The aim of this study was to compare the pathogenicity potential of Staphylococcus pseudintermedius strains isolated from healthy dogs and dogs with evident symptoms of infection. Nearly all examined strains synthesized β-hemolysin, clumping factor, coagulase, DNase, protein A, and lipase. The only statistically significant intergroup difference pertained to protein A, synthesized more frequently in infected dogs. All strains were positive for siet and lukS/F genes. No sea, seb, sed, see and tst genes were detected. The sec gene was detected in 1/71 (1.4%) strains obtained from a healthy dog and in 2/120 (1.6%) strains from infected animals. The subsequent DNA sequencing analysis revealed that the amplified sec genes in all the S. pseudintermedius isolates were subgrouped into the type SECcanine.
In 1976, V. Hajek identified a new species, Staphylococcus intermedius, within animal biotype of S. aureus (E and F), based on its biochemical properties and G+C content.1 This species was considered a predominant cause of canine infections until 2005 when Devriese et al.2 identified another new species, S. pseudintermedius, based on the sequential analysis of 16S rRNA. In 2007, sequential analysis of sodA and hsp60 genes confirmed close relatedness of three species, S. intermedius, S. pseudintermedius and S. delphini, which are currently referred to as the S. intermedius group.3 Recent studies confirmed that S. pseudintermedius, corresponding to previous S. intermedius species, is the most commonly isolated canine staphylococcus.4 S. pseudintermedius can be obtained from the nose, oral mucosa, anus, groin, and skin of healthy dogs. Moreover, it often leads to opportunistic purulent dermatitis, otitis externa and conjunctivitis.4,5
Similarly to S. aureus, S. pseudintermedius synthesizes an array of invasion and virulence factors. These include factors enabling adhesion to host’s cells or extracellular matrix (clumping factor and biofilm), toxins, and factors modulating host’s immune system (hemolysins, leukotoxin, exfoliative toxins and enterotoxins). Another group includes factors enabling the microorganism to spread within host’s body, such as coagulase, DNase, protein A or lipases.6
However, our knowledge on the pathogenesis of S. pseudintermedius is very limited; to date, the majority of virulence factors have not been characterized in detail. Consequently, the aim of this study was to compare the pathogenicity potential of S. pseudintermedius isolated from healthy dogs with no history of any infection-related symptoms for at least one year preceding the study, to that obtained from dogs with evident symptoms of infection.
The study included 71 isolates of S. pseudintermedius obtained from healthy dogs and 120 S. pseudintermedius isolates from diseased dogs. The strains were obtained from 369 dogs of both genders from ten veterinary practices, between February 2008 and December 2011, in four cities of Northern Poland (Gdansk, Gdynia, Lębork and Pruszcz Gdanski). The study included 172 completely healthy dogs with no history of any infection-related symptoms for at least one year prior to sampling. Three swabs were taken from each dog from this group. One cotton-tipped culture swab was taken from one anterior nostril, the second from the mouth, and the third from the perineal region. Additionally, samples from 197 diseased dogs were obtained by swabbing diseased sites with a sterile cotton swab or by urinary bladder catheterization in the case of cystitis. The samples were taken only from dogs with evident symptoms of infection: dermatitis (superficial pyodermatitis, abscess, and deep pyodermatitis, n = 109), external otitis (n = 31), conjunctivitis (n = 18), vaginitis (n = 12), rhinitis (n = 11), pharyngitis (n = 10) and cystitis (n = 6). In order to avoid duplication of results, only one isolate was considered if various isolates belonged to the same species and characterized by similar susceptibility were obtained from a particular dog. The examined strains were preserved with Tryptic Soy Bullion (TSB) and 15% glycerol at a temperature of –70°C.
Specimens were subcultured onto Columbia blood agar and incubated at 35°C for 24 h. Suspected staphylococcal isolates were identified on the basis of colony characteristics, Gram-stained appearance, and hemolysis. For the identification of S. pseudintermedius species, polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method was used. This method is based on MboI restriction gene of the pta fragment.7
The abilities to synthesize β-hemolysin, coagulase and clumping factor (CF) were tested as previously described.8 DNase activity was detected by flooding DNase test agar with toluidine blue O (Merck).9 Lypolytic properties of examined strains were tested in TSA medium (Difco) with 1% Tween 80 and 0.1% CaCl2 (Serva).10
Protein A was estimated with the dot blot method. Namely, 10 µL of overnight broth cultures were spotted onto nitrocellulose (Schleicher and Schuell) sheets and air-dried. Non-specific binding sites were blocked with 3% bovine serum albumin (Serva) in PBS for 1 h at room temperature; next, peroxidase-conjugated rabbit anti-human IgG (DAKO) was added to a concentration of 1/1,000 for another hour. After washing in PBS and in TBS (10 mM, pH 7.4), the sheet was immersed in freshly prepared 4-chloronaphtol reagent to develop the color. The amount of color was assumed to be dependent on the binding of gamma globulin by protein A.11 S. aureus Cowan I strain served as positive control strain.
Detection of enterotoxins (sea, seb, sec, sed and see) and tst (toxic shock syndrome toxin) genes was performed as described previously.12 The amplification of lukS/F (leukotoxin) and siet (exfoliative toxin) genes was performed according to Futagawa-Saito et al.13 and Lautz et al.14 respectively (Table 1). DNA amplification was performed in a Perkin Elmer 2400 thermocycler (Norwalk). The PCR products were analyzed on 2% agarose gel (Sigma) in the presence of ethidium bromide.
Table 1. Primer sequences and thermal conditions of PCR analysis.
| Gene | Oligonucteotide sequence (5′-3′) | Temperature of annealing | Size of PCR product (bp) | Reference |
|---|---|---|---|---|
|
Pta |
AAA GAC AAA CTT TCA GGT AA GCA TAA ACA AGC ATT GTA CCG |
53°C |
320 |
Bannoehr et al.7 |
|
Sea |
CCT TTG GAA ACG GTT AAA ACG TCT GAA CCT TCC CAT CAA AAA C |
55°C |
127 |
Becker et al.15 |
|
Seb |
TCG CAT CAA ACT GAC AAA CG GCA GGT ACT CTA TAA GTG CCT GC |
55°C |
477 |
Becker et al.15 |
|
Sec |
CTC AAG AAC TAG ACA TAA AAG CTA GG TCA AAA TCG GAT TAA CAT TAT CC |
55°C |
271 |
Becker et al.15 |
|
Sed |
CTA GTT TGG TAA TAT CTC CTT TAA ACG TTA ATG CTA TAT CTT ATA GGG TAA ACA TC |
55°C |
319 |
Becker et al.15 |
|
See |
CAG TAC CTA TAG ATA AAG TTA AAA CAA GC TAA CTT ACC GTG GAC CCT TC |
55°C |
178 |
Becker et al.15 |
|
Tst |
AAG CCC TTT GTT GCT TGC G ATC GAA CTT TGG CCC ATA CTT T |
55°C |
445 |
Becker et al.15 |
|
Siet |
ATG GAA AAT TTA GCG GCA TCT GG CCA TTA CTT TTC GCT TGT TGT GC |
56°C |
359 |
Lautz et al.14 |
|
lukS |
TGT AAG CAG CAG AAA ATG GGG GCC CGA TAG GAC TTC TTA CAA |
57°C |
503 |
Futagawa-Saito et al.13 |
| luk F | CCT GTC TAT GCC GCT AAT CAA AGG TCA TGG AAG CTA TCT CGA |
57°C | 572 | Futagawa-Saito et al.13 |
For sequencing the 271-bp sec, amplicons of the three S. pseudintermedius strains were purified by gel filtration (Centri-Sep columns, Princeton Separations). The sequencing was performed by DNA sequencer (PerkinElmer Corp.). The investigated sequences were compared with seccanine and sec1–3 sequences.15
The ability of the isolates to form biofilm was investigated by a method described by Stapanovic et al.16 with some modifications. Bacteria were cultivated overnight in TBS (Gras). Each culture was diluted 1:100 in the same medium and, subsequently, 200 μL of the diluted bacterial suspension was transferred, in triplicates, into the wells of sterile 96-well polystyrene microtiter plates (Becton Dickinson) and incubated at 37°C for 24 h. The negative control contained only the growth medium. The plates were washed twice with 200 μL of PBS (pH 7.2) and dried at room temperature prior to adding 1% Hucker crystal violet solution. The plates were incubated at room temperature for 15 min before excess dye was removed by washing with water. The bound dye was dissolved in 150 μL of 95% ethanol. The absorbance of the adherent biofilm at 570 nm (OD570) was measured in a microplate reader. For each isolate, the result was calculated by subtracting the median OD570 of the triplicates of the negative control from the median OD570 of the triplicates of the samples. Three independent experiments were performed and the mean of the three experiments was calculated based on the median from each experiment. Based on the OD produced by bacterial films, strains were classified into the following categories: no biofilm producers (OD ≤ ODc), weak biofilm producers (ODc < OD ≤ 2 × ODc), moderate (2 × ODc < OD ≤ 4 × ODc) and strong biofilm producers (4 × ODc < OD), as proposed by Stapanovic et al.16 The cutoff OD (ODc) was defined as three standard deviations above the mean OD of the negative control.
The fractions of staphylococcal isolates were presented as number and percentage distributions and compared among the studied groups of dogs with Pearson’s chi-square test and Fischer’s exact test. All calculations were performed with the Statistica 10 (StatSoft®) package, with the level of significance set at p ≤ 0.05.
Both S. pseudintermedius strains isolated from healthy dogs and those obtained from infected dogs synthesized β-hemolysin, CF, coagulase, DNase, protein A and lipase. The only statistically significant intergroup difference pertained to protein A (Table 2).
Table 2. Expression of phenotypic pathogenicity factors in S. pseudintermedius isolates.
| Source | Beta-hemolysin | Clumping factor | Coagulase | DNase | Protein A | Lipase |
|---|---|---|---|---|---|---|
| Healthy dogs (n = 71) |
70 (98.6%) |
10 (14.1%) |
66 (92.9%) |
71 (100%) |
1 (1.4%) |
70 (98.6%) |
| Infected dogs (n = 120) |
118 (98.3%) |
23 (19.2%) |
116 (96.6%) |
120 (100%) |
17 (14.2%) |
117 (97.5%) |
| p | 0.890 | 0.396 | 0.242 | - | 0.004 | 0.611 |
All isolates were positive for siet and lukS/F genes. No sea, seb, sed, see and tst genes were detected. The sec gene was detected in 1/71 (1.4%) strains obtained from a healthy dog and in 2/120 (1.6%) strains from infected animals (p = 0.689). The subsequent DNA sequencing analysis revealed that the amplified sec genes in all the S. pseudintermedius isolates were subgrouped into the type SECcanine (Fig. 1).
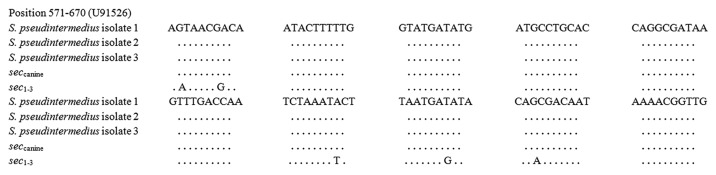
Figure 1. Alignment of DNA sequences for sec genes of three investigated isolates of S. pseudintermedius and reference sequences of S. intermedius canine subtype, and S. aureus sec 1–3 subtype. The EMBL accession numbers of the sec nucleotide sequences used for the alignment are as follows: seccanine, U91526; sec1, X05815; sec2, DQ192646; sec3, X51661. The sequences of the three S. aureus subtypes were merged, since there were not differences in the aligned region as showed here. Dots indicate identity.
No significant differences with regards to biofilm formation were documented between the strains isolated from healthy and infected dogs; most of these strains synthesized medium or high amounts of biofilm (Table 3). All strains isolated from conjunctivitis synthesized large amounts of biofilm. No association between the isolation site and the intensity of biofilm synthesis was observed for the strains isolated from other clinical materials.
Table 3. Formation of biofilm by S. pseudintermedius isolates.
| Formation of biofilm | Healthy dogs (n = 71) | Infected dogs (n = 120) | p |
|---|---|---|---|
| No biofilm producer |
0 |
0 |
- |
| Weak biofilm producer |
3 (4.2%) |
8 (6.6%) |
0.484 |
| Moderate biofilm producer |
45 (63.4%) |
63 (52.5%) |
0.143 |
| Strong biofilm producer | 23 (32.4%) | 49 (40.8%) | 0.245 |
Analysis of the prevalence of phenotypic virulence factors among the examined isolates of S. pseudintermedius revealed that nearly all of them were characterized by the presence of β-hemolysin, coagulase, DNase and lipase. The presence of these factors is consistent with the characteristic of new S. pseudintermedius species, published by Devriese et al.2 According to this description, S. pseudintermedius strains are CF-negative. In contrast, our study revealed that nearly 20% of isolates are CF-positive. On the other hand, Futagawa-Saito et al.17 analyzed strains of S. intermedius group and revealed that more than half of them were CF-positive. Plausibly, the discrepancies can result from insufficient evidence with regards to the pathogenicity of newly established species S. pseudintermedius; further research is required in this matter.
Staphylococcal protein A is a wall-anchored surface protein with four or five domains that can each bind to the Fc region of IgG. The interaction between protein A and IgG coats the surface of the cell with IgG molecules that cannot be recognized by the neutrophil Fc receptor and activate the complement by the classical pathway as a result of incorrect orientation. This explains the anti-phagocytic effect of protein A in vitro and the reason for constituting a virulence factor in several models of animal infection.18 Protein A is encoded by spa gene, located on chromosome. Despite wide utilization of the polymorphism of this gene for epidemiological studies of staphylococci, including S. pseudintermedius,5 the synthesis of protein A has not been studied in S. pseudintermedius isolates thus far. Futagawa-Saito et al.17 were the only ones to observe that 54.5% of canine S. intermedius strains show the expression of protein A and CF on latex agglutination test. Our study is the first to examine the expression of protein A by means of dot blot in a large number of canine strains. We have unambiguously confirmed that the strains from infected dogs synthesize protein A markedly more frequently than those from healthy dogs. Protein A was the only phenotypic pathogenicity factor that distinguished infected and non-infected dogs. This finding is not surprising in view of the evidence that protein A is a significant and established pathogenicity factor of S. aureus.18 Our findings suggest that the same pertains to the newly established S. pseudintermedius species.
Panton-Valentine leukocidin of S. aureus is a cytotoxin that causes leukocyte destruction and tissue necrosis. A similar toxin, biocomponent leukotoxin Luk-I, encoded by two genes, lukS/F, is also produced by S. pseudintermedius.17 All strains analyzed in our study, both from healthy and infected dogs, had both leukotoxin genes, lukS/F. Also, in research published by other authors all analyzed clones were positive for leukotoxin genes, suggesting clone-specific ability of toxin synthesis.5,13 However, in view of our findings, the determination of given pathogenicity of S. pseudintermedius based on the presence of lukS/F genes seems less unambiguous.
All strains of S. pseudintermedius included in our study, isolated from both healthy and infected dogs, possessed exfoliative toxin genes. S. pseudintermedius exfoliative toxin (SIET), first described by Terauchi et al.,19 plays a potential role in the pathogenesis of canine pyoderma and chronic otitis.19 The dog injected with SIET developed clinical signs such as erythema, exfoliation, and crusting, which are similar to symptoms seen in canine pyoderma and human staphylococcal scaled skin syndrome.19 However, similarly to our study, a number of other authors confirmed the presence of siet gene in all analyzed strains rather than only in those isolated from skin lesions.5,14,20 Our study adds to this evidence, revealing that this gene is present in all S. pseudintermedius, even those isolated from healthy dogs. The role of SIET in pathogenicity of the species in question is even more unclear since, in a recent study, the intradermal injection of recombinant SIET in three dogs did not cause any clinically- or histopathologically evident lesions.6
Superantigens are a class of antigens that cause non-specific activation of T cells resulting in polyclonal T cell proliferation and massive cytokine release. Staphylococcal superantigens include enterotoxins, responsible for the signs of food poisoning, as well as the toxic shock syndrome toxin (TSST-1). Similar to previous studies,20-22 our study analyzing the presence of genes encoding the most important enterotoxins A-E (SEA-SEE) and TSST-1, revealed solely the sec gene. In previous studies, the prevalence of sec ranged between 24.3%20 and 0.6%;21 this gene was identified in 1.6% of all dogs included in our study. Edwards et al.23 have identified a canine type SEC from canine pyoderma isolates, distinct from other staphylococcal enterotoxins, but sharing their ability to induce vomiting and T cell proliferation. Our DNA sequencing analysis revealed that the amplified sec genes in all the S. pseudintermedius isolates represented SECcanine type, which confirmed their enteropathogenic potential.
Staphylococci form biofilm on the surface of host’s tissues as well as on abiotic surfaces, where bacterial cells are immersed within polysaccharide excretion of amorphic mucus. Biofilm promotes the survival of bacteria, protecting them against physicochemical factors and immune mechanisms of the host. As a result of biofilm fragmentation and detachment, the bacteria spread throughout the body and colonize new sites, leading to the chronic and recurrent character of resultant infection. Bacteria that are immersed in biofilm show higher resistance to antibiotics, whose effective concentrations have to be many times higher than in the case of planktonic cells.24 While the synthesis of biofilm by various strains of S. aureus and S. epidermidis has been quite extensively studied, only sparse studies have dealt with the problem in question in staphylococci isolated from dogs.25 We tested a large number of S. pseudintermedius isolates for their biofilm formability. The method of microtiter plates used in this study is one of the most frequently applied screening methods, and is considered both reliable and sensitive. Our study revealed that the majority of S. pseudintermedius strains generated moderate or large amounts of biofilm. However, there were no statistically significant differences in this matter between the strains obtained from healthy and infected dogs. An important finding of our study is the observation that all strains isolated from conjunctivitis cases synthesized large amounts of biofilm. S. intermedius group is one of the most frequently encountered staphylococcal species in the conjunctival sac of clinically normal dogs as well as in dogs with ulcerative keratitis.26,27 Previous studies confirmed that biofilm plays an undisputable role in various ophthalmic infections in humans, including staphylococcal infections.24 However, to the best of our knowledge this study is the first to document the synthesis of large amounts of biofilm by S. pseudintermedius strains isolated from canine conjunctivitis. Previous studies revealed that bacterial biofilms may participate in ocular infections by allowing bacteria to persist on abiotic surfaces that come in contact with, or are implanted, in the eye and by direct biofilm formation on the biotic surface of the eye.24 Since the abiotic surfaces were not found in any dogs with conjunctivitis included in our study, it can be supposed that the enhanced synthesis of biofilm by studied S. pseudintermedius strains could play a role in the course of ocular inflammation in vivo.
In conclusion, our comparative analysis of pathogenicity factors of S. pseudintermedius has shown that infected and healthy dogs differed significantly in terms of protein A formation; this suggests that this protein may potentially play a role in the pathogenicity of S. pseudintermedius. Moreover, we have documented for the first time that S. pseudintermedius strains isolated from canine conjunctivitis are able to synthesize large amounts of biofilm.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/23526
References
- 1.Hajek V. Staphylococcus intermedius, a new species isolated from animals. Int J Syst Bacteriol. 1976;26:401–8. doi: 10.1099/00207713-26-4-401. [DOI] [Google Scholar]
- 2.Devriese LA, Vancanneyt M, Baele M, Vaneechoutte M, De Graef E, Snauwaert C, et al. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int J Syst Evol Microbiol. 2005;55:1569–73. doi: 10.1099/ijs.0.63413-0. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki T, Kikuchi K, Tanaka Y, Takahashi N, Kamata S, Hiramatsu K. Reclassification of phenotypically identified staphylococcus intermedius strains. J Clin Microbiol. 2007;45:2770–8. doi: 10.1128/JCM.00360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garbacz K, Zarnowska S, Piechowicz L, Haras K. Staphylococci isolated from carriage sites and infected sites of dogs as a reservoir of multidrug resistance and methicillin resistance. Curr Microbiol. 2013;66:169–73. doi: 10.1007/s00284-012-0254-9. [DOI] [PubMed] [Google Scholar]
- 5.Ruscher C, Lübke-Becker A, Semmler T, Wleklinski CG, Paasch A, Soba A, et al. Widespread rapid emergence of a distinct methicillin- and multidrug-resistant Staphylococcus pseudintermedius (MRSP) genetic lineage in Europe. Vet Microbiol. 2010;144:340–6. doi: 10.1016/j.vetmic.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Bannoehr J, Guardabassi L. Staphylococcus pseudintermedius in the dog: taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet Dermatol. 2012;23:253–66, e51-2. doi: 10.1111/j.1365-3164.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- 7.Bannoehr J, Franco A, Iurescia M, Battisti A, Fitzgerald JR. Molecular diagnostic identification of Staphylococcus pseudintermedius. J Clin Microbiol. 2009;47:469–71. doi: 10.1128/JCM.01915-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devriese LA. A simplified system for biotyping Staphylococcus aureus strains isolated from animal species. J Appl Bacteriol. 1984;56:215–20. doi: 10.1111/j.1365-2672.1984.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 9.Waller JR, Hodel SL, Nuti RN. Improvement of two toluidine blue O-mediated techniques for DNase detection. J Clin Microbiol. 1985;21:195–9. doi: 10.1128/jcm.21.2.195-199.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sierra G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek. 1957;23:15–22. doi: 10.1007/BF02545855. [DOI] [PubMed] [Google Scholar]
- 11.Kerr S, Kerr GE, Mackintosh CA, Marples RR. A survey of methicillin-resistant Staphylococcus aureus affecting patients in England and Wales. J Hosp Infect. 1990;16:35–48. doi: 10.1016/0195-6701(90)90047-R. [DOI] [PubMed] [Google Scholar]
- 12.Becker K, Roth R, Peters G. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J Clin Microbiol. 1998;36:2548–53. doi: 10.1128/jcm.36.9.2548-2553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Futagawa-Saito K, Sugiyama T, Karube S, Sakurai N, Ba-Thein W, Fukuyasu T. Prevalence and characterization of leukotoxin-producing Staphylococcus intermedius in Isolates from dogs and pigeons. J Clin Microbiol. 2004;42:5324–6. doi: 10.1128/JCM.42.11.5324-5326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lautz S, Kanbar T, Alber J, Lämmler C, Weiss R, Prenger-Berninghoff E, et al. Dissemination of the gene encoding exfoliative toxin of Staphylococcus intermedius among strains isolated from dogs during routine microbiological diagnostics. J Vet Med B Infect Dis Vet Public Health. 2006;53:434–8. doi: 10.1111/j.1439-0450.2006.00999.x. [DOI] [PubMed] [Google Scholar]
- 15.Becker K, Keller B, von Eiff C, Brück M, Lubritz G, Etienne J, et al. Enterotoxigenic potential of Staphylococcus intermedius. Appl Environ Microbiol. 2001;67:5551–7. doi: 10.1128/AEM.67.12.5551-5557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Cirković I, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115:891–9. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 17.Futagawa-Saito K, Ba-Thein W, Sakurai N, Fukuyasu T. Prevalence of virulence factors in Staphylococcus intermedius isolates from dogs and pigeons. BMC Vet Res. 2006;2:4. doi: 10.1186/1746-6148-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmqvist N, Foster T, Tarkowski A, Josefsson E. Protein A is a virulence factor in Staphylococcus aureus arthritis and septic death. Microb Pathog. 2002;33:239–49. doi: 10.1006/mpat.2002.0533. [DOI] [PubMed] [Google Scholar]
- 19.Terauchi R, Sato H, Hasegawa T, Yamaguchi T, Aizawa C, Maehara N. Isolation of exfoliative toxin from Staphylococcus intermedius and its local toxicity in dogs. Vet Microbiol. 2003;94:19–29. doi: 10.1016/S0378-1135(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 20.Yoon JW, Lee KJ, Lee SY, Chae MJ, Park JK, Yoo JH, et al. Antibiotic resistance profiles of Staphylococcus pseudintermedius isolates from canine patients in Korea. J Microbiol Biotechnol. 2010;20:1764–8. [PubMed] [Google Scholar]
- 21.Futagawa-Saito K, Suzuki M, Ohsawa M, Ohshima S, Sakurai N, Ba-Thein W, et al. Identification and prevalence of an enterotoxin-related gene, se-int, in Staphylococcus intermedius isolates from dogs and pigeons. J Appl Microbiol. 2004;96:1361–6. doi: 10.1111/j.1365-2672.2004.02264.x. [DOI] [PubMed] [Google Scholar]
- 22.Youn JH, Koo HC, Ahn KJ, Lim SK, Park YH. Determination of staphylococcal exotoxins, SCCmec types, and genetic relatedness of Staphylococcus intermedius group isolates from veterinary staff, companion animals, and hospital environments in Korea. J Vet Sci. 2011;12:221–6. doi: 10.4142/jvs.2011.12.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards VM, Deringer JR, Callantine SD, Deobald CF, Berger PH, Kapur V, et al. Characterization of the canine type C enterotoxin produced by Staphylococcus intermedius pyoderma isolates. Infect Immun. 1997;65:2346–52. doi: 10.1128/iai.65.6.2346-2352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zegans ME, Becker HI, Budzik J, O’Toole G. The role of bacterial biofilms in ocular infections. DNA Cell Biol. 2002;21:415–20. doi: 10.1089/10445490260099700. [DOI] [PubMed] [Google Scholar]
- 25.Osland AM, Vestby LK, Fanuelsen H, Slettemeås JS, Sunde M. Clonal diversity and biofilm-forming ability of methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother. 2012;67:841–8. doi: 10.1093/jac/dkr576. [DOI] [PubMed] [Google Scholar]
- 26.Prado MR, Rocha MF, Brito EH, Girão MD, Monteiro AJ, Teixeira MF, et al. Survey of bacterial microorganisms in the conjunctival sac of clinically normal dogs and dogs with ulcerative keratitis in Fortaleza, Ceará, Brazil. Vet Ophthalmol. 2005;8:33–7. doi: 10.1111/j.1463-5224.2005.04061.x. [DOI] [PubMed] [Google Scholar]
- 27.Varges R, Penna B, Martins G, Martins R, Lilenbaum W. Antimicrobial susceptibility of Staphylococci isolated from naturally occurring canine external ocular diseases. Vet Ophthalmol. 2009;12:216–20. doi: 10.1111/j.1463-5224.2009.00701.x. [DOI] [PubMed] [Google Scholar]


