doi: 10.1071/rd9930639. doi:10.1071/RD9930639. [DOI] [PubMed] [Google Scholar]
- Wildt DE, Rall WF, Critser JK, Monfort SL, Seal US. Genome resource banks: living collections for biodiversity conservation. Bioscience. 1997;47:689–698. doi:10.2307/1313209. [Google Scholar]
- Woods EJ, Benson JD, Agca Y, Critser JK. Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 2004;48:146–156. doi: 10.1016/j.cryobiol.2004.03.002. doi:10.1016/J.CRYOBIOL.2004.03.002. [DOI] [PubMed] [Google Scholar]