Abstract
D8/17, an alloantigen found on B lymphocytes, has been reported to be elevated in patients susceptible to rheumatic fever and may be associated with autoimmune types of neuropsychiatric disorders. The pediatric-autoimmune-neuropsychiatric-disorders-associated-with-streptococci model is a putative model of pathogenesis for a group of children whose symptoms of obsessive-compulsive disorder and Tourette's disorder (TD) are abrupt and may be triggered by an infection with group A streptococci. As a test of this model, we have examined D8/17 levels on the B cells of patients with TD and acute rheumatic fever (ARF) along with those on the B cells of normal controls by flow cytometry. We have utilized several different preparations of D8/17 antibody along with a variety of secondary antibodies but have been unable to show an association with an elevated percentage of D8/17-positive, CD19-positive B cells in either ARF or TD. We did find, however, that the percentages of CD19-positive B cells in ARF and TD patients were significantly elevated compared to those in normal controls. Group A streptococcal pharyngitis patients also had an elevated percentage of CD19 B cells, however. These studies failed to confirm the utility of determining the percentage of B cells expressing the D8/17 alloantigen in ARF patients or our sample of TD patients. In contrast, the percentage of CD19-positive B cells was significantly elevated in ARF and TD patients, as well as group A streptococcal pharyngitis patients, suggesting a role for inflammation and/or autoimmunity in the pathogenesis of these disorders.
D8/17 is an alloantigen found on B lymphocytes and has been reported to be elevated in patients with acute rheumatic fever (ARF) (7, 8, 11). It is detected using a murine monoclonal antibody, derived by immunization with isolated B lymphocytes obtained from patients with rheumatic fever or rheumatic heart disease (4, 20), and has been reported to be expressed on elevated percentages of B cells in over 90% of rheumatic fever patients (11). This has led to the hypothesis that D8/17 may be a susceptibility marker for the development of rheumatic fever. D8/17 expression on B cells has also been reported to be elevated in patients with pediatric autoimmune neuropsychiatric disorders associated with streptococci (PANDAS) (17, 19). The PANDAS model was proposed by Swedo et al. (19) for children whose symptoms of obsessive-compulsive disorder appear abruptly and are triggered by an infection with group A beta-hemolytic streptococci similar to that of Sydenham chorea, a manifestation of ARF. These authors reported that the D8/17 marker was expressed on elevated percentages of B cells in 85% of PANDAS patients and 89% of Sydenham chorea patients compared to 17% of healthy controls. Subsequently, Murphy et al. (17) indicated that patients with obsessive-compulsive disorder and/or Tourette's disorder (TD) had a mean percentage of D8/17-positive B cells of 22% but that in control subjects the mean percentage of D8/17-positive B cells was 9%. However, more recently, a study reported failure to replicate the association between an elevated level of D8/17 expression on B cells and PANDAS (10).
Elevated percentages of B cells have been reported in a number of autoimmune diseases, and this condition has been shown to be related to genetic quantitative trait loci (5, 13).
In the present study, we have examined D8/17 expression as a percentage of the total number of lymphocytes expressing D8/17 and as a percentage of CD19-positive B cells expressing D8/17 in patients with ARF and TD (as a test of the PANDAS model) and have compared these results with those for normal controls. In addition, we have quantitated the percentages of total CD19-positive B cells in controls and the two patient groups, along with a second control group of patients with group A streptococcal pharyngitis. In contrast to the results in several previous reports, we could not document elevated percentages of D8/17-positive B cells in patients with ARF and TD compared to those in controls but we did find higher percentages of CD19-positive B cells in each patient group.
MATERIALS AND METHODS
Sample collection.
The levels of D8/17 and CD19 expression on B cells were determined for 24 ARF patients, 33 TD patients, 17 streptococcal pharyngitis patients, and 98 unaffected controls. University of Utah Institutional Review Board-approved consent was obtained for all individuals. ARF was confirmed by the Jones criteria. TD subjects were recruited from an ongoing family genetics study. TD cases were required to meet diagnostic criteria specified in the Diagnostic and Statistical Manual (fourth edition [text revision]) of the American Psychiatric Association (1) and the Yale Global Tic Severity Scale (14). While other childhood psychiatric disorders (such as anorexia nervosa [18] and autism [9]) have been reported to be associated with elevated D8/17 expression, only subjects with TD were included in our sample. Patients with pharyngitis had culture-documented group A streptococcal throat infections at the time of blood collection. The controls were normal individuals without a history of rheumatic fever or tics. All controls reported being healthy at the time of the blood draw. Blood was drawn in anticoagulant-citrate-dextrose solution A or B Vacutainer tubes (Becton Dickinson, San Jose, Calif.).
D8/17 staining protocol.
Staining was performed according to the methodology first described by Chapman et al. (4) by adding 100 μl of D8/17 monoclonal antibody to 300 μl of whole blood and incubating for 1 h at 4°C. The D8/17 monoclonal antibody was originally supplied to us by John Zabriskie of the Rockefeller University. The concentration of this exact antibody was not specified, but we used it at a final dilution of 1:8, which we found to be optimal. We later used a commercial preparation from Goodwin Biotechnology Incorporated (Plantation, Fla.) at a concentration of 7.5 μg/ml, again found to be optimal. Once we discovered that this preparation actually had a very low antibody content, we obtained the original D8/17 cell line from the American Type Culture Collection (ATCC; Rockville, Md.). This cell line was maintained in our laboratory at 37°C with 5% CO2 by using RPMI 1640 (BioWhittaker, Walkersville, Md.) containing 10% fetal bovine serum (Atlanta Biologicals, Norcross, Ga.), 1% gentamicin (BioWhittaker), and 4 mM l-glutamine (BioWhittaker)/ml. Supernatant from this cell line was found to have 30 μg of immunoglobulin M (IgM) antibody/ml. When we tested the supernatant against the positive (VWB) and negative (LG2) cell lines, we determined that a concentration of 3 μg/ml worked most consistently. Mouse IgM (Dako, Carpinteria, Calif.) was used in place of the D8/17 antibody as the negative control at the same concentration as the D8/17 monoclonal antibody. Following incubation, 3 ml of phosphate-buffered saline with 0.5% bovine serum albumin and 0.1% NaN3 was added to the sample, which was then centrifuged for 5 min at 200 × g. Forty microliters of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgM (Caltag, Burlingame, Calif.), diluted to a concentration of 70 μg/ml, was added to the pellet, mixed, incubated for 30 min at 4°C, and then washed as before. Forty microliters of phycoerythrin-labeled CD19 antibody (Becton Dickinson), diluted to a concentration of 26 μg/ml, was then added to the pellet, mixed, and incubated for 20 min at 4°C. Following the third incubation, the red blood cells were lysed with 3 ml of Becton Dickinson fluorescence-activated cell sorter lysing solution at a dilution of 1:10. The samples were incubated at room temperature for 5 min and then centrifuged for 5 min at 200 × g. The cell pellet was washed as before to remove any residual red blood cells. The samples were resuspended in 300 μl of phosphate-buffered saline and analyzed within 2 h by flow cytometry (4).
Flow cytometry.
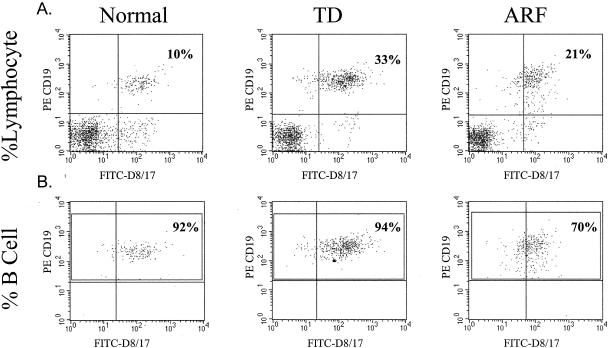
We employed the flow cytometric method of Chapman et al. (4) to determine the level of D8/17 expression on B cells. The samples were analyzed on a Becton Dickinson FACScan using Cellquest software. Five thousand lymphocytes were gated using a forward-scatter and side-scatter dot plot. From this, a second dot plot of FL1 (D8/17-FITC) and FL2 (phycoerythrin-CD19) with quadrants was used to analyze the cells. Double-staining CD19-positive B cells in the upper right quadrant were considered positive for D8/17. This dot plot was used to calculate the percentage of total lymphocytes staining for both CD19 and D8/17 (Fig. 1A) as well as the percentage of total lymphocytes expressing CD19. To calculate the percentage of CD19-positive B cells expressing D8/17, we gated the upper two quadrants of the FL1-FL2 dot plot to encompass only the CD19-positive B cells (Fig. 1B). We also gated monocyte and neutrophil populations, and neither expressed D8/17 or CD19 as expected.
FIG. 1.
Percentage of total lymphocytes expressing D8/17 versus percentage of CD19-positive B cells expressing D8/17. Flow cytometry histograms for a normal control, a TD patient, and an ARF patient show the different results obtained by analyzing the percentage of total lymphocytes expressing D8/17 (A) and the percentage of CD19-positive B cells expressing D8/17 (B). D8/17 antibody was from Goodwin (7.5 μg/ml). PE, phycoerythrin.
Streptococcal antibody measurement.
Throat swabs and sera were also obtained from some of the ARF and TD subjects at the time blood was drawn for the D8/17 assay. Throat swabs were plated onto 5% Columbia sheep's blood agar and incubated for 24 to 48 h at 37°C. Anti-streptolysin O (ASO) titers were measured using the Colorcard ASO kit from Wampole (Cranbury, N.J.). Anti-DNase B titers were measured using the Streptonase-B kit, also from Wampole. Instructions included with each kit were followed. ASO titers of ≥200 IU/ml were considered to be elevated, while an anti-DNase B titer of ≥1:170 for subjects ages 6 to 17 and a titer of ≥1:60 for subjects under 6 years of age were considered to be elevated. The normal values were obtained from ARUP Laboratories, the University of Utah exoteric reference laboratory.
Statistical analysis.
Statistical analysis was performed using the Student t test. Analysis of variance was also performed on all comparisons and resulted in essentially the same statistical significance. Given the variability of the results with each of the monoclonal D8/17 antibodies and the two methods of calculating the level of D8/17 expression (as a percentage of the total number of lymphocytes expressing D8/17 or as a percentage of CD19-positive B cells expressing D8/17), we were unable to arrive at a figure to be used as a cutoff for positive patients or controls.
RESULTS
Initially, we analyzed the samples for the percentage of total lymphocytes expressing both the D8/17 and CD19 antigens. Employing this method of calculation and monoclonal antibodies obtained from both Goodwin and Zabriskie, we obtained highly significant results for the patient groups and controls (Table 1). The percentages were very comparable to what has been reported before in the literature (8, 11, 16, 17, 19). We then analyzed the data to determine the percentage of CD19-positive B cells expressing the D8/17 marker by using the flow cytometry method as originally described (4). When the level of D8/17 expression as a percentage of CD19-positive B cells expressing D8/17 was calculated, all three subject groups had much higher percentages of B cells expressing the D8/17 antigen. There was no significant difference, however, between the controls and either patient group (Table 1).
TABLE 1.
Comparison of levels of D8/17 expression found in ARF and TD patients and normal control subjects by using D8/17 monoclonal antibody preparations from three different sources
| Group | % of total lymphocytes expressing D8/17 as determined with D8/17 monoclonal antibody froma:
|
% of CD19-positive B cells expressing D8/17 as determined with D8/17 monoclonal antibody froma:
|
||||
|---|---|---|---|---|---|---|
| Zabriskieb | Goodwinc | ATCCd | Zabriskieb | Goodwinc | ATCCd | |
| Controls | 8.57 ± 2.99 (36) | 9.03 ± 2.14 (42) | 1.23 ± 0.79 (16) | 83.78 ± 15.28 (36) | 73.38 ± 17.19 (40) | 8.04 ± 4.55 (16) |
| ARF patients | 15.53 ± 5.93 (7; 0.02) | 16.79 ± 2.68 (5; 0.002) | 1.09 ± 0.87 (10; 0.69) | 87.89 ± 8.11 (8; 0.31) | 77.43 ± 5.47 (4; 0.32) | 8.50 ± 3.55 (10; 0.77) |
| TD patients | 12.40 ± 4.31 (22; 0.0008) | 17.59 ± 4.17 (6; 0.004) | ND | 76.92 ± 12.31 (23; 0.07) | 83.89 ± 10.36 (8; 0.06) | ND |
Values are means ± standard deviations. Values in parentheses are n values (where two values appear in parentheses, the second value is the P value [for comparison with controls]). ND, not done.
Unspecified concentration; diluted 1:8.
Concentration, 7.5 μg/ml.
Concentration, 3 μg/ml.
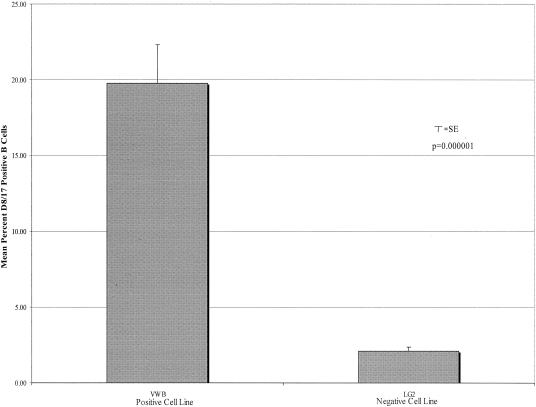
The much higher percentage of D8/17-positive B cells observed with the Goodwin and Zabriskie monoclonal antibodies led us to obtain the original D8/17 cell lines from the ATCC. Using supernatant from the D8/17 cell line grown in our laboratory, we have documented a high percentage of D8/17-positive cells in the VWB positive cell line (mean ± standard deviation [SD], 19.79% ± 10.93%; n = 19). In contrast, the percentage of D8/17-positive cells in the negative LG2 cell line was much lower (mean ± SD, 2.09% ± 1.24%; P = 0.000001; n = 19) (Fig. 2). Employing supernatants from this ATCC D8/17 cell line, however, we were still unable to document differences in the percentages of CD19-positive B cells expressing D8/17 between ARF patients and controls (Table 1).
FIG. 2.
Percentage of cells in positive (VWB) cell line versus percentage of cells in negative (LG2) cell line expressing D8/17 antigen. D8/17 antibody was from the ATCC (3 μg/ml). Bar graphs compare the mean percentages of D8/17-positive B cells in the VWB cell line (mean ± SD, 19.79% ± 10.93%; n = 19) and the LG2 cell line (mean ± SD, 2.09% ± 1.24%; n = 19; P = 0.000001). SE, standard error.
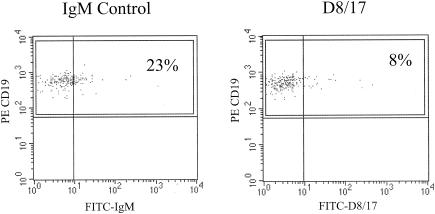
We had difficulties with other reagents used in the assay besides the D8/17 antibody. We had to switch from a mouse IgM control from Sigma (St. Louis, Mo.) to one from Caltag because of unreliable results. In later experiments, we obtained higher values with the Caltag mouse IgM negative control (concentration, 3 μg/ml) than with the ATCC D8/17 monoclonal antibody (concentration, 3 μg/ml) (Fig. 3). For this reason, we settled on a mouse IgM negative control from Dako (3 μg/ml), which has given us the most reliable results. We also tried several different secondary FITC-conjugated antibodies before settling on one from Caltag utilized at 70 μg/ml.
FIG. 3.
Flow cytometry histograms comparing mouse IgM negative control (3 μg/ml; Caltag) and D8/17 monoclonal antibody (3 μg/ml; ATCC) expression on CD19-positive B cells from the same subject. PE, phycoerythrin.
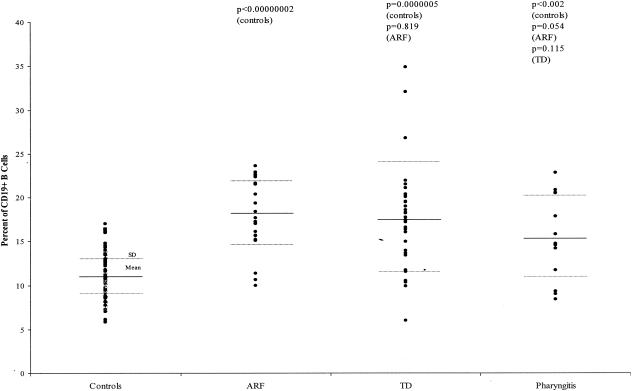
Since the percentages of B cells expressing D8/17 were so high in the normal subjects and ARF and TD patients, even though the differences between the groups were not significant, we decided to look more closely at the total populations of CD19-positive B cells in these groups. When we reanalyzed the data to determine simply the percentages of CD19-positive B cells in both patient groups and controls, we found highly significant increases in the percentages of CD19-positive B cells in both the ARF and TD patients compared to those in controls. Eighty-nine normal controls had a mean percentage of CD19-positive B cells of 11.06% (SD, ±2.52%), while 23 ARF patients had a mean percentage of 18.01% (SD, ±3.99%; P, <0.00000002 against normal controls). Thirty-three TD patients had a mean percentage of CD19-positive B cells of 17.70% (SD, ±5.95%; P, 0.0000005 against normal controls and 0.819 against ARF patients) (Fig. 4). Thus, both patient groups had highly significant increases in the percentages of CD19-positive B cells compared to normal, healthy controls. We then looked at a group of patients presenting with only documented group A streptococcal throat infections to determine whether the increase in CD19-positive B cells was related to ARF or TD or whether this was a nonspecific occurrence related to inflammation. Seventeen pharyngitis patients had a mean percentage of CD19-positive B cells of 15.25% (SD, ±4.38%; P, <0.002 against normal controls, 0.054 against ARF patients, and 0.115 against TD patients) (Fig. 4).
FIG. 4.
Percentage of total lymphocytes expressing the CD19 B cell marker in ARF, TD, and pharyngitis patients and controls. The dot plot shows the differences among 89 controls (mean ± SD, 11.106% ± 2.52%), 23 ARF patients (mean ± SD, 18.01% ± 3.99%; P, <0.00000002 against normal controls), 33 TD patients (mean ± SD, 17.70% ± 5.95%; P, 0.0000005 against normal controls and 0.819 against ARF patients), and 17 pharyngitis patients (mean ± SD, 15.25% ± 4.38%; P, <0.002 against normal controls, 0.054 against ARF patients, and 0.115 against TD patients).
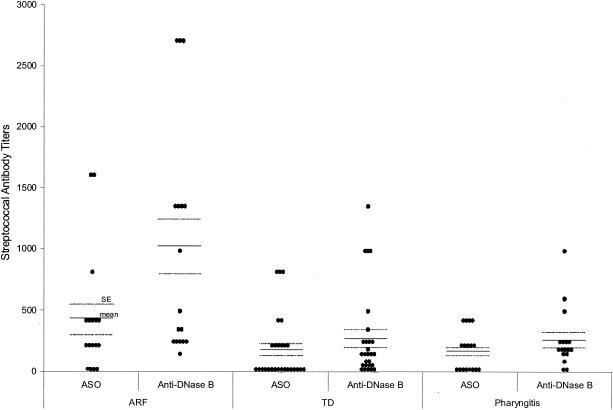
At the time of the D8/17 assay, 11 of the ARF subjects had throat swabs taken, all of which were negative. Seventeen had ASO and anti-DNase B titers measured. Ninety-four percent of these subjects had either an elevated ASO titer or an elevated anti-DNase B titer; 71% had elevated titers of both antibodies. At the time that the level of D8/17 was measured, 16 TD subjects had throat swabs taken and all swabs were negative. Twenty-three subjects had ASO and anti-DNase B titers measured. Seventy-four percent had either an elevated ASO titer or an elevated anti-DNase B titer; 26% had elevated titers of both antibodies. Thus, most of the ARF and TD patients had cultural or serological evidence of a recent group A streptococcal infection. Ninety-four percent of pharyngitis subjects had either an elevated ASO titer or an elevated anti-DNase B titer; 35% had elevated titers of both antibodies. When tested again 4 weeks later, 77% showed an increase in either their ASO or anti-DNase B titers. As shown in Fig. 5, the mean ASO and anti-DNase B titers in the ARF patients (412 ± 119 IU/ml [ASO] and 1,002 ± 228 [anti-DNase B]) were higher than the mean titers in TD patients (163 ± 50 IU/ml [ASO] and 267 ± 70 [anti-DNase B]) and acute pharyngitis patients (153 ± 40 IU/ml [ASO] and 250 ± 59 [anti-DNase B]). Thus, these titers paralleled the elevation in CD19-positive-B cell numbers (Fig. 4), suggesting that this response may be a nonspecific one related to a preceding streptococcal infection.
FIG. 5.
ASO and anti-DNase B titers in ARF, TD, and pharyngitis patients. The dot plot shows the differences in titers among 17 ARF patients (mean ASO titer, 412 ± 119 IU/ml; mean anti-DNase B titer, 1,002 ± 228), 27 TD patients (mean ASO titer, 163 ± 50 IU/ml [P, <0.07 against ARF patients]; mean anti-DNase B titer, 267 ± 70 [P, 0.006 against ARF patients]), and 17 pharyngitis patients (mean ASO titer, 153 ± 40 IU/ml [P, 0.054 against ARF patients and 0.877 against TD patients]; mean anti-DNase B titer, 250 ± 59 [P, 0.005 against ARF patients and 0.859 against TD patients]).
DISCUSSION
In our laboratory, we have been unable to reproduce the published D8/17 flow cytometric data for ARF patients. The commercial D8/17 antibody purchased was found to have very little antibody content, while other D8/17 antibody preparations, including ours from the original D8/17 cell line, failed to identify an increased percentage of D8/17-positive B cells in our ARF patients. Even employing supernatant obtained from the original D8/17 cell line grown in our laboratory, we were unable to find a significantly higher percentage of CD19-positive B cells expressing the D8/17 marker in patients with ARF. One possible reason for the problems we have had in reproducing the original published D8/17 data may be that the D8/17 monoclonal antibody has changed. The D8/17 alloantigen was originally discovered using the serum from one multiparous woman from Bogota, Colombia. Since this serum supply was eventually exhausted, the D8/17 monoclonal antibody was prepared by immunizing mice with the B cells of multiple ARF patients who were originally determined to be positive for the alloantigen by using this woman's serum (20). To our knowledge, there is no data confirming the identity of the alloantigen recognized by the woman's serum with the D8/17 monoclonal antibody, although the patients' cells did react with the monoclonal antibody. Because we had such difficulties with our first two monoclonal antibodies, we obtained the original D8/17 ATCC cell line. We did not find significant differences between ARF patients and controls with supernatant from this cell line, even though the antibody performed adequately against the positive and negative control cell lines. Other laboratories have also noted difficulty in standardizing the D8/17 assay, particularly in developing standardized controls, antibody dilutions, and reagents (6, 10, 15, 16). The exact nature of the alloantigen recognized by the D8/17 antibody has not been adequately defined, although Carreno-Manjarrez et al. (3) have reported that it reacts with a 95-kDa protein on Western blots. The antigen, to our knowledge, is not available to determine the specificity of our antibodies. In fact, Kumar and colleagues (12) have developed three monoclonal antibodies by immunizing mice with B cells from patients from northern India with rheumatic heart disease. They report that these antibodies have different specificities than the D8/17 monoclonal antibody. Thus, we are unable to define the specificity of our three D8/17 preparations, but all were derived from the same initial hybridoma cell lines deposited by Zabriskie in the ATCC.
Initially, we were very encouraged by our results when we analyzed D8/17 expression as a percentage of total lymphocytes expressing D8/17, since the actual numbers seemed to agree with those published in the literature (4, 17, 19) and yielded highly significant differences between controls and patient groups (J. C. Moore, N. H. Augustine, H. R. Hill, and W. M. McMahon, Abstr. J. Investig. Med., abstr. 49:49A, 2001). Although our histograms looked very similar to those published in the literature, others were reporting their results as the percentage of D8/17-positive B cells, even though they seemed in many cases to be expressing their data as the percentage of D8/17-positive lymphocytes (7). Once our data were reanalyzed to determine the percentage of D8/17-positive, CD19-positive B cells, we could find no significant difference between patient groups and controls.
These results led us to explore the possibility that the differences we were seeing in the percentages of D8/17-positive lymphocytes were actually due to increased numbers of CD19-positive B cells in our patient populations. We went back to our original histograms and determined percentages of CD19-positive B cells in our patient populations and controls. We found that there were highly significant differences in the percentages of CD19-positive B cells in ARF and TD patients compared to those in controls. We believe that these results may shed some light on the discrepancies we and others have noted in results of the flow cytometric D8/17 assay. It is possible that ARF and TD patients do not actually have an increase in the percentage of B cells expressing the D8/17 antigen but that rather they simply have an increase in the number of CD19-positive B cells in their peripheral blood, as has been described in other autoimmune disorders such as rheumatoid arthritis (13) and even, in one report, in ARF (2). The fact that most of our ARF and TD patients had serological evidence of a recent group A streptococcal infection and that patients with acute streptococcal pharyngitis also had elevated percentages in their peripheral blood suggests that this is a nonspecific phenomenon related to infection or postinfection inflammatory responses. Although CD19-positive B cells were measured acutely in only the pharyngitis patients, one may expect that the percentages of these cells would increase even more several weeks out from the infection, like those in ARF and/or TD patients, since the CD19-positive-B cell numbers shown in Fig. 4 paralleled the elevations in ASO and anti-DNase B titers shown in Fig. 5. We are currently attempting to monitor the patients in each group serially for several months, which we hope will give us a better indication of how long this immunological response may last.
Acknowledgments
This research was supported in part by U.S. Public Health Service grant MH58868 and NIAID contract NO1-AO-2274.
We thank John Zabriskie for his antibody and technical advice.
REFERENCES
- 1.American Psychiatric Association. 2000. Diagnostic and statistical manual of mental disorders, 4th ed. (text revision). American Psychiatric Association, Washington, D.C.
- 2.Bhatia, R., J. Narula, K. S. Reddy, M. Koicha, A. N. Malaviya, R. B. Pothineni, R. Tandon, and M. L. Bhatia. 1989. Lymphocyte subsets in acute rheumatic fever and rheumatic heart disease. Clin. Cardiol. 12:34-38. [DOI] [PubMed] [Google Scholar]
- 3.Carreno-Manjarrez, R., A. Viteri-Jackson, K. Visvanathan, and J. B. Zabriskie. 2000. Characterization of B-cell marker D8/17, p. 529-532. In D. R. Martin and J. R. Tagg (ed.), Streptococci and streptococcal diseases entering the new millennium. Proceedings of the XIV Lancefield International Symposium on Streptococci and Streptococcal Diseases. SecuraCopy, Auckland, New Zealand.
- 4.Chapman, F., K. Visvanathan, R. Carreno-Manjarrez, and J. B. Zabriskie. 1998. A flow cytometric assay for D8/17 B cell marker in patients with Tourette's syndrome and obsessive compulsive disorder. J. Immunol. Methods 219:181-186. [DOI] [PubMed] [Google Scholar]
- 5.Hall, M. A., P. J. Norman, B. Thiel, H. Tiwari, A. Peiffer, R. W. Vaughan, S. Prescott, M. Leppert, N. J. Schork, and J. S. Lanchbury. 2002. Quantitative-trait loci on chromosomes 1, 2, 3, 4, 8, 9, 11, 12 and 18 control variation in levels of T and B lymphocyte subpopulations. Am. J. Hum. Genet. 70:1172-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton, C. S., M. A. Garvey, and S. E. Swedo. 2003. Sensitivity of the D8/17 assay. Am. J. Psychiatry 160:1193-1194. [DOI] [PubMed] [Google Scholar]
- 7.Harel, L., A. Zeharia, Y. Kodman, R. Straussberg, J. B. Zabriskie, and J. Amir. 2002. Presence of the D8/17 B-cell marker in children with rheumatic fever in Israel. Clin. Genet. 61:293-298. [DOI] [PubMed] [Google Scholar]
- 8.Herdy, G. V., J. B. Zabriskie, F. Chapman, A. Khanna, and S. Swedo. 1992. A rapid test for the detection of a B-cell marker (D8/17) in rheumatic fever patients. Braz. J. Med. Biol. Res. 25:789-794. [PubMed] [Google Scholar]
- 9.Hollander, E., G. DelGiudice-Asch, L. Simon, J. Schmeidler, C. Cartwright, C. M. DeCaria, J. Kwon, C. Cunningham-Rundles, F. Chapman, and J. B. Zabriskie. 1999. B lymphocyte antigen D8/17 and repetitive behaviors in autism. Am. J. Psychiatry 156:317-320. [DOI] [PubMed] [Google Scholar]
- 10.Inoff-Germain, G., R. S. Rodriguez, S. Torres-Alcantara, M. J. Diaz-Jimenez, S. E. Swedo, and J. L. Rapoport. 2003. An immunological marker (D8/17) associated with rheumatic fever as a predictor of childhood psychiatric disorders in a community sample. J. Child Psychol. Psychiatry 44:782-790. [DOI] [PubMed] [Google Scholar]
- 11.Khanna, A. K., D. R. Buskirk, R. C. Williams, Jr., A. Gibofsky, M. K. Crow, A. Menon, M. Fotino, H. M. Reid, T. Poon-King, and P. Rubinstein. 1989. Presence of a non-HLA B cell antigen in rheumatic fever patients and their families as defined by a monoclonal antibody. J. Clin. Investig. 83:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar, D., S. Kaur, A. Grover, H. Bali, K. L. Khanduja, E. L. Kaplan, E. D. Gray, and N. K. Ganguly. 2000. Further observations and characterization of monoclonal antibodies reacting with B cell alloantigens associated with rheumatic fever and rheumatic heart disease. J. Lab. Clin. Med. 135:287-293. [DOI] [PubMed] [Google Scholar]
- 13.Lanchbury, J., M. Hall, and S. Steer. 2002. Progress and problems in defining susceptibility genes for rheumatic diseases. Rheumatology 41:361-364. [DOI] [PubMed] [Google Scholar]
- 14.Leckman, J. F., M. A. Riddle, M. T. Hardin, S. I. Ort, K. L. Swartz, J. Stevenson, and D. J. Cohen. 1989. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J. Am. Acad. Child Adolesc. Psychiatry 28:566-573. [DOI] [PubMed] [Google Scholar]
- 15.Murphy, T., and W. Goodman. 2002. Genetics of childhood disorders. XXXIV. Autoimmune disorders, part 7: D8/17 reactivity as an immunological marker of susceptibility to neuropsychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry 41:98-100. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, T. K., N. Benson, A. Zaytoun, M. Yang, R. Braylan, E. Ayoub, and W. K. Goodman. 2001. Progress toward analysis of D8/17 binding to B cells in children with obsessive compulsive disorder and/or chronic tic disorder. J. Neuroimmunol. 120:146-151. [DOI] [PubMed] [Google Scholar]
- 17.Murphy, T. K., W. K. Goodman, M. W. Fudge, R. C. Williams, Jr., E. M. Ayoub, M. Dalal, M. H. Lewis, and J. B. Zabriskie. 1997. B lymphocyte antigen D8/17: a peripheral marker for childhood-onset obsessive-compulsive disorder and Tourette's syndrome? Am. J. Psychiatry 154:402-407. [DOI] [PubMed] [Google Scholar]
- 18.Sokol, M. S., P. E. Ward, H. Tamiya, D. G. Kondo, D. Houston, and J. B. Zabriskie. 2002. D8/17 expression on B lymphocytes in anorexia nervosa. Am. J. Psychiatry 159:1430-1432. [DOI] [PubMed] [Google Scholar]
- 19.Swedo, S. E., H. L. Leonard, B. B. Mittleman, A. J. Allen, J. L. Rapoport, S. P. Dow, M. E. Kanter, F. Chapman, and J. B. Zabriskie. 1997. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Am. J. Psychiatry 154:110-112. [DOI] [PubMed] [Google Scholar]
- 20.Zabriskie, J. B., D. Lavenchy, R. C. Williams, Jr., S. M. Fu, C. A. Yeadon, M. Fotino, and D. G. Braun. 1985. Rheumatic fever-associated B cell alloantigens as identified by monoclonal antibodies. Arthritis Rheum. 28:1047-1051. [DOI] [PubMed] [Google Scholar]