Abstract
Oropharyngeal Candida albicans (C. albicans) infection usually occurs in patients with altered cell-mediated immune response. Many animal models have been developed for studying the pathogenesis of disease. Here we describe a new model for real-time monitoring of oral candidiasis. Mice were rendered susceptible to oral candidiasis by injection with cortisone acetate. Oral infection was performed by placing a swab saturated with genetically engineered bioluminescent strain of C. albicans sublingually. An in vivo imaging technique, exploiting stably trasformed C. albicans that costitutively express luciferase, was adopted. This novel longitudinal study represents a powerful tool to: (1) test real-time progression of infection, (2) identify the target site of C. albicans in specific organs, (3) evaluate the efficacy of antifungal therapies and (4) explore the spread of C. albicans from the local to systemic compartment in a new way.
Keywords: OPC, real-time, bioluminescence, imaging, longitudinal study, C. albicans
Introduction
Oral candidiasis is the most common human fungal infection. It is caused by an overgrowth of Candida that can lead to local discomfort, taste alterations, and dysphagia from esophageal overgrowth, resulting in poor nutrition, slow recovery and prolonged hospital stays.
The incidence varies depending on age and certain predisposing factors. Risk factors include a high carbohydrate diet, drugs, impaired salivary gland function, dentures or a sign of systemic disease such as diabetes mellitus. Moreover, Candida albicans (C. albicans) causes at least 80% of oropharyngeal candidiasis (OPC) cases in patients with HIV/AIDS.1,2
A number of animal models of oral candidiasis involving immunosuppressive treatment have been described.3,4 Under normal conditions this fungal infection is not stable and is usually cleared within 10 d.5 A reliable and novel mouse model of OPC has recently been described by Solis et al.6 Mice were rendered susceptible to oral infection by injection with cortisone acetate and then inoculated by placing a swab saturated with C. albicans sublingually. By combining this model and a new in vivo imaging technique, exploiting genetically engineered, luminescent C. albicans,7,8 we obtained unprecedented tools for longitudinal monitoring of the course of OPC in live animals. Furthemore, Doyle et al.9 described a method for real-time monitoring vaginal and systemic C. albicans infections by using strains transformed with the firefly luciferase gene. However, this methods, differently from our experimental approach, was unable to reveal hyphal cells. Moreover, no studies of OPC infection have been undertaken by using this model.
Results
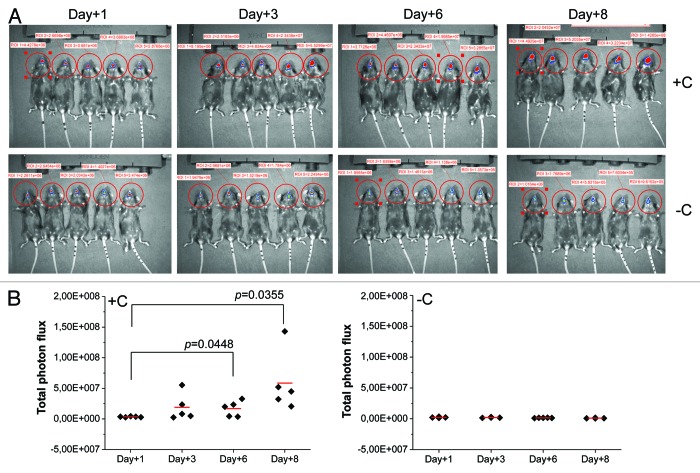
C57BL/6 mice were left untreated or treated subcutaneously (s.c.) with cortisone acetate every two days starting at day -1 relative to infection. The infection was performed as described by Solis et al.6 and the course of OPC was monitored 1, 3, 6 and 8 d after infection. One day after challenge the infection appeared to go through a bottleneck in which only a small number of C. albicans cells were evidenced in the oral cavity (Fig. 1A and B). No differences in fungal load were evidenced between the untreated mice and those treated with corticosteroids. A modest rise of fungal load, evaluated as photon emission, was observed 3 d after infection only in mice treated with corticosteroids. A significant increase of oral fungal burden was observed 6 d post-infection again in these mice only (p = 0.0448). The fungal load continued to rise through to the end of the monitoring period (day +8; p = 0.0355). Mice were killed on day 8 for humane reasons. Corticosteroid-untreated mice did not show a significant fungal load in any determination performed. As a consequence, in subsequent experiments the determinations were performed only in corticosteroid-treated mice.
Figure 1. In vivo imaging of OPC. Mice treated (+C) and not treated (-C) with cortisone acetate were infected with C. albicans gLUC59 (1 × 106/ml). 1, 3, 6 and 8 d post-infection anesthetized mice were treated sublingually with 10 µl of coelenterazine (0.5 mg/ml) and imaged in the IVIS-200TM Imaging system. Data are from one of three experiments with similar results. Total photon flux from oral areas in the images (ROI) of each mouse was quantified with Living ImageR software package (A). The statistical significance of total photon flux from ROI was evaluated with the Student's t test. p = 0.0448 (day +6 post challenge vs day +1); p = 0.0355 (day +8 post challenge vs day +1) (B).
Corticosteroid-treated and infected mice were also monitored by using the standard assessment of CFU in the tongue, esophagus and stomach. Figure 2 shows that there are no differences between the CFU recovery 3 and 6 d after challenge, while a significant increase of fungal load was observed 8 d post-infection. Given that the coelenterazine substrate was added sublingually and anesthetized mice were immediately imaged with IVIS system, the substrate was not able to reach the pharynx, esophagus and stomach; as a consequence, we performed an ex vivo analysis from infected animals. Ex vivo bioluminescence imaging of pharynx, esophagus and stomach was performed at various times. No bioluminescence was manifested 3 d after infection; however, 6 d post-infection a clear and well-defined bioluminescent signal was observed in the first tract of the pharynx and esophagus. The progression of infection was also monitored 8 d after challenge and at this time we observed that C. albicans had also gained access to the lower part of the esophagus and the stomach (Fig. 3).
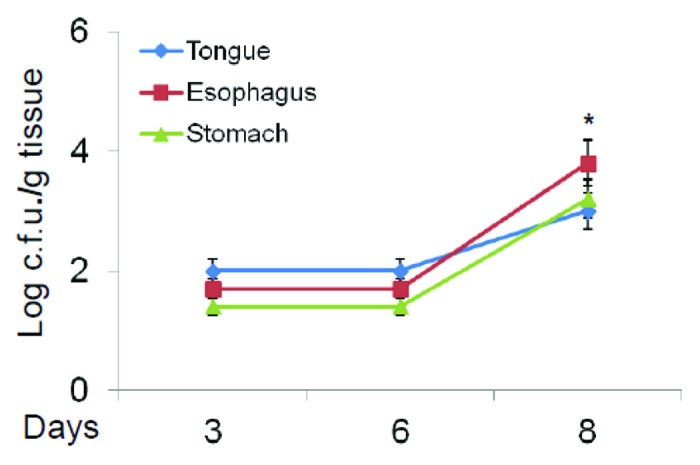
Figure 2. Fungal burden in target organs. Fungal burden of cortisone acetate treated mice with OPC was evaluated 3, 6 and 8 d post-infection in tongue, esophagus and stomach. *p < 0.05 (day +8 post challenge vs day +3) according to Mann-Whitney U test.
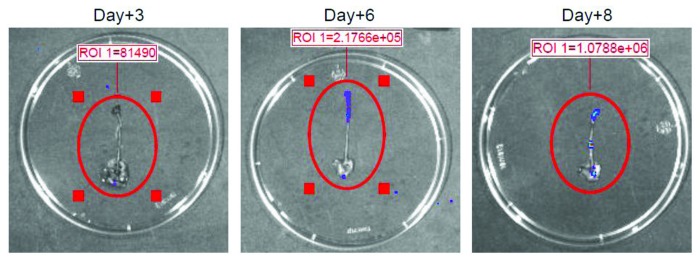
Figure 3. Ex vivo analysis of target organs. Ex vivo analysis of infected pharynx, esophagus and stomach from cortisone acetate treated mice with OPC was shown. 3, 6 and 8 d post-infection, mice were euthanized, gastric tracts were excised and 10 µl of coelenterazine (0.5 mg/ml) were injected through the pharynx into esophagus lumen to visualize the fungal burden and localization by the IVIS-200TM Imaging system. Data are from one of three experiments with similar results. ROI was quantified with Living ImageR software package.
The histological analysis of the tongue, esophagus and stomach of corticosteroid-treated mice was performed 8 d after infection and compared with corticosteroid-untreated mice. No histological modifications were evident in the untreated animals (Fig. 4A–C, left panels). Conversely, corticosteroid-treated mice showed an extensive colonization of the epithelium of the dorsal surface of the tongue by numerous hyphae and blastospores, with consequent complete destruction of papillary architecture and hyperplastic mucosal reaction (Fig. 4A, right panels). The esophagus lumen appeared obstructed by a mass of yeasts and hyphae and inflammatory cells including polymorphonuclear leukocyte (PMNL), and micro abscesses with hyphae and hyperplastic reaction were evident in the mucosa (Fig. 4B, right panels). Stomach sections showed an ulcerative gastric lesion in the glandular mucosa near the cardium atrial fold with infiltrates of yeasts and inflammatory cells and large abscesses containing hyphae and blastospores in the submucosal area (Fig. 4C, right panels). A progressive decline of body weight, reaching a maximum after 8 d, was observed in cortisone treated mice with OPC. The decline of body weight was not observed in cortisone untreated and infected mice (Fig. 5).
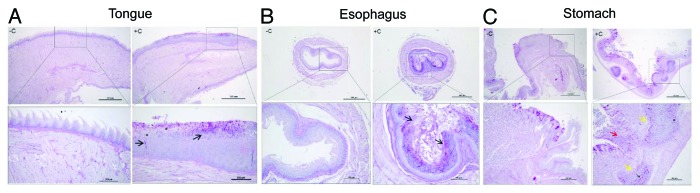
Figure 4. Histopathology. The tissue sections from non-cortisone treated (-C) and cortisone treated (+C) mice were shown. Tongue (A, left panels), esophagus (B, left panels) and stomach (C, left panels) sections from non-cortisone treated mice did not show signs of fungal burden or mucosal modification; conversely, tongue (A, right panels) section of cortisone treated mice showed yeasts, hyphae (arrows) and blastoconidial forms (asterisk) that invade the whole superficial layer of dorsal and ventral surfaces of the organ, completely destroying the papillary architecture (dorsal). Extensive hyperplastic reaction of spinous layer (acantosis) and aggregates of PMNL are evident. The lumen of esophagus (B, right panels) appeared obstructed by masses of fungi (arrows), inflammatory cells, and keratin; enlarged views show micro abscesses with hyphae (arrows) and hyperplastic mucosal reaction. Finally, in the stomach (C, right panels) ulcerative lesions (red arrows) in the cardium atrial fold with massive infiltrates of inflammatory cells (mononuclear cells and rare PMNL), hyphae, blastospore (asterisk), large abscesses (yellow arrows) of inflammatory cells and candidal burden (arrows) are evident in the submucosal area under lamina propria. Scale bars are in μm. Data are representative of two mice and nine tissue sections for treatment.
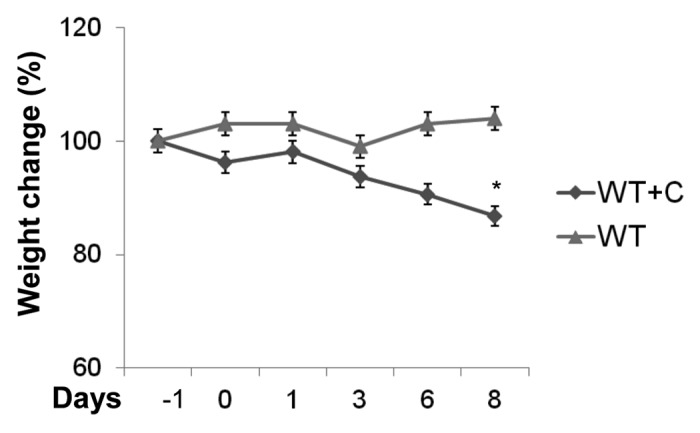
Figure 5. Effect of infection on body weight. Time course of weight change (%) in cortisone acetate treated (WT+C) or untreated (WT) mice with OPC was shown. *p < 0.05 (day +8 post challenge vs. day -1) according to Mann-Whitney U test.
Discussion
With this model we describe a new approach for longitudinal monitoring of the dynamics of oral C. albicans infection in C57BL/6 mice. C57BL/6 is the most widely used inbred strain and it provides a permissive background for maximal expression of most mutations. Recently, by using mice with C57BL/6 background it has been discovered that Th17-deficient mice, which lack either interleukin (IL)-23p19 or the IL-17 receptor, are susceptible to OPC without additional immunosuppression.4 Notably, in our experimental system CFU recovery from the tongue 6 d after infection did not show a significant increase with respect to the level observed 3 d after infection; conversely, when the fungal load was measured by photon emission, a significant increase was detected 6 d after challenge compared with 1 d after infection. The discrepancy between the two methods was not observed when the fungal load was measured 8 d post challenge. At this time both methods evidenced an increased growth of C. albicans with respect to the first post challenge determination. Moreover, an ex vivo analysis demonstrated that 6 d post-infection a clear and well-defined bioluminescent signal was observed, and at 8 d post challenge C. albicans had also gained access to the lower part of the esophagus and the stomach. This suggests that determination of fungal burden by measurement of photon emission permits a more rapid and sensitive evaluation of infection than standard CFU counting. A possible explanation is that the increase of fungal load which is detectable in the whole oral mucosa 6 d after infection is not appreciable with the standard assessment of CFUs from the tongue.6 Since the oral mucosa includes the tongue, gingiva, soft palate and pharynx, CFU determination might not be completely accurate for expressing the real fungal load of the whole oral cavity. Indeed, a previous study has shown a consistent colonization of C. albicans in gingival tissues.10 In addition it has been reported that CFU of C. albicans in cell suspensions or homogenates prepared from infected tissues are not always accurate indicators of the severity of infection in mucosal tissues.11 An additional explanation of the observed discrepancy between the traditional CFU test and this novel method could be the ability of bioluminescence to assess hyphae7,12 that are critical for the invasion process,13,14 but might not be accurately determined by the CFU method.
The extent of disease was also determined by the histological analysis of the infected tongue, esophagus and stomach, demonstrating the hyphal penetration into the deep layer of the tissue and the accumulation of leukocytes around the infecting organisms. Another method for estimating disease severity is monitoring individual mice for weight loss. We have found that it is correlated with the progression of the disease.
In conclusion, our original combination of Solis et al.’s model of OPC and the gLUC59 reporter represents a powerful tool for several reasons: (1) it gives an accurate estimation of the Candida load in the whole oral cavity, including the load of invasive hyphae, (2) it allows for the longitudinal monitoring of oral candidiasis in individual animals, providing a unique possibility to determine the progression or resolution of OPC in real-time and avoiding individual variations when the time course of CFU is assessed, (3) it allows the monitoring of C. albicans dissemination from the oral cavity to the gastrointestinal tract and (4) it decreases the need to sacrifice a large number of animals. Hence, this novel method provides a flexible and efficient approach to the real-time monitoring of oral candidiasis in live animals and will undoubtedly facilitate the evaluation of fungal and host parameters in the study of severity of infection in specific tissues.
Methods
C. albicans strain and culture
C. albicans CA1398 carrying the ACT1p-gLUC59 fusion (gLUC59) and C. albicans CA1399 that did not express gLUC59 (control strain) were used.7 The gLUC59 luciferase reporter has previously been described.7C. albicans gLUC59 and the control strain were cultured in YPD as described by Solis et al.6
Mouse model of OPC
Female, 6–8 weeks old, inbred C57BL/6 mice (Harlan Nossan Laboratories, Milan, Italy) were housed at the Animal Facilities of the University of Perugia. The procedures involving the animals and their care were conducted in conformity with the national and international laws and policies. Where indicated in the text, mice were treated with 225 mg/kg cortisone acetate (Sigma-Aldrich) and all were infected by placing a calcium alginate swab (Fisher Scientific) saturated with 1 × 106/ml C. albicans suspension sublingually for 75 min as previously described.6 Infections were conducted under anesthesia with a s.c. injection of a mixture of Tiletamine:Zolazepam-Xylazine (50 mg/kg:5 mg/kg).
Real-time monitoring of OPC
At selected days post-infection, starting 1 d after challenge, 10 µl (0.5 mg/ml in 1:10 methanol:H2O) of coelenterazine (Synchem, OHM) was added sublingually. Mice were then imaged in the IVIS-200TM Imaging system (Xenogen Inc.) under s.c. anesthesia. The total photon emission from oral areas within the images (region of interest, ROI) of each mouse was quantified with Living ImageR software package. An ex vivo analysis of infected pharynx, esophagus and stomach from mice with OPC was performed. After 3, 6 and 8 d post-infection mice were euthanized, the gastric tracts (pharynx, esophagus and stomach) were excised and 10 µl (0.5 mg/ml) of coelenterazine (Synchem) was injected through the pharynx into the esophagus lumen, to visualize the fungal burden using the IVIS-200TM Imaging system as described above. None of the mice exhibited detectable carriage of oral C. albicans based on an oral swab taken before the first infection.
CFU assay
The fungal burden of the tongue, esophagus and stomach 3, 6 and 8 d post-infection was evaluated by plating serial dilutions of organ homogenates onto YPD agar plus chloramphenicol (50 µg/ml) (both from Sigma-Aldrich) and counting CFUs. The disease severity was also evaluated by monitoring individual mice for weight loss.
Histological analysis
Eight days post-infection we analyzed tongue, esophageal and stomach tissues for pathological signs. The tissues were excised and fixed immediately in 10% formalin and successively embedded in paraffin. The tongues and stomachs were sectioned longitudinally to verify the extension of the lesions, the esophagi were sectioned transversally. The 3–5 µm thick sections were stained using the periodic acid-Schiff (PAS) procedure to visualize fungi, and examined by light microscopy (Leica DM2500). The scale bars are in μm.
Statistical analysis
The data are reported as the mean ± s.e.m. from triplicate samples of three experiments. The photon flux emission was compared using Student’s t test. CFU counts and weight loss were compared using Mann-Whitney U test. A value of p < 0.05 was considered significant.
Acknowledgments
We are grateful to Catherine Macpherson for editorial assistance. This work was supported by Fondazione Cassa di Risparmio di Perugia, Italy, project no. 2010.011.0398.
Glossary
Abbreviations:
- OPC
oropharyngeal candidiasis
- ROI
region of interest
- PMNL
polymorphonuclear leukocyte
- s.c.
subcutaneous
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/23529
References
- 1.Sangeorzan JA, Bradley SF, He X, Zarins LT, Ridenour GL, Tiballi RN, et al. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97:339–46. doi: 10.1016/0002-9343(94)90300-X. [DOI] [PubMed] [Google Scholar]
- 2.Revankar SG, Dib OP, Kirkpatrick WR, McAtee RK, Fothergill AW, Rinaldi MG, et al. Clinical evaluation and microbiology of oropharyngeal infection due to fluconazole-resistant Candida in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26:960–3. doi: 10.1086/513950. [DOI] [PubMed] [Google Scholar]
- 3.Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis. 2002;185:521–30. doi: 10.1086/338836. [DOI] [PubMed] [Google Scholar]
- 4.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elahi S, Pang G, Clancy R, Ashman RB. Cellular and cytokine correlates of mucosal protection in murine model of oral candidiasis. Infect Immun. 2000;68:5771–7. doi: 10.1128/IAI.68.10.5771-5777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solis NV, Filler SG. Mouse model of oropharyngeal candidiasis. Nat Protoc. 2012;7:637–42. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enjalbert B, Rachini A, Vediyappan G, Pietrella D, Spaccapelo R, Vecchiarelli A, et al. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect Immun. 2009;77:4847–58. doi: 10.1128/IAI.00223-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.d’Enfert C, Vecchiarelli A, Brown AJ. Bioluminescent fungi for real-time monitoring of fungal infections. Virulence. 2010;1:174–6. doi: 10.4161/viru.1.3.11119. [DOI] [PubMed] [Google Scholar]
- 9.Doyle TC, Nawotka KA, Kawahara CB, Francis KP, Contag PR. Visualizing fungal infections in living mice using bioluminescent pathogenic Candida albicans strains transformed with the firefly luciferase gene. Microb Pathog. 2006;40:82–90. doi: 10.1016/j.micpath.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Farah CS, Ashman RB, Challacombe SJ. Oral candidosis. Clin Dermatol. 2000;18:553–62. doi: 10.1016/S0738-081X(00)00145-0. [DOI] [PubMed] [Google Scholar]
- 11.Hayama K, Maruyama N, Abe S. Cell preparation method with trypsin digestion for counting of colony forming units in Candida albicans-infected mucosal tissues. Med Mycol. 2012;50:858–62. doi: 10.3109/13693786.2012.683538. [DOI] [PubMed] [Google Scholar]
- 12.Pietrella D, Enjalbert B, Zeidler U, Znaidi S, Rachini A, Vecchiarelli A, et al. A luciferase reporter for gene expression studies and dynamic imaging of superficial Candida albicans infections. Methods Mol Biol. 2012;845:537–46. doi: 10.1007/978-1-61779-539-8_39. [DOI] [PubMed] [Google Scholar]
- 13.Bartie KL, Williams DW, Wilson MJ, Potts AJ, Lewis MA. Differential invasion of Candida albicans isolates in an in vitro model of oral candidosis. Oral Microbiol Immunol. 2004;19:293–6. doi: 10.1111/j.1399-302X.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 14.Malic S, Hill KE, Ralphs JR, Hayes A, Thomas DW, Potts AJ, et al. Characterization of Candida albicans infection of an in vitro oral epithelial model using confocal laser scanning microscopy. Oral Microbiol Immunol. 2007;22:188–94. doi: 10.1111/j.1399-302X.2007.00344.x. [DOI] [PubMed] [Google Scholar]