Abstract
While modern immunoassays provide sensitive and specific means for the quantitation of cytokines in biological fluids, heterophile antibodies are still a well-recognized cause of interference in the measurement of cytokines in these assays. We have developed a multiplexed fluorescent microsphere immunoassay for the simultaneous quantification of 10 cytokines in only 75 μl of serum. During the development of this multiplexed assay, the amount of assay interference due to heterophile antibodies was also determined, and methods for detecting heterophile interference and minimizing its effect were incorporated into the assay. Heterophile antibodies resulted in significantly elevated cytokine values compared to those of normal blood bank samples. These falsely elevated values, and thus the components of the assay the heterophile antibodies were binding to, were identified through the use of internal controls. This information was then used to design assay-specific blockers and absorbents that were shown to significantly reduce falsely elevated cytokine values while not affecting the standard and control values. The fluorescent multiplexed microsphere-based immunoassay can be used to quantitate multiple cytokines from a single sample and should be a useful tool in furthering our understanding of the role of cytokines in disease processes.
Cytokines are hormonelike polypeptides which are secreted in the course of immunologic and inflammatory responses. They function as intercellular signals, are produced by a variety of different cell types, and regulate both local and systemic inflammatory responses. Cytokines are also important immunoregulators in the processes of wound healing, immunity, hematopoiesis, and perhaps even atherogenesis. Individual cytokines can have multiple effects on the growth and differentiation of many cell types and may exhibit considerable overlap with other cytokines in their biologic effects on these cells. The analysis and measurement of cytokine concentrations in various body fluids has become a commonly used procedure in research and an emerging field of interest in clinical laboratory medicine (2) and has clearly enhanced our knowledge of many immunologic and inflammatory disorders.
Cytokines are involved in numerous immunological functions, having both antagonistic and synergetic effects on many different cell types as well as enhancing the production of other cytokines. The optimal manner in which to correlate a specific disease process with changes in cytokine concentrations requires analyzing individual samples for multiple cytokines. The enzyme-linked immunosorbent assay (ELISA) is the most commonly reported method for the quantitation of secreted cytokines. These assays, however, must all be run individually, which in turn requires substantially more reagents, more technician time, and a larger sample volume. Employing a multiplexed fluorescent microsphere immunoassay system (Luminex 100; Luminex Corp., Austin, Tex.), we have developed a sandwich capture assay to simultaneously assess the production of nine different cytokines (interleukin-1β [IL-1β], IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 p70, gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) and one cytokine receptor (IL-2 soluble receptor alpha [IL-2r]) in human serum. Also included in the multiplexed assay were internal controls for detecting interfering levels of heterophile antibodies.
Heterophile antibodies are a well-recognized cause of interference in immunoassays (4, 12, 14, 19) and are present in 5 to 40% of normal blood donors (7, 8, 11). Heterophile antibodies (antibodies which cross phyla in their reactivity) are produced against poorly defined antigens and generally show weak avidity and are multispecies specific. Other interfering antibodies may be specific human anti-animal antibodies, which may be produced against animal immunoglobulins (Ig). For example, this process occurs when a patient makes antibody to OKT3, a mouse monoclonal against CD3 used for preventing or reversing allograft transplant rejections.
Heterophile antibodies can interfere by causing false-positive results in two-site immunoassays by bridging the capture and detection antibodies. Likewise, heterophile antibodies can cause false-negative results by binding directly to the capture antibody and thus blocking the reactive site from binding the analyte of interest. Many immunoassays, including ELISA and Luminex-based assays, use animal proteins such as bovine serum albumin and casein to block reactive sites of the microtiter plate or polystyrene microspheres. These blockers provide another potential source of assay interference, as elevated values, false-positive results, and high background readings may occur as a result of heterophile and human anti-animal antibodies binding directly to the blocking protein.
The Luminex Multi-Analyte Profiling (LabMAP) system is a flow cytometry-based instrument that allows multiple analytes to be assayed simultaneously in a single sample (6, 10). The technology uses 5.6-μm-diameter polystyrene particles called microspheres that are internally labeled with two fluorescent dyes. As the microsphere passes through the flow cell, it is interrogated by two lasers. One laser identifies the microsphere on the basis of the ratio of the two fluorophores contained within the microsphere, while the other laser quantitates the amount of analyte bound to the microsphere on the basis of the intensity of the reporter fluorescence. The surface of each microsphere contains multiple carboxyl groups that function as sites for covalent ligand attachment.
In this report, we describe the use of the Luminex system for the development of an immunoassay for the simultaneous quantification of 10 cytokines in human serum. In addition, we have examined 10 heterophile serum samples shown to initially cause falsely elevated cytokine levels in our multiplexed cytokine assay. By identifying which component of the assay the heterophile antibodies were binding to, assay-specific blockers and absorbents were developed to significantly reduce falsely elevated cytokine values.
MATERIALS AND METHODS
Clinical samples.
This study included 10 serum samples shown to have heterophile antibodies reactive to goat, mouse, and rat proteins. These samples were first identified by their reaction to the control antibody in immunodiffusion assays. An abnormal line of precipitation between the patient's serum and the goat-derived positive control antibody was seen, suggesting the presence of human anti-goat antibodies in the patient's serum. A case report on a serum sample found in this manner and used in our investigation showed it to contain heterophile antibodies that were cross-reactive with bovine and caprine proteins (18). These heterophile antibodies were also shown to cause false-positive immunoassay results for human immunodeficiency virus type 1 and in several other infectious disease serology tests. This particular sample, as well as nine other serum samples reactive to the goat-derived controls in double-immunodiffusion assays, was used to determine the effects of heterophile antibodies in our multiplexed cytokine assay.
Also included in this study were 25 blood bank samples used as normal controls. All patient samples included in this study were de-identified according to the University of Utah Institutional Review Board approved protocol (protocol no. 7275) to meet the Health Information Portability and Accountability Act patient confidentiality guidelines.
Development of the multiplexed cytokine assay.
The multiplexed cytokine assay was developed by using a standard sandwich capture format which has been described previously (10, 13). Briefly, monoclonal antibodies to human IL-2, IL-4, IL-6, IL-8, IL-10, and TNF-α were purchased from Pharmingen/BD Biosciences (San Diego, Calif.), IL-2r and IL-12 p70 were purchased from R&D Systems (Minneapolis, Minn.), and IL-1β and IFN-γ were purchased from Pierce-Endogen (Rockford, Ill.). These products were used as capture antibodies and coupled to carboxylated Luminex microspheres by using a two-step carbodiimide reaction (15). Internal controls were created by coupling of pooled normal mouse and rat IgG (50 μg/ml; Sigma, St. Louis, Mo.) and pooled mouse serum (100 μg/ml; Sigma) to additional microspheres. A standard curve for each cytokine was made by mixing known concentrations of recombinant human cytokines IL-4, IL-6, IL-8, IL-10, and TNF-α (Pharmingen/BD Biosciences), human cytokines IL-2 and IL-12 cytokine receptor IL-2r (R&D Systems), and human cytokines IFN-γ and IL-1β (Pierce-Endogen) in serum diluent. The patient serum sample was diluted 1:2 (75 μl of serum diluent plus 75 μl of patient serum) and incubated for 10 min on a rotator before the addition of the coupled microspheres to allow the absorption of heterophile antibodies. The cytokine standards, controls, serum samples, and microspheres were incubated for 2 h at room temperature on a rotator by using 96-well filter bottom microtiter plate (Millipore, Bedford, Mass.) to allow subsequent washing. This step was followed by the addition of 100 μl of biotinylated monoclonal antibodies to human IL-2, IL-4, IL-6, IL-8, IL-10, TNF, and IL-2r (Pharmingen/BD Biosciences), IL-12 (R&D Systems), and IL-1β and IFN-γ (Pierce-Endogen) to each well of the microtiter plate. The final concentrations of the biotinylated detection antibodies ranged from 1 to 2.5 μg/ml. For the studies conducted to determine the amount of heterophile binding directly to the specific cytokine monoclonal antibody-coupled microspheres, as well as the mouse and rat protein-coupled internal controls, an anti-human IgG-conjugated R-phycoerythrin detection antibody was used instead. Following a second 30-min incubation on the rotator and washing, 100 μl of streptavidin (10 μg/ml)-conjugated R-phycoerythrin (Molecular Probes, Eugene, Oreg.) was added to each well. After a 15-min incubation and a final wash, the microspheres were resuspended in 100 μl of phosphate-buffered saline with Tween 20 (PBST), and the 96-well microplate was placed in a Luminex 100 instrument with an XY platform (automated microtiter plate handler). The amount of cytokine bound to the microspheres by this antibody sandwich technique is determined by the median fluorescence intensity (MFI) of the reporter molecule, phycoerythrin. The MFI of the unknown sample is then converted into a picograms-per-milliliter value based on the known cytokine concentrations of the standard curve by using a five-parameter regression formula. Since the analyte specificity and position of each microsphere classification in the array is known, a single fluorescent reporter molecule can be used to measure all 10 cytokine concentrations.
Heterophile-blocking serum diluent.
Reagents used for the formulation of the serum diluent used in our multiplexed assay for blocking heterophile interference were purchased from Sigma and included fetal bovine serum (FBS; product no. F2442, lot nos. 80K8400, 41K8406, and 52K8411), pooled normal mouse serum (product no. M5905, lot nos. 89H9020 and 073K9305), reagent-grade purified rat IgG (product no. R9759), pooled normal rat serum (product no. I4131), and reagent-grade purified mouse IgG (product no. I5381).
RESULTS
Investigation of heterophile antibody interference causing falsely elevated cytokine values.
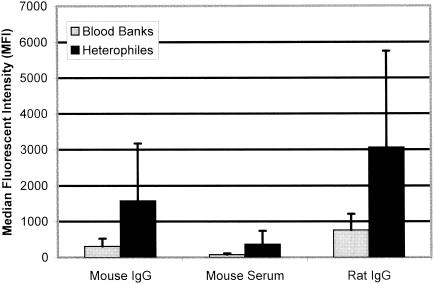
Ten serum samples containing heterophile antibodies were tested in our multiplexed cytokine assay to determine whether the heterophile antibodies would cause significantly elevated cytokine values by binding to the rat- and mouse-derived monoclonal capture antibodies coupled to the microspheres. To assess this possibility, three internal control microspheres coupled with either mouse IgG, mouse serum, or rat IgG were included in our multiplexed assay in addition to the 10 cytokine-specific monoclonal antibody-coupled microspheres. The reactivities of the 10 heterophile serum samples to the three internal controls were compared to those of 25 blood bank normal serum samples. A significant increase in binding for the heterophile samples compared to that for the blood bank normal samples was observed for the mouse IgG internal control microspheres (an MFI of 1,583 versus an MFI of 309, P = 0.0007), the mouse serum internal control microspheres (an MFI of 367 versus an MFI of 79, P = 0.001), and the rat IgG internal control microspheres (an MFI of 3,063 versus an MFI of 763, P = 0.0004) (Fig. 1). Since the cytokine-specific monoclonal antibodies used in our assay are also mouse and rat derived, it was determined that these heterophile antibodies caused falsely elevated cytokine values by binding to the capture antibodies (results presented below).
FIG. 1.
Mean MFI values for 25 blood bank serum samples (gray bars) and 10 heterophile serum samples (black bars) with mouse and rat protein-coupled microspheres. Reactivities of heterophile samples to mouse IgG, mouse serum, and rat IgG were significant (with P values of 0.0007, 0.001, and 0.0004, respectively) compared with those of blood bank samples. Error bars indicate standard deviations.
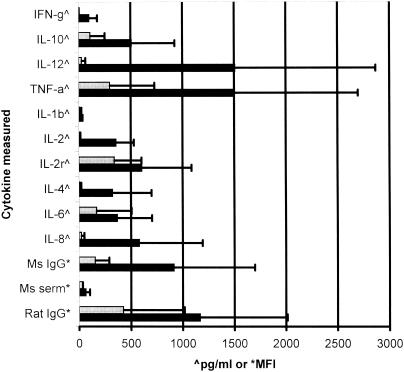
In an effort to reduce the falsely elevated cytokine values due to heterophile antibody interference, five formulations of different blocking reagents were developed and investigated. The five different formulations of serum diluents were 10% (vol/vol) mouse serum, 10% (vol/vol) rat serum, 10% (vol/vol) FBS, 50 μg of nonimmune rat IgG/ml, and 50 μg of nonimmune mouse IgG/ml. The 10 heterophile samples were diluted in the five different blocking diluents and PBST and incubated with the 10 cytokine-specific monoclonal antibody-coupled microspheres and three internal control microspheres. The MFI results for heterophile sample 4 are shown in Table 1. The data show variability in the effectiveness of the blocking diluents compared with that of PBST. The IFN-γ monoclonal antibody-coupled microsphere, for instance, shows considerable heterophile antibody binding in the PBST diluent (MFI, 1,195), and this binding was greatly reduced by the five blocking diluents, 10% mouse serum (MFI, 91), 10% rat serum (MFI, 170), 10% fetal bovine serum (MFI, 101), nonimmune rat IgG (MFI, 254), and nonimmune mouse IgG (MFI, 149). The most effective blockers appear to be the mouse serum and the nonimmune mouse IgG, which is logical because the IFN-γ capture antibody coupled to the bead is a mouse-derived immunoglobulin. This correlation was not always found to be the case, however. For the rat-derived IL-10 monoclonal antibody-coupled microsphere (Table 1), the 10% rat serum diluent had little effect in reducing heterophile binding relative to that occurring with PBST (an MFI of 2,098 versus an MFI of 2,496). The nonimmune rat IgG diluent also seemed to have little effect in reducing binding, with an MFI of 2,014 versus an MFI of 2,469 for PBST. For this specific sample (heterophile sample 4), the most effective blocking diluent was 10% FBS, which reduced the amount of heterophile binding by 86% (an MFI of 2,498 for PBST versus an MFI of 349 for 10% FBS). The specific results for the five blocking diluents for the remaining eight monoclonal antibody-coupled microspheres and three internal controls are shown in Table 1. The results of the blocking diluent study for the rest of the heterophile serum samples are summarized in Table 2. There was an insufficient sample volume to include heterophile sample 8 in this portion of the study. Shown in Table 2 is the most effective blocking diluent for every combination of heterophile sample and coupled microsphere and the percentage of binding reduction. In 48% of the reactions (50 out of 104), the 10% FBS diluent was the most effective diluent in reducing heterophile binding to the coupled microspheres. The serum diluent composed of 10% mouse serum was the second most effective blocker, showing the greatest reduction of heterophile antibody binding in 39 of 104 (37.5%) of the reactions. The 10% rat serum diluent was the third most effective blocker, showing the greatest binding reduction in 15 of 104 (14.4%) of the reactions. The nonimmune rat and mouse IgG-containing diluents were the least effective diluents in reducing heterophile antibody binding. These data show that for a multiplexed assay involving different species of monoclonal capture antibodies, a serum diluent composed of only one species of blocking reagent is not sufficient for reducing heterophile antibody interference. For this reason, titration studies of diluents containing the three most effective blockers, FBS, mouse serum, and rat serum, were conducted. For the cytokine-specific monoclonal antibodies used in our multiplexed assay, the most effective serum diluent for blocking heterophile interference was composed of 10% (vol/vol) FBS, 5% (vol/vol) pooled normal mouse serum, and 2.5% (vol/vol) pooled normal rat serum. Results show that the optimized diluent containing the FBS, mouse serum, and rat serum significantly (P < 0.05) reduced heterophile interference with 8 of the 10 measured cytokines and all three internal controls compared to that occurring with the PBST diluent (Fig. 2). To insure that the blocking effects of the commercial serum were not dependent on a certain production lot, three different lots of FBS and two different lots of mouse and rat sera were examined. Differences in results for the heterophile samples and the blood bank controls between different serum lots were all less than 10% of the coefficient of variation and therefore within normal interassay variance.
TABLE 1.
Comparison of MFI values for five formulations of blocking reagents (three different animal sera and two different nonimmune globulins) to those for PBST to evaluate their effectiveness in reducing heterophile antibody binding to microsphere-bound capture antibodies and proteins for heterophile sample 4
| Diluent | MFI value for bead-bound reagent (host)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-IFNγ (mouse) | α-IL-10 (rat) | α-IL-12 (mouse) | α-IL-1b (mouse) | α-IL-2 (mouse) | α-IL-2r (mouse) | α-IL-4 (mouse) | α-IL-6 (rat) | α-IL-8 (mouse) | α-TNF-α (mouse) | IgG (mouse) | Serum (mouse) | IgG (rat) | |
| PBST | 1,195 | 2,496 | 3,348 | 6,784 | 1,817 | 424 | 2,012 | 2,578 | 544 | 6,683 | 4,698 | 611 | 5,500 |
| 10% mouse serum | 91 | 2,098 | 349 | 128 | 172 | 70 | 1,409 | 1,680 | 92 | 3,682 | 148 | 48 | 802 |
| 10% rat serum | 170 | 1,978 | 734 | 134 | 361 | 93 | 1,376 | 1,584 | 100 | 3,694 | 197 | 117 | 548 |
| 10% FBS | 101 | 349 | 190 | 260 | 204 | 125 | 122 | 349 | 128 | 548 | 259 | 180 | 856 |
| 50 μg of rat IgG/ml | 254 | 2,014 | 1,415 | 1,359 | 660 | 101 | 1,527 | 1,585 | 122 | 3,872 | 647 | 377 | 693 |
| 50 μg of mouse IgG/ml | 149 | 2,113 | 718 | 1,514 | 313 | 109 | 1,466 | 1,901 | 133 | 3,827 | 638 | 455 | 1,573 |
TABLE 2.
Summary of results of heterophile antibody-blocking diluentsa
| Heterophile sample | Most effective blocker(s) (% MFI reductionb) for bead-bound reagent (host)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-IFNγ (mouse) | α-IL-10 (rat) | α-IL-12 (mouse) | α-IL-1b (mouse) | α-IL-2 (mouse) | α-IL-2r (mouse) | α-IL-4 (mouse) | α-IL-6 (rat) | α-IL-8 (mouse) | α-TNF-α (mouse) | IgG (mouse) | Serum (mouse) | IgG (rat) | |
| 1 | FBS (45.5) | FBS (71.1) | FBS (74.6) | MS (82.2) | FBS (60.7) | FBS (41.5) | FBS (83.3) | FBS (74.9) | FBS (80.6) | FBS (65.3) | MS (47.5) | FBS (76.1) | RS (29.7) |
| 2 | MS (92.4) | FBS (86) | FBS (94.3) | MS (98.1) | MS (90.5) | MS (83.5) | FBS (94) | FBS (86.5) | MS (83.1) | FBS (91.8) | MS (96.8) | MS (92.1) | RS (90) |
| 3 | FBS (72.9) | FBS (79.1) | FBS (87.9) | MS (90.3) | FBS (77.4) | FBS (75.3) | FBS (91.7) | FBS (76) | MS (35) | FBS (92.6) | MS (91.2) | MS (43.8) | RS (90.4) |
| 5 | FBS (74.5) | FBS (85.5) | FBS (89.9) | MS (83.8) | FBS (82.4) | FBS (44.6) | FBS (94.7) | FBS (89.1) | FBS (61) | FBS (87.7) | MS (80.2) | MS (79.9) | RS (87.4) |
| 6 | FBS (26) | FBS (67) | FBS (65) | MS (73) | FBS (47) | FBS (46) | FBS (63) | FBS & RS (48) | FBS (38) | FBS (9) | MS (63) | MS (86) | MS & RS (41) |
| 7 | MS & RS (86) | FBS (90) | FBS (89) | MS (96) | MS & RS (87) | MS (63) | FBS (90) | FBS (91) | MS & RS (53) | FBS (92) | MS (93) | MS (72) | RS (93) |
| 9 | MS (63) | MS (62) | MS (63) | MS (85) | MS (63) | MS (63) | MS (60) | MS (61) | MS (65) | MS (63) | MS (73) | MS (63) | RS (54) |
| 10 | RS (42) | RS (52) | FBS (55) | MS (75) | FBS (21) | RS (59) | RS (53) | RS (40) | RS (49) | MS & FBS (34) | MS (35) | MS (32) | RS (27) |
FBS, 10% fetal bovine serum; MS, 10% mouse serum; RS, 10% rat serum.
Percentage of reduction of MFI value compared to that of PBST.
FIG. 2.
Comparison of the mean concentrations or MFI values of 10 heterophile antibody-containing serum samples in a multiplexed cytokine immunoassay. Most reductions in falsely elevated cytokine levels in the optimized serum diluent (gray bars) compared with the levels in PBST (black bars) were significant (IFN-γ, P = 0.006; IL-10, P = 0.035; IL-12, P = 0.024; TNF-α, P = 0.040; IL-1β, P = 0.062 [not significant]; IL-2, P = 0.006; IL-2r, P = 0.064 [not significant]; IL-4, P = 0.021; IL-6, P = 0.023; IL-8, P = 0.035; mouse IgG, P = 0.013; mouse serum, P = 0.026; rat IgG, P = 0.006). Error bars indicate standard deviations.
While most of the heterophile interference observed caused falsely elevated cytokine values by presumed bridging of the capture antibody to the biotinylated detection antibody, there were a few cases in which measured cytokine values were higher in the serum diluent than in PBST. Heterophile sample 5 had a value of 384 U/ml for IL-2r in the sample diluent versus 216 U/ml in the PBST diluent. Likewise, heterophile sample 6 measured 502 pg/ml for TNF-α in the sample diluent versus 315 pg/ml in the PBST. The heterophile antibodies in these samples may bind directly to the reactive site of the IL-2r and TNF-α capture antibodies, thus partially blocking the binding of the cytokine and causing falsely depressed values.
While the optimized serum diluent proved to be effective in reducing, and in some cases eliminating, heterophile antibody interference, we also wanted to ensure that the diluent did not adversely affect specific cytokine results by increasing or reducing measurements. To achieve this objective, seven standards containing various concentrations of recombinant cytokines and three controls known to contain native cytokines were run in both diluents. Linear regression and paired type 2 t test analysis for the 10 samples (serum diluent versus PBST) were carried out. The R2 values were all 0.994 and greater (average, 0.999), and the slopes ranged from 0.96 to 1.45 (average, 1.096). The P values ranged from 0.110 to 0.684, with an average of 0.333. These data show that there is no significant difference between results achieved with the two diluents, indicating that the serum diluent does not adversely affect the measurement of known quantities of cytokines.
DISCUSSION
Since circulating cytokines are usually present in low concentrations (picograms per milliliter), it is necessary to dilute the serum sample as little as possible to achieve a maximal assay sensitivity of less than 10 pg/ml. By minimally diluting the patient sample, however, other factors, such as the effects of the sample matrix, may affect the accuracy and specificity of the assay. The presence of heterophilic antibodies in the serum matrix has been well documented as a source of interference in cytokine assays (1, 7, 9, 10, 17). In our studies, a panel of 10 serum samples containing heterophile antibodies was examined to determine whether, and then how much, our multiplexed cytokine assay was susceptible to heterophile interference. Heterophile antibody interference can usually be reduced by adding normal nonimmune animal serum to the serum diluent used in the assay to absorb the heterophilic antibodies. In traditional cytokine ELISAs, in which only one cytokine is measured per assay, a different composition of serum diluent can be developed for each individual assay. This feature allows the use of the most effective blocker and its concentration to be adjusted for each individual assay. In the multiplexed format of the Luminex system, whereby all cytokines are measured from the same sample dilution, there is the disadvantage of having to use only one formulation of diluent for all cytokines. By reacting the heterophile antibody-containing samples directly with the microsphere-bound monoclonal captured antibodies used in our multiplexed assay, we were able to determine that there was significant binding of heterophile antibodies to the coupled microspheres (Tables 1 and 2, PBST). These same heterophile samples were also shown to cause falsely elevated cytokine concentrations in our multiplexed assay when the samples were diluted in PBST, a diluent containing no blockers (Fig. 2). By examining the species from which each monoclonal antibody was derived, a serum diluent was developed by using the most relevant animal serums and the most effective concentration of each blocker for our specific assay. This serum diluent was found to significantly reduce or eliminate falsely elevated cytokine values in the 10 heterophile samples. There were also a few cases in which heterophile interference caused falsely depressed cytokine values by presumed blocking of the capture antibody. Even though our assay contained no bovine-derived capture or detection antibodies, FBS was found to be the most effective single blocker (Table 2). Other investigations have also shown that in some cases, assay interference with immunoglobulins from one species could be blocked better by nonimmune globulin from a different species (3, 4, 7).
While the described serum diluent proved to be very effective in reducing heterophile interference in our limited sample population, a single formulation may not be effective for all individuals because the effects of the sample matrix are often dependent on the particular individuals involved and their various exposures to different heterophile-inducing immunogens. It has in fact been demonstrated that not all heterophile interference can be blocked by nonimmune globulin, even if pooled globulins from several species are used (5, 10, 16). It is for this reason that we included the rat and mouse IgG and mouse serum microsphere-coupled internal controls in our multiplexed serum cytokine assay. These controls allow the detection of potential interfering antibodies in each individual patient sample that are not eliminated by the normal animal serums contained in our optimal serum diluent.
The multiplexing capability of the Luminex instrument is proving to be a powerful platform for the development of multiple analyte profiles which require lower patient sample volumes and fewer reagents and cost less than traditional methodologies. We have found the Luminex instrument to be an accurate and reliable system for simultaneously quantitating 10 cytokines in only 75 μl of patient serum. Determining exactly which components of the assay were being affected by heterophile interference allowed the addition of true internal controls to each individual patient assay. Because of the efficiency and lower costs of the multiplexed assay, cytokine profile testing is now more feasible to perform in a clinical laboratory setting. Studies are increasingly showing correlations between cytokine production and immunologic and inflammatory disorders in numerous disease processes. With the ability to measure multiple cytokines from a single sample source, we now have a powerful research and clinical tool for assessing cytokine profiles in patient serum samples.
REFERENCES
- 1.Banks, R. E. 2000. Measurement of cytokines in clinical samples using immunoassays: problems and pitfalls. Crit. Rev. Clin. Lab. Sci. 37:131-182. [DOI] [PubMed] [Google Scholar]
- 2.Bienvenu, J. A., G. Monneret, M. C. Gutowski, and N. Fabien. 1998. Cytokine assays in human sera and tissues. Toxicology 129:55-61. [DOI] [PubMed] [Google Scholar]
- 3.Boerman, O. C., M. F. Segers, L. G. Poels, P. Kenemans, and C. M. Thomas. 1990. Heterophilic antibodies in human sera causing falsely increased results in the CA 125 immunofluorometric assay. Clin. Chem. 36:888-891. [PubMed] [Google Scholar]
- 4.Boscato, L. M., and M. C. Stuart. 1988. Heterophilic antibodies: a problem for all immunoassays. Clin. Chem. 34:27-33. [PubMed] [Google Scholar]
- 5.Boscato, L. M., and M. C. Stuart. 1986. Incidence and specificity of interference in two-site immunoassays. Clin. Chem. 32:1491-1495. [PubMed] [Google Scholar]
- 6.Fulton, R. J., R. L. McDade, P. L. Smith, L. J. Kienker, and J. R. Kettman, Jr. 1997. Advanced multiplexed analysis with the FlowMetrix system. Clin. Chem. 43:1749-1756. [PubMed] [Google Scholar]
- 7.Heney, D., and J. T. Whicher. 1995. Factors affecting the measurement of cytokines in biological fluids: implications for their clinical measurement. Ann. Clin. Biochem. 32:358-368. [DOI] [PubMed] [Google Scholar]
- 8.Hennig, C., L. Rink, U. Fagin, W. J. Jabs, and H. Kirchner. 2000. The influence of naturally occurring heterophilic anti-immunoglobulin antibodies on direct measurement of serum proteins using sandwich ELISAs. J. Immunol. Methods 235:71-80. [DOI] [PubMed] [Google Scholar]
- 9.Herzyk, D. J., and M. D. Wewers. 1993. ELISA detection of IL-1 beta in human sera needs independent confirmation. False positives in hospitalized patients. Am. Rev. Respir. Dis. 147:139-142. [DOI] [PubMed] [Google Scholar]
- 10.Kellar, K. L., R. R. Kalwar, and K. A. Dubois. 2001. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry 45:27-36. [DOI] [PubMed] [Google Scholar]
- 11.Kricka, L. J. 1999. Human anti-animal antibody interferences in immunological assays. Clin. Chem. 45:942-956. [PubMed] [Google Scholar]
- 12.Levinson, S. S. 1992. Antibody multispecificity in immunoassay interference. Clin. Biochem. 25:77-87. [DOI] [PubMed] [Google Scholar]
- 13.Martins, T. B., B. M. Pasi, J. W. Pickering, T. D. Jaskowski, C. M. Litwin, and H. R. Hill. 2002. Determination of cytokine responses using a multiplexed fluorescent microsphere immunoassay. Am. J. Clin. Pathol. 118:346-353. [DOI] [PubMed] [Google Scholar]
- 14.Rotmensch, S., and L. A. Cole. 2000. False diagnosis and needless therapy of presumed malignant disease in women with false-positive human chorionic gonadotropin concentrations. Lancet 355:712-715. [DOI] [PubMed] [Google Scholar]
- 15.Staros, J. V., R. W. Wright, and D. M. Swingle. 1986. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 156:220-222. [DOI] [PubMed] [Google Scholar]
- 16.Thompson, R. J., A. P. Jackson, and N. Langlois. 1986. Circulating antibodies to mouse monoclonal immunoglobulins in normal subjects—incidence, species specificity, and effects on a two-site assay for creatine kinase-MB isoenzyme. Clin. Chem. 32:476-481. [PubMed] [Google Scholar]
- 17.Tsang, M. L., and J. A. Weatherbee. 1996. Cytokine assays and their limitations. Aliment. Pharmacol. Ther. 2(Suppl. 10):55-61. [DOI] [PubMed] [Google Scholar]
- 18.Willman, J. H., T. B. Martins, T. D. Jaskowski, H. R. Hill, and C. M. Litwin. 1999. Heterophile antibodies to bovine and caprine proteins causing false-positive human immunodeficiency virus type 1 and other enzyme-linked immunosorbent assay results. Clin. Diagn. Lab. Immunol. 6:615-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zweig, M. H., G. Csako, C. C. Benson, B. D. Weintraub, and B. B. Kahn. 1987. Interference by anti-immunoglobulin G antibodies in immunoradiometric assays of thyrotropin involving mouse monoclonal antibodies. Clin. Chem. 33:840-844. [PubMed] [Google Scholar]