Abstract
Nitric oxide (NO) is known to be an important inflammatory mediator with a potential role in gastrointestinal diseases. We prospectively studied the luminal NO levels in 51 patients with infectious gastroenteritis, 35 patients with nonenteric bacterial infections, and 11 healthy control subjects. The levels of proinflammatory cytokines were simultaneously measured in the stools of patients with gastroenteritis. Rectal gas was sampled with balloon catheters made of silicone and was analyzed for NO levels by chemiluminescence. The median rectal NO level was 2,450 ppb in the acute phase of gastroenteritis and gradually decreased to 225 ppb after 3 to 8 weeks, whereas the median NO values were 150 ppb in patients with nonenteric bacterial infections and 100 ppb in healthy control subjects. Patients with Salmonella, Shigella, and Campylobacter infections generally had more severe symptoms and a higher median NO level (17,250 ppb) than patients with Clostridium difficile-associated diarrhea (median NO value, 275 ppb). Interleukin-1β levels were elevated in 82% of the patients at disease onset and decreased during the convalescent phase. In contrast, gamma interferon was detected in only 16% of the patients and was predominantly collected in stool samples collected during the subacute and convalescent stages. Our data point to the possibility of using this easy, minimally invasive method for luminal NO measurement as a diagnostic tool, among others, to evaluate the degree of intestinal inflammation in patients with infectious gastroenteritis.
The clinical spectrum of infectious gastroenteritis is wide, ranging from asymptomatic carriage or mild enteritis to severe watery diarrhea or colitis (4-6). The severity of symptoms reflects the degree of intestinal inflammation. Noninflammatory enteritis, which occurs as a result of, for example, cholera and infections caused by enterotoxigenic Escherichia coli, is characterized by frequent, voluminous, watery diarrhea, while inflammatory enteritis, which is caused by invasive organisms such as Shigella species and Campylobacter species, is characterized by symptoms of colitis with tenesmus and bloody, mucoid stools.
Usually, the degree of inflammation in the gut is diagnosed through invasive methods such as direct inspection of the intestinal mucosa by colonoscopy. A more simple means of measuring intestinal inflammation, besides microbiological diagnostic procedures, would be valuable for distinguishing between colonization and active disease.
Nitric oxide (NO) is a pluripotent biological messenger with a potential role in gastrointestinal diseases. During intestinal inflammation various mediators, such as arachidonic acid metabolites and cytokines, are produced at increased levels. Some of the proinflammatory cytokines, e.g., tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and gamma interferon (IFN-γ), as well as bacterial toxins are known to upregulate inducible NO synthase (iNOS) and thus trigger the production of excessive and potentially toxic NO levels in the intestine. Direct measurement of NO levels is difficult to perform in most biologic systems because of the short-lived nature of this gas, especially in the presence of proteins, e.g., hemoglobin. Therefore, NO is usually measured by evaluation of the levels of its more stable oxidation products, nitrite and nitrate. NO in the gaseous form is, however, fairly stable, which has enabled direct measurement of its levels in air-filled hollow organs, such as the intestine and airways (14, 15). A novel minimally invasive technique for measurement of NO levels in the rectum has been developed. By this technique, increased rectal NO levels have been shown in patients with intestinal inflammatory disorders such as ulcerative colitis, Crohn's disease, collagenous colitis, and celiac disease (7, 8, 16, 19). We have recently shown that rectal NO levels are greatly increased in patients with infectious gastroenteritis, including Clostridium difficile-associated diarrhea (CDAD) (9). This indicates that measurement of rectal NO levels might be a simple, noninvasive, and useful method for evaluating the degree of inflammation in patients with infectious gastroenteritis.
The aim of this study was to determine rectal NO levels as well as the levels of proinflammatory cytokines in stools and their variation over time in patients with infectious gastroenteritis.
MATERIALS AND METHODS
Fifty-one adult patients who were admitted to the Department of Infectious Diseases at Huddinge University Hospital (Stockholm, Sweden) because of infectious diarrhea were included in the study. Data regarding the date of onset of diarrhea, stool frequency, fever, and other concomitant symptoms were registered. Blood and stool samples were collected; and rectal NO levels were measured on admission (acute phase), 2 to 7 days after admission (subacute phase), and at follow-up 3 to 8 weeks later (convalescent phase). Infectious diarrhea was defined as a change in bowel habits during the previous 14 days with an acute onset and with three or more loose stools during the previous 24 h not attributable to any other known cause. The study was approved by the ethics committee of the Karolinska Institute, Stockholm, Sweden.
Control subjects.
Thirty-five patients (infected controls) who had nonenteric bacterial infections (erysipelas, pneumonia, pyelonephritis) but no symptoms of intestinal disease and who were treated in the same department as the study subjects, as well as 11 healthy individuals (volunteer medical staff), were recruited as control subjects. Each individual was examined once for measurement of rectal NO levels.
Laboratory investigations.
Stool specimens were analyzed by established methods for enteropathogenic bacteria, including Salmonella species, Shigella species, Campylobacter species, Yersinia enterocolitica, Aeromonas species, and C. difficile. If the initial stool sample was positive for an enteropathogen, another fecal specimen was obtained at follow-up. Slide preparations were examined for ova and parasites by established methods. The samples were analyzed for C-reactive protein (CRP) at the hospital laboratory.
Rectal NO measurements.
NO sampling was done by using a thin all-silicone Foley catheter (10 Argyle; Sherwood Medical, Tullamore, Ireland) inserted approximately 10 cm into the rectum. The catheter balloon was inflated with 10 ml of ambient air containing less than 5 ppb of NO and was left in place for 10 min to equilibrate with the gases in the rectum. The balloon was then emptied and the gas was immediately analyzed for NO content by using a chemiluminescence analyzer (Aerocrine AB, Stockholm, Sweden), as described by Herulf et al. (7). Analyses of the results of the in vitro experiments have shown that within this equilibration period the level of recovery of NO reaches a reproducible plateau (7).
Cytokine assays.
Stool samples were stored at −70°C until they were processed for cytokine analysis, as previously described in detail (21). The levels of IL-1β, IL-4, TNF-α, and IFN-γ in processed stool samples were determined by multiplex cytokine analyses with Fluorokine MAP kits (R&D, Minneapolis, Minn.) and the Luminex100 instrument (Luminex Corp., Austin, Tex.). The lower limits of detection for IL-1β, IL-4, TNF-α, and IFN-γ ranged between 3 and 20 pg/ml.
Statistical methods.
A normal distribution was achieved by logarithmic transformation. Student's t test was used for comparison between groups. NO levels over time were studied by analysis of variance by the Tukey-Kramer honestly significant difference test as the post hoc test for paired comparisons.
RESULTS
Patient characteristics and microbiological findings.
The age and sex distributions of the patients and the control subjects as well as the median CRP and rectal NO levels on admission are shown in Table 1. Patients with CDAD were generally older than the remaining patients (P < 0.007) (Table 1).
TABLE 1.
Characteristics of 51 patients with infectious diarrhea, 35 patients with nonenteric bacterial infections, and 11 healthy control subjects
| Subject | Median (range) age (yr) | Sex (no. of males/ no. of females) | Median (range) CRP level (mg/liter) | Median (range) rectal NO level (ppb) |
|---|---|---|---|---|
| Patients with: | ||||
| Infectious diarrhea (n = 51) | 54 (19-89) | 27/24 | 132 (13-412) | 2,450 (100-53,900) |
| CDAD (n = 12) | 83 (32-89) | 6/6 | 86 (13-293) | 275 (100-46,700) |
| Bacterial enteritisa (n = 22) | 50 (30-89) | 15/7 | 137 (20-292) | 17,250 (500-53,900) |
| Negative stool culture and C. difficile toxin B (n = 17) | 54 (19-86) | 6/11 | 137 (18-412) | 1,000 (100-19,050) |
| Nonenteric bacterial infectionsb (n = 35) | 64 (28-86) | 16/19 | 92 (11-515) | 150 (50-8,250) |
| Controls (n = 11) | 43 (28-59) | 10/1 | NDc | 100 (50-300) |
Caused by Salmonella spp., Campylobacter spp., Shigella spp., or Aeromonas spp.
Erysipelas, pneumonia, or pyelonephritis.
ND, not determined.
Of the 51 patients with infectious diarrhea, 34 (67%) were found to shed potential enteropathogens (double infections were found in three patients): C. difficile (n = 12), Salmonella species (n = 9), Campylobacter species (n = 12), Aeromonas species (n = 2), and Shigella species (n = 2). For all patients with a positive stool culture on admission, the stool specimens obtained at follow-up were negative for enteropathogens. No parasites were found in slide preparations.
Intense diarrhea, defined as >10 bowel movements per day, was noted in 31 of 47 patients (66%) (Table 2) and was noted more often in patients with bacterial enteritis excluding CDAD and patients with negative stool cultures (16 of 22 [73%] and 12 of 17 [71%], respectively) than in patients with CDAD (3 of 12 [25%]) (P < 0.001). A fever (temperature, >38°C) was noted in 34 of 47 (72%) patients (Table 2). Of 12 patients with CDAD, 7 (58%) had a fever, whereas 16 of 22 (73%) patients with other bacterial enteritis and 15 of 17 (88%) patients with negative stool cultures had a fever.
TABLE 2.
Rectal NO and IL-1β levels in relation to symptoms and features of patients with infectious diarrhea
| Feature or symptom | No. (%) of patientsa | Median (range) rectal NO level (ppb) | Median (range) IL-1β level (pg/ml) |
|---|---|---|---|
| No. of bowel movementsb | |||
| <3/day | 1 (2) | 100 | 37 |
| 3-10/day | 15 (32) | 1,000 (100-46,700) | 135 (0-93,885) |
| >10/day | 31 (66) | 2,700 (100-43,750) | 3,539 (0-107,425) |
| Bloody stoolsb | |||
| Yes | 8 (17) | 125 (100-28,750) | 3,386 (37-9,108) |
| No | 39 (83) | 3,125 (100-46,700) | 1,356 (0-107,425) |
| Fever (temp [°C]) | |||
| <38 | 13 (28) | 600 (100-46,700) | 5,115 (37-47,255) |
| 38-39 | 15 (32) | 1,000 (100-28,750) | 3,693 (0-93,885) |
| >39 | 19 (40) | 8,525 (100-43,750) | 1,399 (4-107,425) |
| CRP level (mg/liter) | |||
| <10-100 | 19 (40) | 2,250 (100-28,750) | 2,730 (11-33, 391) |
| 100-200 | 19 (40) | 7,875 (100-38,250) | 2,250 (13-107, 425) |
| >200 | 9 (20) | 1,000 (100-53,900) | 291 (0-93, 885) |
A total of 47 patients were tested.
As observed by the patient.
All patients gradually improved during the subacute phase of the disease and recovered during the convalescent phase.
Rectal NO levels over time.
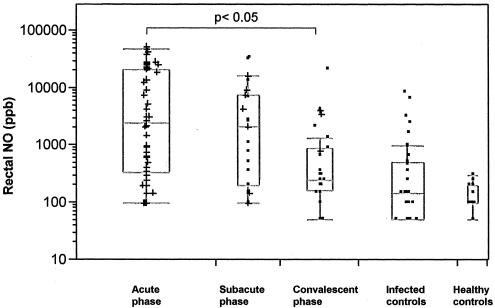
The kinetics of rectal NO levels in patients with infectious diarrhea during the acute, subacute, and convalescent phases of the disease as well as in control subjects are illustrated in Fig. 1. Although large interindividual differences were seen during the acute phase, generally high rectal NO levels were found (median value, 2,450 ppb; range, 100 to 53,900 ppb), but these gradually decreased in the subacute phase (median value, 2,375 ppb; range, 100 to 32,500 ppb) and approximated the levels in the healthy control subjects (median value, 100 ppb; range, 50 to 300 ppb) during the convalescent phase (median value, 225 ppb; range, 50 to 21,500 ppb). The NO levels obtained during the acute and subacute phases of the disease were significantly higher than those obtained during the convalescent phase (P < 0.05), which were in the same range as those found in the infected controls and the healthy control subjects. Thus, rectal NO levels generally decreased with the clinical improvement of the patients. However, a few patients also had high NO levels in the convalescent phase, even though they had recovered clinically (Fig. 1).
FIG. 1.
Rectal NO concentrations over time in 51 patients with infectious diarrhea, 35 patients with nonenteric bacterial infections (infected controls), and 11 healthy control subjects. The acute phase (n = 51) was from day 0 to 1 after admission to the hospital, the subacute phase (n = 22) was from days 2 to 7 after admission to the hospital, and the convalescent phase (n = 28) was from days 22 to 56 after admission to the hospital. The plus signs indicate the presence of diarrhea at the time of examination.
There was no evident correlation between NO levels and clinical symptoms during the acute stage of disease. Although patients with intense diarrhea during the acute phase had a higher median NO level than those with milder symptoms (2,700 and 1,000 ppb, respectively), the difference was not significant. There was also a tendency (not statistically significant) for NO levels to be higher in patients with a fever (temperature, >38°C; median value, 3,100 ppb) than in those with no fever (median value, 600 ppb) (P = 0.8). There was no correlation between NO levels and CRP levels (Table 2). Patients who reported bloody stools tended to have lower NO levels (median value, 125 ppb; range, 100 to 28,750 ppb) than the remaining patients (median value, 3,125 ppb; range, 100 to 46,700 ppb) (Table 2).
Patients with nonenteric bacterial infections (infected controls), including those with extremely high CRP levels (>300 mg/liter), generally had low NO levels (median value, 150 ppb; range, 50 to 8,250 ppb), comparable to the NO levels in the healthy controls (Fig. 1; Table 1).
The kinetics of rectal NO levels over time in patients with CDAD and other forms of bacterial enteritis, as well as those with negative stool cultures, showed that NO levels decreased concurrently with clinical improvement, as was the case in all patients with infectious diarrhea. The NO levels during the acute phase of the disease were generally higher in patients with bacterial enteritis (median value, 17,250 ppb; range, 500 to 53,900 ppb) and lower in patients with CDAD (median value, 275 ppb; range, 100 to 46,700 ppb) compared to those in all patients (median value, 2,450 ppb; range, 100 to 53,900 ppb) (P < 0.04 and P = 0.5 for bacterial enteritis and CDAD, respectively) (Table 1). Patients with negative stool cultures also generally had lower NO levels (median value, 1,000 ppb; range, 100 to 19,050 ppb) compared to those in all patients (P < 0.03). There were no significant differences between acute-phase NO levels in patients with Campylobacter, Salmonella, Shigella, or Aeromonas infections (data not shown).
Stool cytokine levels over time.
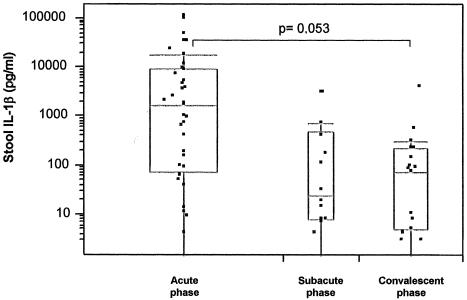
Stool IL-1β levels during the acute, subacute, and convalescent phases of the disease in patients with infectious diarrhea are illustrated in Fig. 2. IL-1β levels were elevated in 37 of 45 (82%) patients, and the levels decreased concurrently with the clinical improvement. High levels (median value, 1,721 pg/ml; range, 0 to 107,425 pg/ml) were found during the acute phase but rapidly decreased during the subacute phase (median value, 24.5 pg/ml; range, 0 to 2,973 pg/ml) and the convalescent phase (median value, 75 pg/ml; range, 0 to 3,810 pg/ml) (P = 0.053). As in the case of NO, large interindividual differences were seen. The IL-1β levels were generally higher, although not statistically significantly so, among patients with bacterial enteritis than among patients with CDAD or negative stool cultures. IL-1β levels in relation to the symptoms and features of the patients are shown in Table 2. There was no correlation between NO and IL-1β levels (data not shown).
FIG. 2.
Stool IL-1β concentrations over time in 37 patients with infectious diarrhea. The acute phase (n = 37) was from day 0 to 1 after admission to the hospital, the subacute phase (n = 14) was from days 2 to 7 after admission to the hospital, and the convalescent phase (n = 19) was from days 22 to 56 after admission to the hospital.
IFN-γ was detected in only 8 of 45 patients (18%) and was predominantly detected in stool samples collected during the subacute and convalescent phases. Detectable levels were found only in patients with bacterial enteritis (n = 5) and patients with CDAD (n = 3).
IL-4 and TNF-α were detected in stool samples from 15 of 45 (33%) and 5 of 45 (11%) patients, respectively. IL-4 was detected in 7 (14%) of the patients with bacterial enteritis and 6 (12%) of the patients with CDAD but in only 2 of the patients (2%) with negative stool cultures. TNF-α was detected only during the acute and subacute phases of the disease and only in patients with bacterial enteritis and CDAD.
DISCUSSION
In this study we have shown increased levels of rectal intraluminal NO in patients during the acute stage of infectious gastroenteritis, regardless of the etiology, compared to those in both patients with nonenteric bacterial infections and healthy volunteers, in accordance with the previous findings by Herulf et al. (9). NO levels were generally higher in patients with Salmonella, Shigella, and Campylobacter infections than in patients with enteritis of unknown etiology and patients with CDAD, who also had less severe clinical symptoms. Thus, the NO levels had a tendency to reflect the severity of disease. We also found that NO levels gradually decreased over time, in general, following clinical improvement of the patient.
The exact site of NO production as well as its role in inflammatory conditions of the intestines remains unclear (11, 23). It is likely that the NO present in the lumen is derived from the mucosa, since NO synthase has been detected there (2, 18). Thus, in patients with inflammatory bowel disease and high luminal NO levels, iNOS is upregulated in the colonic mucosa. It has previously been suggested that luminal NO is produced very superficially, since it would probably be degraded if it was produced in deeper layers due to the wide variety of scavenging systems found in the mucosa. Hemoglobin is a well-known scavenger of NO, and interestingly, we also found that patients with bloody stools tended to have lower NO levels.
There are conflicting results as to the detrimental or beneficial effects of NO. NO or its reaction products may be cytotoxic to host cells when it is produced in excess, and it has been shown to induce diarrhea in experiments with animals (10); on the other hand, however, NO might enhance the local host defense through its bacteriostatic and antiviral properties. Furthermore, McCafferty et al. (17) reported that colitis was more severe and of a longer duration in iNOS-deficient mice in an experimental mouse model of colitis.
Although proinflammatory cytokines like IL-1β have been shown to induce iNOS (23), we were unable to find a significant correlation between stool IL-1β levels and rectal NO levels during the acute stage of the disease. This could, however, possibly be explained by a temporal difference in the production of IL-1β and NO. The lack of correlation between NO and CRP levels could also be explained by this temporal difference in production.
Kinetically, and similar to the rectal NO levels, stool IL-1β levels showed a tendency to decline over time. During the acute stage in patients with infectious gastroenteritis, regardless of the etiology, the elevated stool IL-1β levels subsided with clinical progress, but they subsided much more rapidly than NO levels. TNF-α could be detected in only five patients and only during the acute and subacute phases, in line with its early proinflammatory role in the inflammatory cascade (3)
IFN-γ was found in only a minority of the patients but was found in these patients predominantly during the subacute and convalescent phases. This is in accordance with earlier findings for patients with Shigella infections. Raqib et al. (22) found low IFN-γ-levels at disease onset, and these gradually increased with progression of the disease, with the elevated levels persisting for at least 45 days.
In many enteric infections, asymptomatic carriage of the pathogen is almost as common as the disease itself, at least in some populations. Epidemiological studies have actually shown that the rate of C. difficile carriage might be as high as 10 to 35% in asymptomatic hospitalized patients who are treated with antibiotics (24). Due to high carriage rates in selected populations, the casual relationship between diarrhea and the findings of an enteropathogen in feces is thus not always evident. Treatment with antibiotics in such cases may be directed just toward the microorganism and may have no effect on the clinical disease. Indeed, some patients with CDAD had fairly low NO values and also fairly moderate symptoms. Whether these patients were sick from the C. difficile infection or just colonized with C. difficile remains unclear, however.
A direct inspection by endoscopy would reveal an ongoing inflammatory process, but such examinations are unpleasant for the patient and are also time-consuming. Measurement of rectal NO levels was, however, found to be rapid and simple and involved minimal discomfort to the patient. As we have also shown that other invasive bacterial infections do not result in high rectal NO levels, despite a high fever and elevated CRP levels, we can assume that the high NO levels found in patients with acute enteritis mirror local intestinal inflammation.
One might argue that the measurement of nitrate and nitrite levels in plasma (nitrate and nitrite are the stable end products of NO formation) would be even more simple to perform. Indeed, elevated levels were found in patients with acute gastroenteritis (1, 12) and cholera or shigellosis (20). However, the dietary intake of nitrate influences the concentration in plasma; moreover, the levels of accumulated nitrate and nitrite do not necessarily reflect the current levels of intestinal production.
Our data point to the possibility that the easy, minimally invasive method described here can be used as a tool, among others, for the diagnosis of selected cases of infectious enteritis and can aid the clinician in determining whom to treat with specific antibiotics and, through repeated measurements, in monitoring the clinical course of the illness. It must be kept in mind, however, that we also found patients who had clinical symptoms pointing to invasive enteric disease but whose initial NO levels were quite low. Furthermore, as high NO levels have also been shown in patients with active inflammatory bowel disease, such as Crohn's disease and ulcerative colitis (7, 13), these conditions should always be kept in mind in the differential diagnosis.
Acknowledgments
This work was supported by grants from the SSAC Foundation, the Ruth and Richard Julin Foundation, and the Swedish Foundation for Strategic Research and the Research Council.
We thank Gudmundur Axelsson for help with data processing and Åsa Lagergren and Anita Johansson for expert technical assistance.
REFERENCES
- 1.Åhren, C., L. Jungersten, and T. Sandberg. 1999. Plasma nitrate as an index of nitric oxide formation in patients with acute infectious diseases. Scand. J. Infect. Dis. 31:405-407. [DOI] [PubMed] [Google Scholar]
- 2.Boughton-Smith, N. K., S. M. Evans, C. J. Hawkey, A. T. Cole, M. Balsitis, and B. J. R. Whittle. 1993. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet 342:338-340. [DOI] [PubMed] [Google Scholar]
- 3.Braegger, C. P., S. Nicholls, S. H. Murch, S. Stephens, and T. T. MacDonald. 1992. Tumor necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 339:89-91. [DOI] [PubMed] [Google Scholar]
- 4.Du Pont, H. L. 1994. Infectious diarrhea. Aliment. Pharmacol. Ther. 8:3-13. [DOI] [PubMed] [Google Scholar]
- 5.Guerrant, R. L., J. M. Hughes, N. L. Lima, and J. Crane. 1990. Diarrhea in developed and developing countries: magnitude, special settings and etiologies. Rev. Infect. Dis. 12(Suppl. 1):S41-S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrant, R. L., T. van Gilder, T. S. Steiner, et al. 2001. Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis. 32:31-50. [DOI] [PubMed] [Google Scholar]
- 7.Herulf, M., T. Ljung, P. M. Hellström, E. Weitzberg, and J. O. Lundberg. 1998. Increased luminal nitric oxide in inflammatory bowel disease as shown with a novel minimally invasive method. Scand. J. Gastroenterol. 33:164-169. [DOI] [PubMed] [Google Scholar]
- 8.Herulf, M., L. Blomquist, T. Ljung, E. Weitzberg, and J. O. Lundberg. 2001. Increased rectal nitric oxide in coeliac disease after local challenge with gluten. Scand. J. Gastroenterol. 36:169-173. [DOI] [PubMed] [Google Scholar]
- 9.Herulf, M., B. Svenungsson, Å. Lagergren, et al. 1999. Increased nitric oxide in infective gastroenteritis. J. Infect. Dis. 180:542-545. [DOI] [PubMed] [Google Scholar]
- 10.Izzo, A. A., T. S. Gaginella, N. Mascolo, F. Borrelli, and F. Capasso. 1996. N-G-Nitro-l-arginine methyl ester reduces senna- and cascara-induced diarrhea and fluid secretion in the rat. Eur. J. Pharmacol. 301:137-142. [DOI] [PubMed] [Google Scholar]
- 11.Kubes, P., and D. M. McCafferty. 2000. Nitric oxide and intestinal inflammation. Am. J. Med. 109:150-158. [DOI] [PubMed] [Google Scholar]
- 12.Kukuruzovic, R., R. M. Robins-Browne, N. M. Anstey, and D. R. Brewster. 2002. Enteric pathogens, intestinal permeability and nitric oxide production in acute gastroenteritis. Pediatr. Infect. Dis. J. 21:730-739. [DOI] [PubMed] [Google Scholar]
- 13.Ljung, T., M. Herulf, E. Beijer, et al. 2001. Rectal nitric oxide assessment in children with Crohn disease and ulcerative colitis. Indicator of ileocaecal and colorectal affection. Scand. J. Gastroenterol. 36:1073-1076. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg, J. O., J. M. Lundberg, K. Alving, and E. Weitzberg. 1997. Nitric oxide and inflammation: the answer is blowing in the wind. Nat. Med. 3:30-31. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg, J. O., P. M. Hellström, J. M. Lundberg, and K. Alving. 1994. Greatly induced luminal nitric oxide in ulcerative colitis. Lancet 344:1673-1674. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg, J. O., M. Herulf, M. Olesen, et al. 1997. Increased nitric oxide production in collagenous and lymphocytic colitis. Eur. J. Clin. Investig. 27:869-871. [DOI] [PubMed] [Google Scholar]
- 17.McCafferty, D. M., J. S. Mudgett, M. G. Swain, and P. Kubes. 1997. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology 112:1022-1027. [DOI] [PubMed] [Google Scholar]
- 18.Middleton, S. J., M. Shorthouse, and J. O. Hunter. 1993. Increased nitric oxide synthesis in ulcerative colitis. Lancet 341:465-466. [DOI] [PubMed] [Google Scholar]
- 19.Perner, A., I. Norgaard, P. Madzen, and J. Rask-Madsen. 2002. Colonic production of nitric oxide gas in ulcerative colitis, collagenous colitis and uninflamed bowel. Scand. J. Gastroenterol. 37:183-188. [DOI] [PubMed] [Google Scholar]
- 20.Rabbani, G. H., S. Islam, A. K. Chowdhury, A. K. Mitra, M. J. Miller, and G. Fuchs. 2001. Increased nitrite and nitrate concentrations in sera and urine of patients with cholera or shigellosis. Am. J. Gastroenterol. 96:467-472. [DOI] [PubMed] [Google Scholar]
- 21.Raqib, R., A. Ljungdahl, A. A. Lindberg, U. Andersson, and J. Andersson. 1996. Local entrapment of interferon gamma in the recovery from Shigella dysenteriae type 1 infection. Gut 38:328-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raqib, R., B. Wretlind, P. K. Bardhan, U. Andersson, and J. Andersson. 1995. Cytokine secretion in acute shigellosis is correlated to disease activity and directed more to stool than plasma. J. Infect. Dis. 171:376-384. [DOI] [PubMed] [Google Scholar]
- 23.Salzman, A. L. 1995. Nitric oxide in the gut. New Horizon 3:352-364. [PubMed] [Google Scholar]
- 24.Wiström, C., S. R. Norrby, E. Myhre, et al. 2001. Frequency of antibiotic-associated diarrhea in 2462 antibiotic-treated hospitalized patients: a prospective study. J. Antimicrob. Chemother. 47:43-50. [DOI] [PubMed] [Google Scholar]