Background
Heparin-Induced Thrombocytopenia (HIT) is an acquired immune disorder occurring in 1-5% of patients exposed to drugs belonging to the heparin family of anticoagulant agents {unfractionated heparin (UFH), low-molecular weight heparin (LMWH) or fondaparinux [1]}. HIT is caused by pathogenic autoantibodies directed to complexes of an endogenous platelet protein, Platelet Factor 4 (PF4) and heparin, a glycosaminoglycan (GAG) derived from bovine or porcine mucosal tissues. Although PF4/heparin antibodies are uniformly detected in patients who meet clinical diagnostic criteria for HIT [2], antibodies can also be found in heparinized patients in the absence of clinical disease [3, 4].
Prospective investigations into the biologic basis of the PF4/heparin immune response in humans have been hampered by the life-threatening nature of disease. To overcome this limitation and to gain insights into the mechanisms governing antibody induction and pathogenicity, we have recently developed a murine model with which to characterize the PF4/heparin immune response in vivo. Unlike previously described murine models which rely on injections of pre-formed human or murine monoclonal antibodies [5], we have developed an animal model in which murine autoantibodies arise de novo upon exposure to murine PF4 (mPF4) and heparin. Using this novel murine immunization model, we have recently shown that autoantibodies to mPF4/heparin (mPF4/H): 1) recognize complexes of mPF4/H, but not mPF4 alone or heparin alone, 2) recapitulate salient serologic and functional properties of human HIT antibodies, including platelet activation 3) require T-cells for optimal induction and 4) occur in response to distinct biophysical properties of antigen, relating to charge and size of particles [6, 7].
In the course of developing this murine immunization model, we have refined several technical aspects of this model (strain, dose, injection routes), and have characterized several important biologic aspects of the immune response (temporal course, platelet counts) that have not been previously reported. In this manuscript, we describe studies relating to optimization of our murine model for studying PF4/H autoantibody formation.
Methods
Murine PF4 expression and isolation
PF4 was expressed and isolated from E.coli using cDNA isolated from murine platelet total RNA, as previously described [8-10]. Species specificity of mPF4 was additionally confirmed by ELISA using a murine monoclonal antibody (KKO), which binds to hPF4/H, but lacks reactivity to mPF4/H (data not shown).
Murine immunization model
Wild-type BALB/c or C57BL/6 mice at 8-10 weeks of age were purchased from Jackson Laboratories (Bar Harbor, ME), Charles River Laboratories (Wilmington, MA) or the National Cancer Institute (Frederick, MD). Mice were injected via intravenous retro-orbital (IV-RO) injection daily for five days unless stated otherwise. For experiments examining the effect of injection route, mice were injected with antigen via intraperitoneal (IP) or intravenous tail vein (IV-TV) routes. Antigen (mPF4, UFH or mPF4/H) or buffer (Hank's Balanced Salt Solution, HBSS) were prepared in HBSS, sterile filtered and injected into animals anesthetized with 3% isoflurane. Unless specified, mPF4 was injected at a concentration of 20μg and heparin 0.4U in a final volume of 100μL (final concentration: PF4 200 μg/ml and heparin 4U/mL; PF4: heparin molar ratio of 2.6:1). The first day of injection was considered D1 of injections. Blood was collected from anesthetized mice from the RO plexus and collected into tubes containing 3.2% sodium citrate. Blood was collected 24-48 hours prior to immunizations (baseline, BL) and on a weekly basis for defined periods ranging from 4 to 12 weeks.
Anti-mPF4/H were detected by mPF4/heparin ELISA as previously described [6, 7, 11]. Briefly, 50μL of a premixed solution mPF4 (10μg/mL) and UFH (0.4 U/mL) were coated on 96-microtiter wells (NUNC Maxisorp, Roskilde, Denmark) and incubated overnight at room temperature (RT). After blocking with 10% fetal calf-serum in PBS (FCS/PBS), wells were serially incubated for one hour at RT with murine antibodies (1:100 dilution in FCS/PBS), followed by detection with an antibody specific for murine IgG (anti-mouse IgG peroxidase, γ-chain specific; Sigma-Aldrich Inc, St. Louis MO). Binding of secondary antibody was detected colorimetrically using TMBZ substrate (KPL Laboratories, Gaithersburg, MD) and measured at 450nm absorbance using a Molecular Devices Plate Reader (Sunnyvale, CA). Seroconversion was defined as detectable anti-mPF4/H signals that were elevated by 5-fold or more from baseline signals (prior to immunizations). Studies were performed with the approval of the Institutional Animal Care & Use Committee (IACUC) at Duke University.
Determination of platelet counts
Platelet counts were determined by CellDyn 3700 (Abbott Diagnostics, Illinois, USA) according to manufacturer's protocol. Briefly, 30μL of whole blood was diluted 1:5 in isotonic saline (0.9% Sodium Chloride) and 130μL of this preparation was used for measurement.
Statistical Analysis
For murine studies, data obtained from groups of mice were compared using the student's t-test for comparisons of two groups of animals or for more than 2 groups by a one-way ANOVA analysis with Bonferroni's post-test or Dunnett's multiple comparisons test. Results of ELISA analysis were expressed as mean ± SEM and analyzed for significance using the Student t test. Statistical analyses were performed using Graph Pad Prism (Graphpad Software, San Diego, CA, USA). Differences were considered significant with a P value < 0·05.
Results
We initiated our experimental studies in mice based on several observations relevant to human heparin sensitization: 1) PF4/heparin antibodies form when an endogenous protein (human PF4) is modified by heparin, 2) seroconversions, in most patients, occur after several days (5-10 days) of heparin exposure [12], and 3) sensitization can be increased in clinical settings such as cardiopulmonary bypass (CPB) [13, 14], which is associated with high levels of PF4 (from platelet activating effects of CPB) and heparin. Based on these observations, we reasoned that mice exposed to high doses of murine PF4 (a native protein) and heparin, intravenously over several days, could potentially become sensitized in an analogous manner to humans.
Effects of murine strain
As previously reported [6], our initial experimental schema was developed in BALB/c mice, as this murine strain is commonly used for immunologic studies and shows robust immune responses to a variety of antigens, including carbohydrates [15]. Our initial studies in BALB/c mice revealed that mice were capable of making high titer anti-mPF4/H in the absence of adjuvant and that mice injected with buffer did not seroconvert [6]. While the immune response in our BALB/c model recapitulated several critical aspects of hPF4/H antibodies [6], we noted that seroconversions were not particularly robust in this murine strain, as only 15-20% of animals met our criteria for seroconversion (defined as antibody levels that were 5-fold or more increased from baseline signals) after immunization.
It is widely known that mice exhibit important strain-dependent differences in susceptibility to a variety of antigens [16-18]. For these reasons, we examined the effects of murine strain on the mPF4/H immune response. We selected C57BL/6 mice to study, as this murine strain is a widely used laboratory strain and often serves as a host strain for genetic manipulation. To study differences in sensitization, parallel cohorts of BALB/c (n=24) and C57BL/6 (n=27) mice were injected with identical doses of mPF4/H by RO injection and monitored for seroconversion. As shown in Figure 1, there were significant strain-dependent differences in the immune response to mPF4/H injections. Whereas BALB/c mice showed low-levels of anti-mPF4/H (mean A450nm ± SD, 0.24 ± 0.05) with ~15% showing seroconversion (4 of 27 mice showed >5-fold change in signal) by day 15 (D15), C57BL/6 mice showed high levels of antibody (mean A450nm ± SD, 1.85 ± 0.23; BALB/c v. C57BL/6, p<0.0001) with ~95% showing seroconversions during the same time interval. Like BALB/c mice, C57BL/6 mice did not develop anti-mPF4/H when injected with buffer or heparin alone (data not shown). These studies indicated that C57BL/6 mice were more immunologically responsive to mPF4/H antigen.
Figure 1. Strain dependent difference in the anti-mPF4/H response.
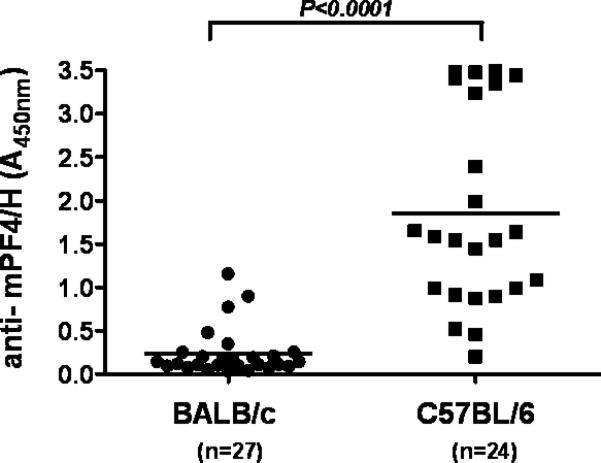
BALB/c and C57BL/6 mice were injected with mPF4/H and monitored for anti-mPF4/H at D15. Individual measurements are shown as well as mean (bar). C57BL/6 not only showed higher rates of seroconversion, but also greater levels of anti-mPF4/H (mean anti-mPF4/H, p<0.0001).
Time course of antibody production varies by strain
Humans manifest PF4/heparin antibodies within 5-10 days of drug exposure, with circulating antibodies decreasing over time to undetectable levels [12]. To determine if time course of antibody production in mice is similar to that of humans, we monitored anti-mPF4/H levels in cohorts of sensitized BALB/c (n=7) and C57BL/6 mice (n=7) over two to three months. Once again, we noted strain-dependent differences in the time course of antibody production, with C57BL/6 mice showing a time course pattern more akin to humans. As shown in Figure 2A, BALB/c mice showed peak antibody responses 30-60 days after immunization. In contrast, C57BL/6 mice showed peak antibody titers by D15 (Figure 2B), with antibody levels decaying to pre-sensitization levels over a three month time period. These kinetic studies, alongside studies in Figure 1, showing a more robust immune response in C57BL/6 mice, indicated that the immune response in the C57BL/6 murine strain was more similar to the human PF4/H immune response and, therefore, all subsequent studies were performed in C57BL/6 mice.
Figure 2. Time course of anti-mPF4/H formation in BALB/c mice v C57BL/6.
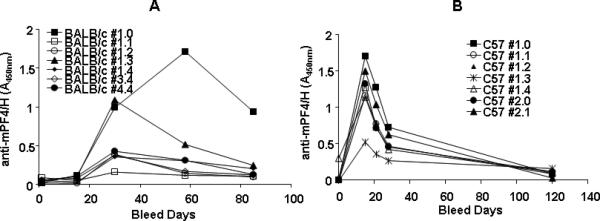
(A) BALB/c or (B) C57BL/6 mice were immunized with mPF4/H and anti-mPF4/H responses were followed over two to three months. Time course for individual mice within each cohort are shown by each line.
Effects of injection route on antibody levels and time course of antibody responses
Our immunization protocol was developed with intravenous administration of antigen via RO injection. Because RO injections require technical expertise and generally are not advised by institutional animal use committees, we examined the effects of immunization route on antibody responses. For these studies, C57BL/6 mice were injected with mPF4/H via IV-TV (n=10), or IV-RO (n=10), or IP (n=10) route. As shown in Figure 3A, at D15 mice immunized by the IP route showed negligible seroconversions as compared to mice immunized by both intravenous routes.
Figure 3. Effects of immunization route and time course of antibody responses.
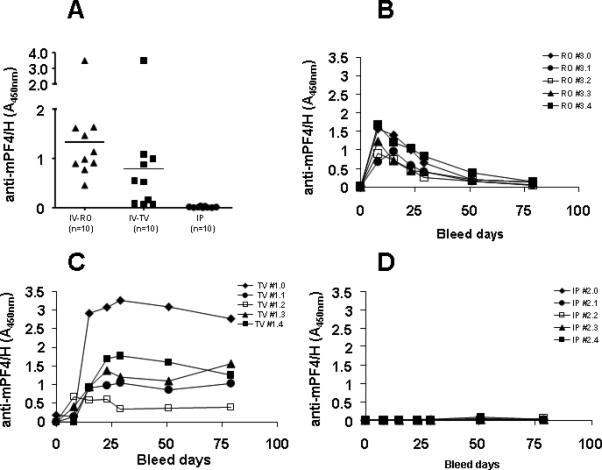
C57BL/6 mice were immunized intravenously {via retro-orbital (RO, n=10) or tail vein sites (TV, n=10)} or intraperitoneally (IP, n=10) with mPF4/H. Anti-mPF4/H levels were monitored at D15 for all mice (A). Antibody levels were followed over 2 ½ months for immunized mice injected by RO (B), TV (C) or IP routes (D) to document any delayed seroconversions.
To determine if mice immunized by the IP route had delayed seroconversions, due to a slow absorption and release of antigen into circulation, five seropositive mice from the IV cohorts (TV and RO) and five mice expressing the highest antibody levels in the IP cohort were monitored for antibody production over time. As shown in Figure 3B-D, temporal kinetics of antibody formation markedly differed among the three cohorts. Contrary to expectations, mice immunized by the IP route did not manifest any delayed seroconversions and had minimal anti-mPF4/H levels over a 30 day period (Fig 3D). Mice immunized by the two IV routes, however, showed striking differences in the kinetics of seroconversion. Mice immunized by the RO route showed peak seroconversions by D8-D15, followed by decreasing antibody levels over a 30 day period. The majority of mice injected by TV (4 of 5 mice), however, displayed persistently elevated antibody levels after peak seroconversion. These findings could possibly be explained by technical limitations of the study. Because of the difficulties encountered in cannulating the TV of mice, it is possible that some TV injections were given intradermally rather than IV.
Duration of antigen exposure
As large amounts of mPF4 are required for these experiments, we were interested in reducing the duration of antigen exposure to reduce amounts of mPF4 required for experiments. We reasoned that a 4 day immunization schedule would reduce the amount of mPF4 required, without significantly compromising the antibody response. For these experiments, animals were injected IV-RO with mPF4/H daily for 5 days, 4 days or 1 day as a negative control. As shown in Figure 4, mice injected with antigen for 5 days had significantly higher antibody levels than mice injected with antigen for 4 days or 1 day (mean anti-mPF4/H A450nm 5D: 0.901 ± 0.685 v. 4D: 0.198 ± 0.202, p<0.05; mean anti-mPF4/H A450nm 5D: v. 1D: 0.05± 0.026, p<0.05). Optimal injection schedule was therefore maintained on a schedule of daily injections for 5 days.
Figure 4. Duration of antigen exposure.
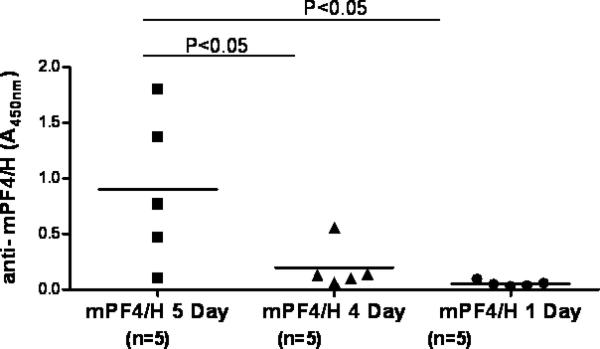
C57BL/6 mice were immunized intravenously (RO) with mPF4/H daily for 5, 4 or 1 day as shown. Anti-mPF4/H levels were monitored at D15 for all mice. Significant differences between cohorts are indicated at the top of the figure.
Effects of PF4 Dose on PF4/heparin immune response
We and others have noted that the size of PF4/heparin ultra-large complexes (ULCs) is critically dependent on PF4:heparin molar ratios (PHRs) [7, 19, 20], with maximal ULC size occurring at molar ratios approaching 1:1. Our in vitro studies indicate that for a fixed PHR, there is a linear relationship between ULC size and mPF4 dose [7] and that high doses of mPF4 alone (200 μg/mL) are not associated with aggregate formation. To determine if there is a dose dependent relationship between mPF4 dose and in vivo immunogenicity, we injected animals with increasing doses of mPF4 (50-200 μg/mL) but adjusted the heparin dose to maintain a constant PHR of 2.6:1. As shown in Figure 5, we detected a dose-dependent effect of mPF4 and heparin on seroconversions. Although PHR remained constant, decreasing the dose of mPF4 had a major impact and was associated with correspondingly lower antibody levels.
Figure 5. Effects of PF4:UFH dose on anti-mPF4/H responses.
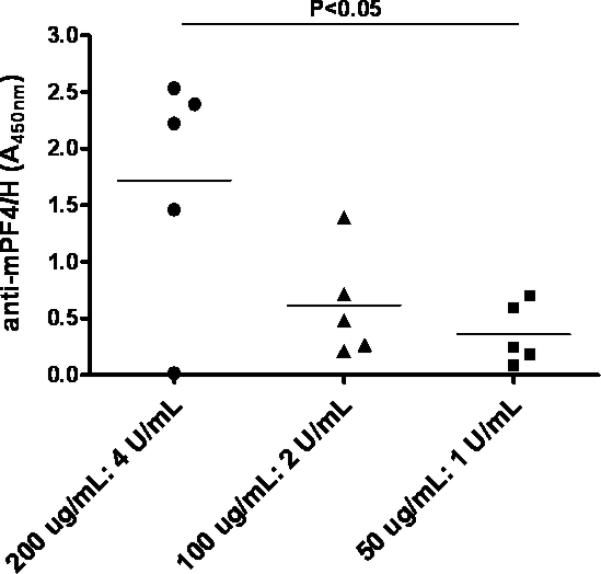
C57BL/6 mice were immunized intravenously (RO) with mPF4/H daily for 5 days with varying doses of PF4 and heparin adjusted to maintain a constant molar ratio of PF4:UFH of 2.6:1(n=5/cohort).
Effects of immunization on murine platelet counts
In humans, thrombocytopenia, manifesting as either an absolute (platelet counts <150,000/μl) or relative (<50% drop from baseline counts) decrease in platelet counts, is often the first clinical harbinger of heparin sensitization [21]. In mice, we have shown that HIT antibody mediated thrombocytopenia and thrombosis occur only in transgenic mice expressing human platelet FcγRIIa [5]. Although WT mice lack platelet Fc receptors, we assessed for thrombocytopenia in WT mice to examine a potential role for endogenous macrophage Fc receptors in mediating antibody-induced thrombocytopenia.
To determine if mPF4/H antibody production in WT mice is associated with development of thrombocytopenia, C57BL/6 mice were injected with mPF4/H (n=10) or buffer (n=5) and monitored for antibody development and platelet counts. As shown in Figure 6, mean platelet counts at baseline were 752, 000 ± 75,000 /μL in mice injected with mPF4/H and 740,000 ± 38,000 /μL in mice injected with buffer. At 8 days from start of immunization, mean platelet counts in both cohorts increased (mPF4/H: 1,468,000 ± 240,000 /μl and buffer: 1,604,000 ± 477,000 /μl), likely reflecting rebound thrombocytosis from baseline blood draws. At D8, 7/10 mPF4/H injected mice seroconverted with anti-mPF4/H levels A450nm>0.45 (solid squares). None of the mice injected with buffer seroconverted (clear circles). Also at D8, there were no statistically significant differences in platelet counts between mPF4/H injected or buffer injected mice, nor were there any statistically significant differences in platelet counts between seropositive (solid squares) or seronegative (open squares) mice injected with mPF4/H. As well, antibody levels did not increase in mice exposed to heparin at D15 (data not shown).
Figure 6. Effects of immunization on platelet counts.
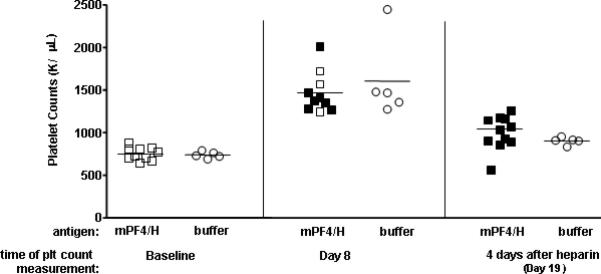
C57BL/6 mice were immunized intravenously with mPF4/H (□, n=10) or buffer (○, n=5). Platelet counts were checked at baseline (“Baseline”) or 8 days after immunization (“Day 8”). On D15, all mice (mPF4/H and buffer injected mice) were given one dose of heparin and platelet counts were checked 4 days after heparin administration on D19 (“4 days after heparin”). Mean platelet counts are indicated by line for each respective cohort and time point. Mice were also concurrently monitored for anti-mPF4/H development. Seropositive mice expressing antibody levels A450nm ≥0.45 are indicated by solid symbols at D8 and D15.
To determine if heparin exposure was necessary for antibody positive animals to develop thrombocytopenia, mPF4/H and buffer cohorts were injected with 50U/mL of heparin by IP injection on D15 from start of immunizations. Platelet counts and antibody levels were monitored 4 days after heparin re-exposure (D19 from start of immunization). As shown in Figure 6, all mPF4/H were seropositive by D19 (solid squares). Once again, on D19, there were no statistically significant differences in platelet counts between the two cohorts that were given heparin (mPF4/H: 1,042,000 ± 135,000 /μl and buffer: 902,000 ± 43,000 /μl; p>0.05), nor were there any statistically significant differences in platelet counts between antibody positive and negative mice (solid v. clear symbols, p>0.05). These studies indicate that an isolated mPF4/H immune response is not sufficient to induce thrombocytopenia in wild-type (WT) C57BL/6 mice.
Demonstration of immune recall in PF4/heparin sensitized mice
It is not known if HIT is associated with immune recall. To determine if the immune response to mPF4/H is associated with recall, we followed previously sensitized mice (from experiments shown in Figure 4) for 50-65 days until antibody levels decreased to 2-3 fold over baseline values. Sensitized mice were given one dose of mPF4/H on day 73 (Figure 7). Seroconversion was monitored over the subsequent 2 weeks by mPF4/H ELISA. As shown in Figure 7, anti-mPF4/H levels in sensitized mice were higher 1 week after re-immunization (mean A450nm ± SD at D79 =1.391 ± 0.377) than at time of primary sensitization either on D8 (mean A450nm± SD = 0.419 ± 0.178; p<0.048 for D8 primary v. secondary by Student t test). Similarly, responses at D15 of primary immunization (A450nm= 0.446 ± 0.164) were lower than at 15 days after secondary immunization, (mean A450nm at D15= 0.91 ± 0.735; p<0.064 for D15 primary v. secondary). Injections of mPF4 alone or heparin alone do not elicit antibody responses (On-line Supplemental Figure 1). Isotype analysis of time points after primary or secondary immunization revealed IgG isotypes occurring within 8 days of immunization, without evidence of IgM precedence (On-line Supplemental Figure 2). These observations showing IgG isotype expression without IgM precedence has been recently described for primary immune responses in human HIT [22, 23].
Figure 7. Presence of immune recall in PF4/heparin sensitized mice.
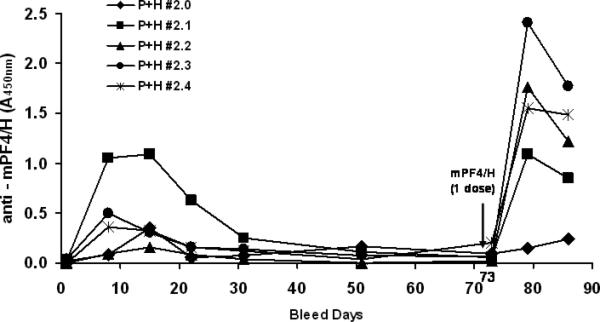
PF4/heparin sensitized C57BL/6 mice were immunized with mPF4/H and followed over time until antibody levels decreased to 2-3 fold over baseline values. Sensitized mice (n=5) were given one dose of mPF4/H on day 73 and seroconversion was monitored at day 8 and day 15 post re-immunization.
Conclusions
In these studies, we describe the effects of several variables on the PF4/heparin immune response, including the influence of host, route of administration, antigen dose and immunization schedule. Our studies indicate that optimal immune responses mimicking that of human heparin sensitization can be elicited in C57BL/6 mice given mPF4/H antigen, by the IV- RO route daily for five days. We also show that immune responses in wild-type mice are not associated with thrombocytopenia or clinical manifestations of thrombosis and that immune recall is preserved to mPF4/H antigen in sensitized mice.
Naturally occurring proteins, in particular, soluble antigens, are poorly immunogenic and therefore require adjuvant, such as alum, complete Freund's adjuvant (CFA) and/or lipopolysaccharide (endotoxin/LPS) for potentiating the immune response. Our past and present studies [6, 7, 24], however, show that PF4/heparin complexes are intrinsically immunogenic and do not require adjuvant for antibody production. LPS levels were extremely low in our mPF4 and buffer preparations and, thus, exclude the effects of endotoxin as an adjuvant in murine seroconversions. It is possible that certain structural features of antigen (size, charge or conserved patterns in the structure) rather than the presence of distinctive epitopes contributes to the immunogenicity of PF4/heparin macromolecular complexes. Indeed, recent studies indicate that immunogenicity of soluble proteins can be enhanced through delivery of antigen in particulate form. It appears that covalent linkage of soluble peptides to ~1μm synthetic microspheres [25, 26] triggers a potent T-cell mediated immune response through enhanced macrophage uptake and/or through direct stimulation of B-cells by cross-linking surface immunoglobulin. It is possible that injections of macromolecular complexes of mPF4/H, which we have shown by in vitro techniques to approximate 0.5-1.5 μm in size [7], have enhanced recognition by the immune system as particulate antigens. Whether the immunogenicity of PF4/heparin particles in these studies is simply due to particle formation, presentation of repeating neoepitopes or other parameters such as charge remains to be determined.
Our studies also validate previous observations on the importance of strain and injection route in humoral responses to antigen. We noted significant differences in the temporal kinetics of antibody formation between BALB/c and C57BL/6 mice. These strain dependent differences have been noted with regards to other immunologic responses, such as experimental autoimmune disease [16], tumorigenesis [17] and infection [18]. To what extent these strain-dependent differences are due to one or several recognized differences in immunoregulatory function [27] remains to be determined. The complexity of immunoregulation, even within a given individual strain, is revealed by our studies of injection route in C57BL/6 mice. As shown in Figure 3, we observed that the same antigen delivered by two parenteral routes (TV or RO) elicited antibody responses with markedly different kinetics of seroconversion. Even if it was presumed that tail-vein injections were unintentionally given intradermally, the striking differences in seroconversion profiles for these two routes of antigen delivery support previous observations that immune responses are influenced by route of inoculation, which in turn reflects distinct antigen-presenting cell compartments [28].
We show that antibodies formed after RO decrease over time. Decay in antibody levels, however, does not necessarily imply a lack of immune recall. Consistent with previous observations for secondary immune responses in animals and humans [29, 30], mPF4/H sensitized animals required far less antigen (1D) to elicit a robust secondary immune response as compared to a primary immune response (see Figures 4 & 7). As shown in Figure 7, when sensitized mice were rechallenged with one dose of antigen at time of antibody decay, we noted a rapid rise in antibody levels within 8 days of re-immunization. We also noted that antibody levels were higher 7 days after mPF4/heparin re-exposure than at primary immunization time points (D8 or D15) and that both primary and secondary immune responses were primarily of IgG isotype (see Supplemental Figure 2) and were accompanied, but not necessarily, preceded by IgM as has been recently described in human HIT [22, 23].
The role of immune recall in human HIT is unresolved [31]. Although it is well-recognized that human anti-PF4/heparin antibodies appear to be transient in the majority of patients with HIT [12] and in some cases, patients have been safely re-exposed to heparin [32], most practitioners remain cautious about heparin re-exposure in patients with a diagnosis of HIT. Whether our findings of immune recall in mice apply to human disease is not clear, as the experimental conditions used in our model differ considerably from conditions under which humans become sensitized or re-exposed. However, the presence of immune recall in our murine model should guide development of cellular or molecular assays with which to ascertain the presence or absence of immunologic memory in humans.
We developed this murine model to study mechanisms relevant to PF4/heparin antibody induction, the knowledge of which is currently lacking. Although our murine model has been optimized to mimic relevant serological properties of human PF4/H antibodies, we recognize several important limitations of our experimental system. We recognize that the murine model does not recapitulate salient manifestations of human HIT, namely thrombocytopenia and thrombosis. The experimental design of our study also does not allow conclusions to be drawn on the biological impact of an isolated PF4/heparin immune response in humans, as our murine model lacks expression platelet FcγRIIa expression, which is vital for IgG dependent platelet activation [5, 33]. Finally, it is important to note that important species differences between mice and humans limit our ability to extrapolate murine findings to human disease. However, due to the medical and ethical challenges of manipulating the PF4/heparin immune response in humans, our murine model fills an important experimental void in understanding the pathogenesis of human heparin sensitization.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health HL081395 (GMA)
References
- 1.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710–5. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- 2.Arepally GM, Ortel TL. Heparin-Induced Thrombocytopenia. N Engl J Med. 2006;355:809–17. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 3.Amiral J, Peynaud-Debayle E, Wolf M, Bridey F, Vissac AM, Meyer D. Generation of antibodies to heparin-PF4 complexes without thrombocytopenia in patients treated with unfractionated or low-molecular-weight heparin. Am J Hematol. 1996;52:90–5. doi: 10.1002/(SICI)1096-8652(199606)52:2<90::AID-AJH4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Bauer TL, Arepally G, Konkle BA, Mestichelli B, Shapiro SS, Cines DB, Poncz M, McNulty S, Amiral J, Hauck WW, Edie RN, Mannion JD. Prevalence of heparin-associated antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation. 1997;95:1242–6. doi: 10.1161/01.cir.95.5.1242. [DOI] [PubMed] [Google Scholar]
- 5.Reilly MP, Taylor SM, Hartman NK, Arepally GM, Sachais BS, Cines DB, Poncz M, McKenzie SE. Heparin-induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcgammaRIIA. Blood. 2001;98:2442–7. doi: 10.1182/blood.v98.8.2442. [DOI] [PubMed] [Google Scholar]
- 6.Suvarna S, Rauova L, McCracken EKE, Goss CM, Sachais BS, McKenzie SE, Reilly MP, Gunn MD, Cines DB, Poncz M, Arepally G. PF4/heparin complexes are T cell-dependent antigens. Blood. 2005;106:929–31. doi: 10.1182/blood-2004-12-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suvarna S, Espinasse B, Qi R, Lubica R, Poncz M, Cines DB, Wiesner MR, Arepally GM. Determinants of PF4/heparin immunogenicity. Blood. 2007;110:4253–60. doi: 10.1182/blood-2007-08-105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li ZQ, Liu W, Park KS, Sachais BS, Arepally G, Cines DB, Poncz M. Defining A Second Epitope For Heparin-Induced Thrombocytopenia/Thrombosis (HIT/T) Antibodies Using KKO, A Murine HIT-Like Monoclonal Antibody. Blood. 2002;99:1230–6. doi: 10.1182/blood.v99.4.1230. [DOI] [PubMed] [Google Scholar]
- 9.Ziporen L, Li ZQ, Park KS, Sabnekar P, Liu WY, Arepally G, Shoenfeld Y, Kieber-Emmons T, Cines DB, Poncz M. Defining an antigenic epitope on platelet factor 4 associated with heparin-induced thrombocytopenia. Blood. 1998;92:3250–9. [PubMed] [Google Scholar]
- 10.Eslin DE, Zhang C, Samuels KJ, Rauova L, Zhai L, Niewiarowski S, Cines DB, Poncz M, Kowalska MA. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood. 2004;104:3173–80. doi: 10.1182/blood-2003-11-3994. [DOI] [PubMed] [Google Scholar]
- 11.Arepally G, Reynolds C, Tomaski A, Amiral J, Jawad A, Poncz M, Cines DB. Comparison of PF4/heparin ELISA assay with the 14C-serotonin release assay in the diagnosis of heparin-induced thrombocytopenia. Am J Clin Pathol. 1995;104:648–54. doi: 10.1093/ajcp/104.6.648. [DOI] [PubMed] [Google Scholar]
- 12.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. New Engl J Med. 2001;344:1286–92. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 13.Visentin GP, Malik M, Cyganiak KA, Aster RH. Patients treated with unfractionated heparin during open heart surgery are at high risk to form antibodies reactive with heparin:platelet factor 4 complexes. J Lab Clin Med. 1996;128:376–83. doi: 10.1016/s0022-2143(96)80009-6. [DOI] [PubMed] [Google Scholar]
- 14.Warkentin TE, Sheppard JA, Horsewood P, Simpson PJ, Moore JC, Kelton JG. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96:1703–8. [PubMed] [Google Scholar]
- 15.Blomberg B, Geckeler WR, Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972;177:178–80. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- 16.Graus YM, van Breda Vriesman PJ, de Baets MH. Characterization of anti-acetylcholine receptor (AChR) antibodies from mice differing in susceptibility for experimental autoimmune myasthenia gravis (EAMG). Clin Exp Immunol. 1993;92:506–13. doi: 10.1111/j.1365-2249.1993.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuraguchi M, Cook H, Williams ED, Thomas GA. Differences in susceptibility to colonic stem cell somatic mutation in three strains of mice. J Pathol. 2001;193:517–21. doi: 10.1002/path.834. [DOI] [PubMed] [Google Scholar]
- 18.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Ann Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 19.Rauova L, Poncz M, McKenzie SE, Reilly MP, Arepally G, Weisel JW, Nagaswami C, Cines DB, Sachais BS. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105:131–8. doi: 10.1182/blood-2004-04-1544. [DOI] [PubMed] [Google Scholar]
- 20.Greinacher A, Gopinadhan M, Gunther J-U, Omer-Adam MA, Strobel U, Warkentin TE, Papastavrou G, Weitschies W, Helm CA. Close Approximation of Two Platelet Factor 4 Tetramers by Charge Neutralization Forms the Antigens Recognized by HIT Antibodies. Arterioscler Thromb Vasc Biol. 2006;26:2386–93. doi: 10.1161/01.ATV.0000238350.89477.88. [DOI] [PubMed] [Google Scholar]
- 21.Greinacher A, Farner B, Kroll H, Kohlmann T, Warkentin TE, Eichler P. Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408 patients. Thromb Haemost. 2005;94:132–5. doi: 10.1160/TH04-12-0825. [DOI] [PubMed] [Google Scholar]
- 22.Greinacher A, Kohlmann T, Strobel U, Sheppard J-AI, Warkentin TE. The temporal profile of the anti-PF4/heparin immune response. Blood. 2008 doi: 10.1182/blood-2008-08-173062. blood-2008-08-173062. 10.1182/blood-2008-08-173062. [DOI] [PubMed] [Google Scholar]
- 23.Warkentin TE, Sheppard J-AI, Moore JC, Cook RJ, Kelton JG. Studies of the immune response in heparin-induced thrombocytopenia. Blood. 2009 doi: 10.1182/blood-2008-10-186064. blood-2008-10-186064. 10.1182/blood-2008-10-186064. [DOI] [PubMed] [Google Scholar]
- 24.Suvarna S, Qi R, Hollingsworth JW, Arepally GM. Platelet Factor 4 (PF4)-Heparin complexes Trigger Immune Responses independently of the MyD88 Pathway. Br J Haematol. 2008;142:671–3. doi: 10.1111/j.1365-2141.2008.07240.x. [DOI] [PubMed] [Google Scholar]
- 25.Gengoux C, Leclerc C. In vivo induction of CD4+ T cell responses by antigens covalently linked to synthetic microspheres does not require adjuvant. Inter Immunol. 1995;7:45–53. doi: 10.1093/intimm/7.1.45. [DOI] [PubMed] [Google Scholar]
- 26.Sedlik C, Rojas M, Leclerc C. Activation of B cells by 1 microm particulate lysozyme or peptides: a Th-dependent pathway requiring CD40-CD40 ligand interaction. Int Immunol. 1998;10:1111–9. doi: 10.1093/intimm/10.8.1111. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Oppenheim JJ, Howard OMZ. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+CD25- responder cells than C57BL/6 mice. J Leukoc Biol. 2005;78:114–21. doi: 10.1189/jlb.0604341. [DOI] [PubMed] [Google Scholar]
- 28.Hocart MJ, Mackenzie JS, Stewart GA. The IgG Subclass Responses to Influenza Virus Haemagglutinin in the Mouse: Effect of Route of Inoculation. J Gen Virol. 1989;70:809–18. doi: 10.1099/0022-1317-70-4-809. 10.1099/0022-1317-70-4-809. [DOI] [PubMed] [Google Scholar]
- 29.Whittingham S, Buckley JD, Mackay IR. Factors influencing the secondary antibody response to flagellin in man. Clin Exp Immunol. 1978;34:170–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Konishi E, Yamaoka M, Khin Sane W, Kurane I, Takada K, Mason PW. The Anamnestic Neutralizing Antibody Response Is Critical for Protection of Mice from Challenge following Vaccination with a Plasmid Encoding the Japanese Encephalitis Virus Premembrane and Envelope Genes. J Virol. 1999;73:5527–34. doi: 10.1128/jvi.73.7.5527-5534.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and Prevention of Heparin-Induced Thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:340S–80. doi: 10.1378/chest.08-0677. 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 32.Potzsch B, Klovekorn WP, Madlener K. Use of heparin during cardiopulmonary bypass in patients with a history of heparin-induced thrombocytopenia [letter]. New Engl J Med. 2000;343:515. doi: 10.1056/NEJM200008173430718. [DOI] [PubMed] [Google Scholar]
- 33.Kelton JG, Sheridan D, Santos A, Smith J, Steeves K, Smith C, Brown C, Murphy WG. Heparin-induced thrombocytopenia: laboratory studies. Blood. 1988;72:925–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


