Abstract
Purpose
National attention has focused on whether urology-radiation oncology practice integration – known as integrated prostate cancer centers (IPCCs) – contributes to use of intensity-modulated radiation therapy (IMRT), a common and expensive treatment for prostate cancer.
Methods
We examined prostate cancer treatment patterns pre- and post-conversion of a urology practice to an IPCC in July, 2006. Using the SEER-Medicare database, we identified patients age ≥ 65 years diagnosed in one state-wide registry with non-metastatic prostate cancer between 2004 and 2007 and classified patients into 3 groups: (1) those seen by IPCC physicians (exposure group); (2) those living in the same hospital referral region (HRR) and not seen by IPCC physicians (HRR-control group); and (3) those living elsewhere in the state (state-control group). We compared changes in treatment among the 3 groups, adjusting for patient, clinical, and socio-economic factors.
Results
Compared with the 8.1 percentage point (ppt) increase in adjusted IMRT use in the state-control group, IMRT increased 20.3 ppts (95% confidence interval [CI] 13.4, 27.1) in the IPCC group and 19.2 ppts (95% CI 9.6, 28.9) in the HRR-control group. Androgen-deprivation therapy (ADT), for which Medicare reimbursement declined sharply, decreased similarly in the IPCC and HRR-control groups. Prostatectomy declined significantly in the IPCC group.
Conclusions
Coincident with the conversion of a urology group practice to an IPCC, we observed increases in IMRT and decreases in ADT among patients seen by IPCC physicians and those seen in the surrounding healthcare market that were not observed in the remainder of the state.
INTRODUCTION
Prostate cancer costs exceed $12 billion annually and account for nearly 10% of the total cost of cancer care to Medicare.1,2 Rapid adoption of new and expensive medical technology for prostate treatment has been an important cost driver.3,4 Medicare reimbursement for intensity-modulated radiation therapy (IMRT), introduced in the late 1990s and now the most common radiotherapy treatment for prostate cancer, is approximately $25,000 per treatment course, compared to $8,000 to $13,000 for prostatectomy and $12,000 for brachytherapy.5,6
National attention has recently focused on the contribution of integrated prostate cancer centers (IPCCs) to use of IMRT.7–12 IPCCs have formed when urology practices acquire radiotherapy equipment and employ radiation oncologists, consolidating the provision of prostatectomy and IMRT within a single group practice, as allowed under the In-Office Ancillary Services Exemption of the Stark Law. The Stark Law prohibits physicians from referring patients to a facility with which they have a financial relationship, while the In-Office Ancillary Services Exemption permits referrals when physicians maintain care oversight of the service within their office setting.13
Integrated care may improve quality through better coordination of specialized multidisciplinary cancer treatment. However, ownership of highly reimbursed medical services by referring physicians has been shown to increase the use and costs of medical care.14,15 Moreover, companies selling turnkey IMRT programs to urology practices explicitly market the potential for increased IMRT revenue to replace lost earnings from androgen-deprivation therapy (ADT), for which reimbursement declined sharply as part of the 2003 Medicare Modernization Act.7,16
To evaluate the impact of urology-radiation oncology practice integration on prostate cancer treatment patterns, investigated the impact of one urology practice’s conversion to an IPCC in June, 2006 on prostate cancer treatment patterns.
METHODS
We identified the month and year of the conversion of a urology practice to an IPCC based on publically available sources. The group practice is a large provider of urologic services in its hospital referral region (HRR). HRRs represent regional healthcare markets for tertiary medical care.17 The practice opened a new radiotherapy center as part of the IPCC, extending the total market capacity for radiotherapy. Other providers of radiotherapy in the HRR include a University-based center and two community cancer cancers. To comply with Surveillance, Epidemiology and End Results (SEER)-Medicare confidentiality guidelines, we redacted identifying information about the IPCC, the HRR in which the IPCC was located, and the state.
Using state-wide cancer registry and administrative claims data from the SEER-Medicare database, we definedpre- and post-conversion periods. Each comprised 18 months for case ascertainment from a state-wide SEER registry, and 9 months of follow-up for treatment ascertainment from Medicare claims (an interval allowing sufficient time for multidisciplinary consultation and treatment initiation). No other IPCCs were formed in the state during the study period.
We identified men age ≥ 65 years with non-metastatic prostate cancer diagnosed from March 1, 2004 to August 31, 2005, with follow-up through May 31, 2006 (pre-period, n=2,076) and from July 1, 2006 to December 31, 2007, with follow-up through September 31, 2008 (post-period, n=1,904).
We identified urology and radiation oncology physician members of the IPCC and obtained their Unique Physician Identifier Numbers (UPINs) and National Provider Identifiers (NPIs) from the Medicare Physician Identification and Eligibility Record (MPIER). We examined physician visits based on Medicare claims occurring between diagnosis date and treatment date (or 9 months after diagnosis) and then classified patients into 3 groups: (1) those seen by IPCC physicians (exposure group), 97% of whom resided in the HRR; (2) those living in the same HRR and not seen by IPCC physicians (HRR-control group); and (3) those living elsewhere in the state (“rest of” state-control group). By comparing changes in treatment use between these three groups, we can distinguish the impact of practice integration on patients seen by physician members of the IPCC versus patients seen by other physicians in the same regional healthcare market from secular trends elsewhere in the state.
The primary outcome was a categorical measure of prostate cancer treatments. Based on Medicare billing codes for each therapy, treatment was defined as the most aggressive therapy first delivered within 9 months following diagnosis and was classified in five categories as 1) prostatectomy (including open and robot-assisted laparoscopic radical prostatectomy, which were both available to patients in each of the 3 groups), 2) IMRT, 3) other radiotherapy (referred to henceforth as ‘other RT’ and including brachytherapy and conformal radiotherapy), 4) ADT or 5) expectant management (if no Medicare codes for definitive treatment were found).15We calculated the proportions of patients receiving treatment in each of the 3 groups in the pre- and post-conversion periods.
We compared changes in the proportions of patients receiving treatments across the 3 patient groups in a differences-in-differences quasi-experimental framework. The unit of analysis was the patient. We estimated a multinomial logit model of patients’ treatment as a function of time period, patient group, and their interaction, adjusting for patient age, race, marital status, SEER-modified National Comprehensive Cancer Network (NCCN) risk group (incorporating Gleason sum, pre-treatment PSA, and tumor stage)18, comorbidity in the 12 months prior to diagnosis19, and census tract median household income. Because IMRT, prostatectomy and other active treatments should be reserved for men with ≥ 10 years life expectancy20, we also evaluated treatment patterns in subgroups based on age (65 – 74 years and ≥ 75 years).
To enhance interpretability, we converted the model output to the probability scale by calculating average partial effects.21 For each of the binary indicators for time period, patient group and their interactions, and for each of the five treatments separately, we used the estimated coefficients to compute the difference between the predicted probabilities of each patient’s receiving that treatment when the covariate took the value 0 and when the covariate took the value 1. We averaged this difference across all patients in the sample, and then bootstrapped the model estimation and average partial effect calculation with 1,000 replicates drawn with replacement to obtain the percentile 95% confidence intervals.22 Statistical modeling was performed using STATA release 11.2 (College Station, TX). The study was approved by the Institutional Review Board.
RESULTS
While patients in the IPCC and state-control groups had largely similar characteristics, patients in the HRR-control group more often were black, had high-risk prostate cancer, and resided in areas with lower median household income (Table 1).
Table 1.
Characteristics of Study Groups
| IPCC | HRR-Control | State-Control | |||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | %) | P value | |
| All patients | 545 | (100) | 279 | (100) | 3,156 | (100) | |
| Time Period | |||||||
| 18 mo pre- | 284 | (52.1) | 140 | (50.2) | 1652 | (52.3) | |
| 18 mo post- | 261 | (47.9) | 139 | (49.8) | 1504 | (47.7) | 0.79 |
| Age at Diagnosis (years) | |||||||
| 65 to 74 | 322 | (59.1) | 160 | (57.3) | 1815 | (57.5) | |
| 75 and older | 223 | (40.9) | 119 | (42.7) | 1341 | (42.5) | 0.27 |
| Race | |||||||
| White | 423 | (77.6) | 181 | (64.9) | 2408 | (76.3) | |
| Non-white* | 122 | (22.4) | 98 | (35.1) | 748 | (23.7) | <0.001 |
| Marital Status | |||||||
| Married | 399 | (73.2) | 191 | (68.5) | 2148 | (68.1) | |
| Not married** | 146 | (26.8) | 88 | (31.5) | 1008 | (31.9) | <0.001 |
| NCCN Risk Group | |||||||
| Low | 157 | (28.8) | 53 | (19) | 918 | (29.1) | |
| Intermediate | 195 | (35.8) | 92 | (33) | 767 | (24.3) | |
| High | 173 | (31.7) | 115 | (41.2) | 1008 | (31.9) | |
| Unknown | 20 | (3.7) | 19 | (6.8) | 463 | (14.7) | <0.001 |
| Comorbidity Index¥ | |||||||
| 0 | 64 | (11.7) | 61 | (21.9) | 436 | (13.8) | |
| 1 | 134 | (24.6) | 69 | (24.7) | 709 | (22.5) | |
| ≥ 2 | 347 | (63.7) | 149 | (53.4) | 2011 | (63.7) | 0.001 |
| Median Household Income in Census Tract of Residence (US$) | |||||||
| 25,000 or less | 147 | (27) | 93 | (33.3) | 713 | (22.6) | |
| > 25,000 to 40,000 | 243 | (44.6) | 124 | (44.4) | 1539 | (48.8) | |
| > 40,000§ | 155 | (28.4) | 62 | (22.2) | 904 | (28.6) | 0.003 |
97% (943/968) of non-white patients in the cohort were Black.
< 9% of patients had unknown race (analyzed as dummy category in regression models but masked in Table 1 per SEER-Medicare confidentiality restrictions as some cell sizes < 11).
Comorbidity score of ≥ 2 represents highest level of comorbidity.
< 1% of patients had unknown median household income (analyzed as dummy category in regression models but masked in Table 1 per SEER-Medicare confidentiality restrictions as some cell sizes < 11).
Unadjusted IMRT use increased in all 3 patient groups (Table 2), but the increase was larger in the IPCC group (from 24% to 46%) and the HRR-control group (16% to 38%) compared to the state-control group (14% to 23%). ADT declined substantially in the IPCC and HRR-control groups but not in the state-control group. Prostatectomy declined in the IPCC group. Brachytherapy use was more common in the state-control group compared to the IPCC group or the HRR-control group (data masked as part of Other RT category per SEER-Medicare confidentiality restrictions).
Table 2.
Unadjusted Treatment Use by Patient Group
| IPCC | HRR-control | State-control | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| IMRT | 23.6 | 45.6 | 15.7 | 38.1 | 14.0 | 22.7 |
| Prostatectomy | 27.5 | 19.5 | 17.1 | 23.0 | 17.3 | 19.3 |
| Androgen-deprivation therapy | 23.9 | 13.4 | 25.7 | 10.8 | 21.0 | 17.3 |
| Other RT | ++ | ++ | ++ | ++ | 28.4 | 21.1 |
| Expectant Management | 16.5 | 15.3 | 33.6 | 25.2 | 19.3 | 19.6 |
| N | 284 | 261 | 140 | 139 | 1,652 | 1,504 |
| P value | <0.001 | <0.001 | <0.001 | |||
For presentation, Other RT category masked per SEER-Medicare confidentiality restrictions (some cell sizes less than 11).
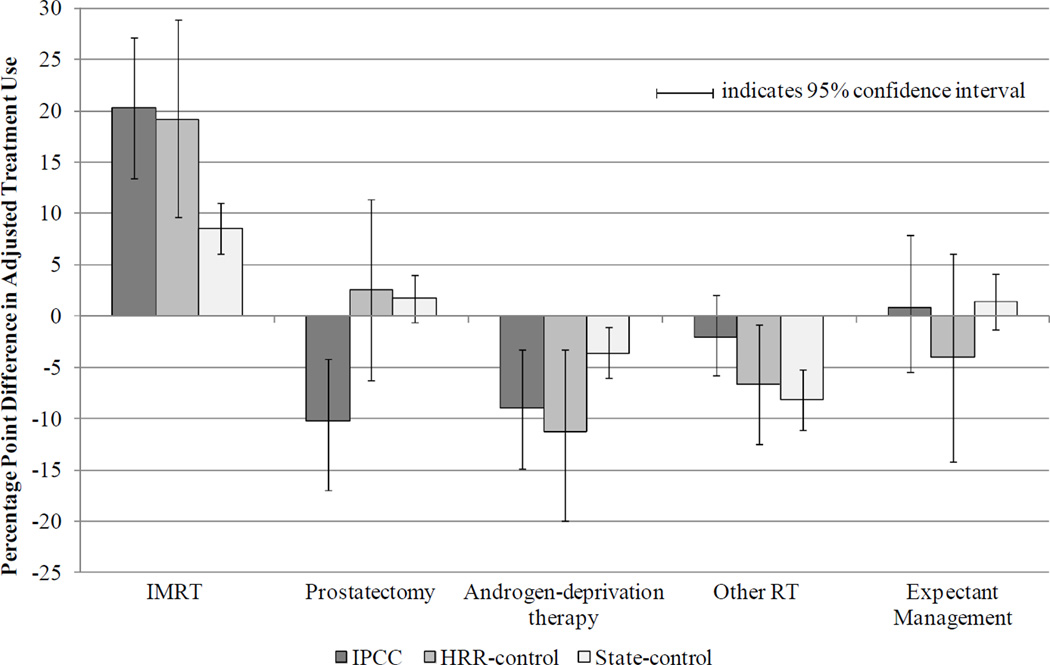
Figure 1 presents adjusted differences in treatment from before to after June 2006 by patient group. Compared with the 8.6 percentage point (ppt) increase in IMRT in the state-control group, IMRT increased 20.3 ppts (95% confidence interval [CI] 13.4, 27.1; net difference 11.7 ppts, 95% CI 3.9, 19.2) in the IPCC group and 19.2 ppts (95% CI 9.6, 28.9; net difference 10.5 ppts, 95% CI 0.9, 20.7) in the HRR-control group. Likewise, compared with a 3.6 ppt decline in ADT in the state-control group, ADT fell 8.9 ppts (95% CI −14.8, −3.2; net difference −5.3 ppts, 95% CI −12.1, 1.3) in the IPCC group and 11.2 ppts (95% CI −19.9, −3.2; net difference −7.5 ppts, 95% CI −16.7, 0.5) in the HRR-control group. Prostatectomy declined significantly in the IPCC group (net difference IPCC vs HRR-control: −12.9 ppts, 95% CI −23.5, −1.9; IPCC vs state-control: −12.0 ppts, 95% CI −19.4, −5.2), while Other RT (predominantly conformal radiotherapy) declined in the HRR- and state-control groups.
Figure 1. Adjusted Differences in Treatment from Before to After July 2006, by Patient Group.
Note: Multinomial logit model of patients’ treatment as a function of time period, patient group, and their interaction, adjusting for patient age, race, marital status, and NCCN risk group (incorporating Gleason sum, pre-treatment PSA, and tumor stage), comorbidity, and census tract median household income. If the 95% confidence interval excludes zero, the adjusted differences between time periods are significant at the < 0.05 level.
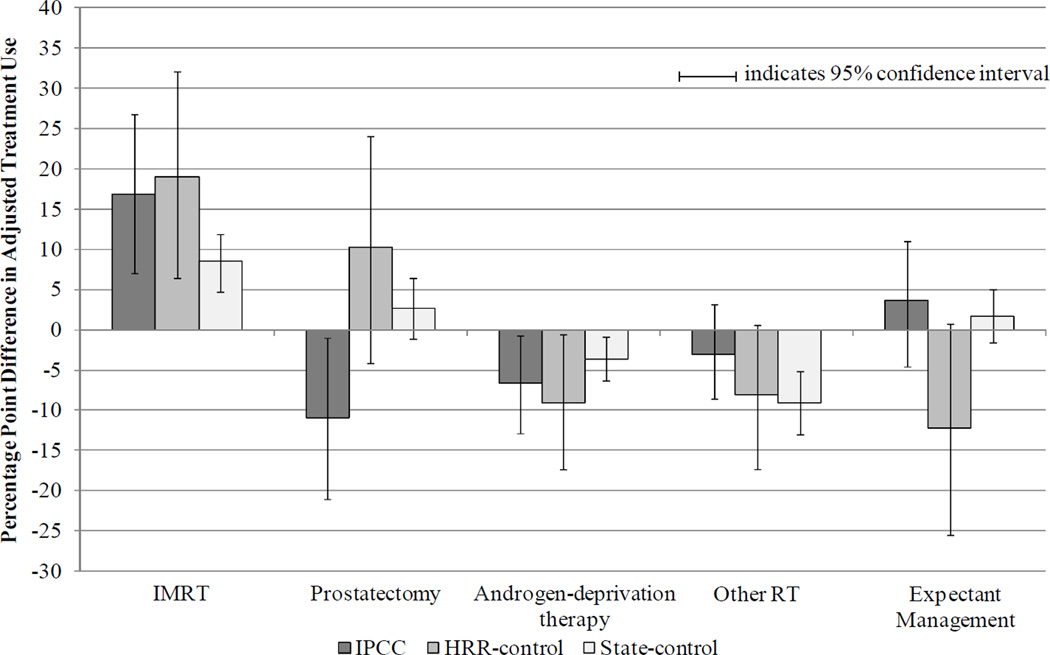
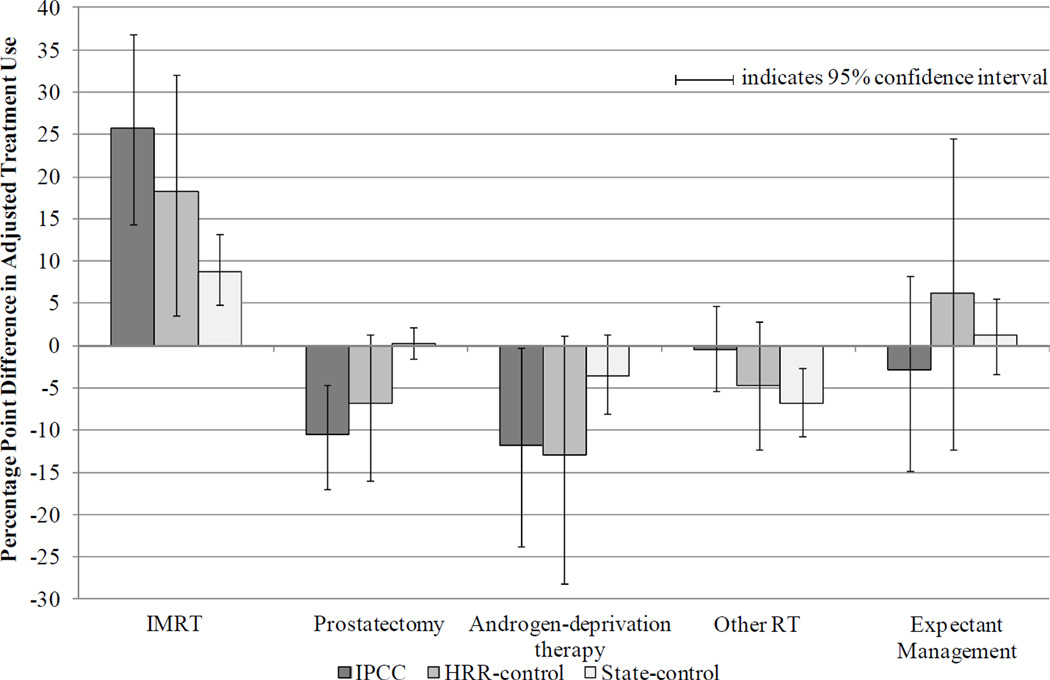
Subgroup analyses by age revealed consistent trends (Figures 2 and 3). Among younger patients age 65 – 74, prostatectomy declined significantly in the IPCC group (net difference IPCC vs HRR-control: −21.1 ppts, 95% CI −38.6, −3.6; IPCC vs state-control: -13.7 ppts, 95% CI −24.3, −2.7). In addition, among younger patients, expectant management declined significantly in the HRR-control group relative to the IPCC group (net difference HRR-control vs IPCC: −15.9 ppts, 95% CI −30.7, −0.6; HRR-control vs state-control: −13.8 ppts, 95% CI −26.8, 0.2).
Figure 2. Adjusted Differences in Treatment Use from Before to After June 2006, by Patient Group (Patients Age 65 – 74 years).
Note: Multinomial logit model of patients’ treatment as a function of time period, patient group, and their interaction, adjusting for patient age, race, marital status, and NCCN risk group (incorporating Gleason sum, pre-treatment PSA, and tumor stage), comorbidity, and census tract median household income. If the 95% confidence interval excludes zero, the adjusted differences between time periods are significant at the < 0.05 level.
Figure 3. Adjusted Differences in Treatment Use from Before to After June 2006, by Patient Group (Patients Age > 75 years).
Note: Multinomial logit model of patients’ treatment as a function of time period, patient group, and their interaction, adjusting for patient age, race, marital status, and NCCN risk group (incorporating Gleason sum, pre-treatment PSA, and tumor stage), comorbidity, and census tract median household income. If the 95% confidence interval excludes zero, the adjusted differences between time periods are significant at the < 0.05 level.
DISCUSSION
We undertook this study to examine the effects of practice integration between urologists and radiation oncologists on treatment patterns for men with prostate cancer. Coincident with the conversion of a urology group practice to an IPCC, we observed increases in IMRT and decreases in ADT among patients seen by IPCC physicians and those seen in the surrounding healthcare market that were not observed in the remainder of the state, where there were no other IPCCs.
One explanation is that practice patterns among all physicians in the IPCC’s healthcare market were more sensitive to IMRT and ADT reimbursement than elsewhere in the state. An alternative explanation is that IPCC formation induced a competitive response among non-IPCC providers in the same market, resulting in greater adoption of IMRT (at the expense of other radiotherapy modalities and ADT). This would be plausible, for example, if non-IPCC radiation oncologists more actively courted referrals from urologists in the HRR as ADT use declined – and would be consistent with evidence of a broader medical arms race and the related competition between specialties to treat similar patients.23,24 However, in this claims-based observational study, it is not possible to verify either of these explanations. Nonetheless, while there are few normative standards of care for prostate cancer treatment, both explanations underscore the concern that, irrespective of IPCC affiliation, financial considerations influence prostate cancer treatment decisions, particularly in some healthcare markets.25
Moreover, among patients seen by IPCC physicians, and unlike patients seen by other physicians, the increase in IMRT after IPCC conversion was partly offset by a decline in prostatectomy. While physician ownership of expensive medical services may to some extent explain the increase in IMRT at the expense of prostatectomy,14 patient preferences also play an important role in prostate cancer treatment decisions. We cannot know from our claims-based study to what extent clinical criteria, patient preferences, physicians’ financial incentives, or other unmeasured factors contributed to an overall decline in surgical management among patients seen by IPCC physicians. Yet, a contraction in treatments considered equally effective but differentiated by their toxicity profile raises concerns about the extent to which patients are offered all available options to treat their cancer. Understanding how treatment decisions are made is an important next step in disentangling the effects of IPCCs, and economic incentives more generally, on prostate cancer treatment patterns.
This study has limitations. First, we cannot draw causal inferences about the effects of urology practice integration on prostate cancer treatment patterns from this observational study. Second, we do not know the profit sharing arrangement between urologists and radiation oncologists at the IPCC under study. Some have contended that economic incentives may differ between urology groups that employ radiation oncologists relative to those in which urologists and radiation oncologists partner and share profits.10 However, in both settings, urologists have a financial incentive to refer patients for IMRT, and radiation oncologists’ compensation remains tied to treatment.10 Third, certain treatments and subgroup analyses had small patient numbers which limit generalizability. Fourth, while at least 37 IPCCs have been established in 16 states, available SEER-Medicare program data limit us to studying the impact of a single urology practice in a single state and to men ≥ 65 years with Medicare coverage (a population that comprises over two-thirds of all men who receive radiation for prostate cancer).
CONCLUSION
Coincident with the conversion of a urology group practice to an IPCC, we observed increases in IMRT and decreases in ADT among patients seen by IPCC physicians and those seen in the surrounding healthcare market that were not observed in the remainder of the state. Prostatectomy declined significantly in the IPCC group. Our findings suggest that multi-specialty consolidation of prostate cancer care without accompanying payment reform may be associated with market-wide changes in practice patterns.
Acknowledgment
This study used the linked Surveillance, Epidemiology, and End Results (SEER)–Medicare database. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute (NCI), the Office of Research, Development and Information, Centers for Medicare & Medicaid Services (CMS), Information Management Services (IMS), Inc., and the SEER Program tumor registries in the creation of the SEER–Medicare database. We thank Robert Sunderland, MS, for programming assistance and Alexandra Lipschultz, BA, for research assistance.
Funding/Support: This study was supported by National Cancer Institute K07-CA163616 (Bekelman) and K07-CA151910 (Pollack).
Role of the Sponsors: The funding agencies did not participate in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr. Bekelman had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bekelman, Suneja, Armstrong, Epstein
Acquisition of data: Bekelman, Suneja
Drafting of the manuscript: Bekelman, Epstein
Critical revision of the manuscript for important intellectual content: Bekelman, Suneja, Guzzo, Pollack, Armstrong, Epstein
Statistical analysis: Epstein, Bekelman
Obtained funding: Bekelman
Administrative, technical, or material support: Bekelman
Study supervision: Bekelman, Epstein
Financial Disclosures: None
Publisher's Disclaimer: Disclaimer: The interpretation and reporting of these data are the sole responsibility of the authors.
REFERENCES
- 1.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, Brown ML. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008 May 7;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto A, Yabroff K, Shao Y, Feuer E, Brown M. Projections of the Cost of Cancer Care in the United States: 2010–2020. JNCI Journal of the National Cancer Institute. 2011;103(2) doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonhardt D. In Health Reform, a Cancer Offers an Acid Test. New York Times. 2009 Jul 7; [Google Scholar]
- 4.Nguyen PL, Gu X, Lipsitz SR, Choueiri TK, Choi WW, Lei Y, Hoffman KE, Hu JC. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011 Apr 20;29(12):1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, Lotan Y. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol. Mar;57(3):453–458. doi: 10.1016/j.eururo.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Management options for low-risk prostate cancer: A report on comparative effectiveness and value. Institute for Clinical and Economic Review; 2009. [Last accessed January 5, 2011]. At www.icer-review.org. [Google Scholar]
- 7.Carreyrou J, Tamman M. A device to kill cancer, lift revenue. [Accessed January 3, 2011];2010 http://online.wsj.com/article/SB10001424052748703904804575631222900534954.html.
- 8.Saul S. Profit and Questions on Prostate Cancer Therapy. New York Times. 2006 [PubMed] [Google Scholar]
- 9.Kapoor DA, Zimberg SH, Ohrin LM, Underwood W, 3rd, Olsson CA. Utilization trends in prostate cancer therapy. The Journal of urology. 2011 Sep;186(3):860–864. doi: 10.1016/j.juro.2011.04.075. [DOI] [PubMed] [Google Scholar]
- 10.Falit BP, Gross CP, Roberts KB. Integrated prostate cancer centers and over-utilization of IMRT: a close look at fee-for-service medicine in radiation oncology. International journal of radiation oncology, biology, physics. 2010 Apr;76(5):1285–1288. doi: 10.1016/j.ijrobp.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 11.Services provided under the in-office ancillary exception to the physician self-referral law. [Accessed December 1, 2011];Medicare Payment Advisory Commission Public Meeting Transcript. 2010 Mar 4 and 5;:223–257. Available at http://www.medpac.gov/transcripts/0304-05MedPAC.final.pdf.
- 12. [Last accessed December 1, 2011];Letter from Representatives Henry Waxman, Sandy Levin, and Pete Stark to Government Accounting Office requesting study of physician self-referral for radiation oncology. services at http://www.auanet.org/content/media/Congressional%20Request_GAO%20Study_Imaging%20.pdf.
- 13.Hollenbeck B, Nallamothu B. Financial Incentives and the Art of Payment Reform. JAMA. 2011;306(18):2028–2030. doi: 10.1001/jama.2011.1630. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell JM, Sunshine JH. Consequences of physicians' ownership of health care facilities--joint ventures in radiation therapy. N Engl J Med. 1992 Nov 19;327(21):1497–1501. doi: 10.1056/NEJM199211193272106. [DOI] [PubMed] [Google Scholar]
- 15.Higher Use of Advanced Imaging Services by Providers Who Self-Refer Costing Medicare Millions. Washington DC: United States Government Accountability Office; 2012. [Accessed January 22, 2013]. at http://democrats.energycommerce.house.gov/sites/default/files/documents/GAO-Medicare-Self-Refer-Report-2012-10-31.pdf. [Google Scholar]
- 16.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010 Nov 4;363(19):1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 17.Wennberg J, Gittelsohn Small area variations in health care delivery. Science. 1973 Dec 14;182(117):1102–1108. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 18.Swisher-McClure SD, Sunderland R, Christodouleas J, Guzzo T, Haas N, Vapiwala N, Bekelman J. Variation in Use of Androgen Suppression with External Beam Radiotherapy for Non-metastatic Prostate Cancer. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.06.1951. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris D, Coffey R. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.The Complete Library of NCCN Clinical Practice Guidelines in Oncology. Jenkintown, Pennsylvania: ©National Comprehensive Cancer Network; 2011. The NCCN Prostate Cancer Clinical Practice Guidelines in Oncology (Version 2.2011) [Google Scholar]
- 21.Wooldridge J. Econometric analysis of cross section and panel data. Cambridge, MA: 2010. [Google Scholar]
- 22.Kleinman L, Norton E. What’s the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health services research. 2009;44(1):288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berenson R, Bodenheimer T, Pham H. Specialty-service lines: salvos in the new medical arms race. Health Affairs. 2006;25:w337–w343. doi: 10.1377/hlthaff.25.w337. [DOI] [PubMed] [Google Scholar]
- 24.Huckman R, Pisano G. Turf battles in coronary revascularization. New England Journal of Medicine. 2005;352(9):857–859. doi: 10.1056/NEJMp048242. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs BL, Zhang Y, Skolarus TA, Hollenbeck BK. Growth of high-cost intensity-modulated radiotherapy for prostate cancer raises concerns about overuse. Health Aff (Millwood) 2012 Apr;31(4):750–759. doi: 10.1377/hlthaff.2011.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]