Abstract
Metastasis is a combination of biological events that makes the difference between cancer and other diseases. Metastasis requires flow of erroneous but precisely coordinated basic cellular activities like cell migration–invasion, cell survival–apoptosis, cell proliferation, etc. All of these processes require efficient regulation of cell attachment and detachment, which recruit integrin receptors in this flow of events. World literatures show several aspects of interrelation of integrins and metastasis. Integrin molecules are being used as prime target to battle metastasis. In this review we are collating the observations showing importance of integrin biology in regulation of metastasis and the strategies where integrin receptors are being used as targets to regulate metastasis.
Keywords: integrin, metastasis, MMP, invasion, adhesion
Introduction
Survival of eukaryotic organisms requires balanced interactions between cells and the extracellular matrix (ECM). Homeostasis is maintained by tight regulation of cell survival, proliferation, differentiation, and cell death (e.g., by apoptosis). Cancer cells disobey the normal restraints on cell division and invade to colonize at other sites that are normally reserved for other cells or other tissue components. If the neoplastic cells cluster together into a single mass forming a benign tumor, surgical removal of this mass can result in a complete cure. But such treatment is complicated if the tumor becomes malignant, acquiring the ability to invade surrounding tissues, blood, and lymphatic vessels, and forming secondary tumors, called metastases, at distant sites in the body. Both the cell proliferation and metastasis require continuous interaction with the ECM components and modulation of cell signaling. The nature of such interactions with ECM and downstream signaling is often quite different in cancer cells than in normal cells. Extracellular matrix is the tissue components apart from the cells and is mainly filled with intricate network of macromolecules like glycoproteins, fibrous proteins, etc. Some important examples of ECM components are fibronectin (FN), laminin (LM), vitronectin (VN), and collagen-IV (C-IV). The adhesion signal of these matrix components can regulate biological processes such as proliferation, differentiation, survival, wound healing, migration, tumorigenesis, etc.,1 all of which are necessary for execution of successful metastasis. These responses require active participation of several signaling proteins, including Rho GTPases, MAPKs, FAK, JNK, etc., which are found to be recruited at the ECM ligand/integrin binding site.2,3 The ways in which these intracellular mediators regulate gene expression and ultimately the effectors responses can vary among cell types. Thus, selection of the appropriate matrix for experiments using cultured cells is crucial for studying ECM-mediated signaling and its effectors response, including metastasis.
The major difference in behavior between normal and tumor cells lies in the fact that many receptors and signaling components are altered such that they become constitutively active when normally they should be turned on in a particular situation only. Research in the field of metastatic pathways helped to identify some factors that can be targeted to achieve possible therapeutic purpose. The present review covers mainly integrin receptor-mediated signaling pathways leading to metastasis and the present status of some therapeutic approaches targeting these pathways.
Integrins
Integrins are the most predominant and well characterized cell surface receptors of various extracellular matrix (ECM) proteins (e.g., LM, FN, C-IV, VN, etc.). The complete receptor molecule is composed of non-covalent, heterodimeric complexes of an α subunit and a β subunit.3 So far 18 α and 8 β subunits have been found and they combine to form 24 various combinations of different specificities.1 Many members of the integrin family, including α5β1, α8β1, αIIbβ3, αVβ3, αVβ5, αVβ6 and αVβ8 recognize an Arg-Gly-Asp (RGD) motif within their ligands, including fibronectin (FN), fibrinogen, vitronectin, von Willebrand factor, and many other large glycoproteins.4 This ligand receptor recognition can achieve the specificity and high affinity for each ligand receptor pair, by specific residues outside the RGD motif. Such novel non-RGD site, appears to act synergistically with the RGD site to promote cell adhesion, is often designated as “synergy site”. This sequence is highly conserved among fibronectin of diverse species and in human it is localized to a Pro-His-Ser-Arg-Asn (PHSRN) peptide sequence within the ninth type III module of fibronectin structure.3
Sequence studies on integrin genes have shown no detectable homology between α and β subunits, but 30% and 45% of sequence identities among α and β subunits respectively indicates possible evolution of these two gene families by gene duplication.5 In several mammalian α subunits (α1, α2, α10, α11, αL, αM, αX, αD, and αE), an I (insertion or interaction) domain is present, which contains a metal-ion-dependent adhesive site (MIDAS), and participates in ligand binding. Integrin α3, α4, α5, α6, α7, α8, α9, αV, and αIIb are non-I-domain subunits. Hence, RGD motif is recognized by integrins having non-I-domain α subunits. However, an I-like domain, which contains a structurally similar metal-binding motif, is present in all human integrin β subunits and amino-acid residues from this domain can interact with the RGD peptide of a ligand.5,6
The general structural features of all α and β subunits of integrins appear to be similar. Both the subunits are transmembrane glycoproteins with large globular NH2-terminal extracellular domains that together make up an ellipsoidal head. Each subunit contains a relatively thin transmembrane domain and a relatively small cytoplasmic tail of less than 60 amino acids,3 only known exception is β4 integrin, which has a cytoplasmic domain of over 1000 amino acids.7 As the cytoplasmic tails of integrins are devoid of enzymatic features, integrins transduce signals by associating with adaptor proteins that connect the integrin to the cytoskeleton, cytoplasmic kinases, and transmembrane growth factor receptors.8 Binding of integrins to ECM allows them to cluster in the plane of the cell membrane and associate with a cytoskeletal and signaling complex that promotes the assembly of actin filaments. The reorganized actin filaments cause more integrin clustering, thus enhancing the matrix binding of integrins in a positive feedback system. As a result, adaptor and cytoskeletal signaling proteins assemble into aggregates at the cytoplasmic tails of integrins so that, integrins can serve as integrators of the ECM and cytoskeleton. Such well-developed aggregates are known as focal adhesion sites.8 These sites provide a structural link between the actin cytoskeleton and the extracellular matrix and are regions of signal transduction that relate to growth control9 and regulate several biological processes such as proliferation, differentiation, survival, wound healing, migration, tumorigenesis, etc.1
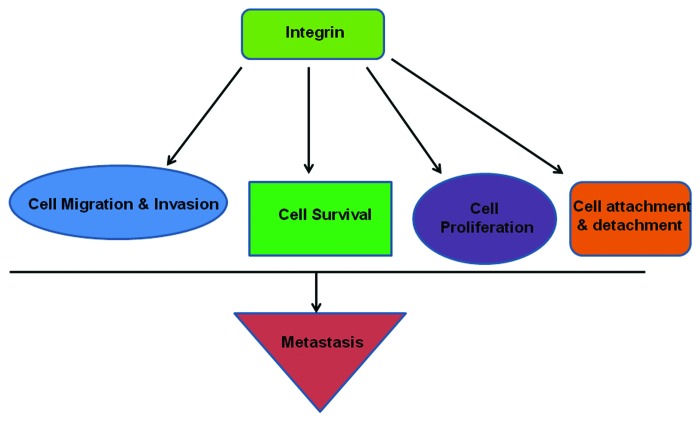
An interesting feature of integrin-mediated signaling is bidirectional signaling, which is also termed as outside-in and inside-out signaling. Integrin-ligand interaction induces several conformational changes of integrin itself. Interaction with ligands occurs at the extracellular head-domain which induces separation of cytoplasmic domains of integrin subunits. This allows interaction of the cytoplasmic domain with different cytoskeletal and/or signal transducer molecules. This chain of response called outside-in signaling. On the other hand separation of cytoplasmic domain interaction by talin or other proteins, promotes interaction of head-domain and ligands, which is termed inside-out signaling. Another interesting report shows mutation near the tail or at the cytoplasmic domain near the membrane of either integrin subunit shows constitutive activation of this receptor. This again shows the interaction of two subunits as well as inside-out signaling.10,11 A communication has proposed that these two conceptual models may be view as two reflections of same allosteric equilibrium.12 The crystallization of unligated specific domain of integrin αVβ3 have given newer dimension to the study of integrin bidirectional signaling.13,14 An important molecular mediator of integrin bidirectional signaling is Talin, which has been studied by several groups.15,16 A recent report has showed Kindlin-2 as regulator of integrin bidirectional signaling. The authors have shown Kindlin-2 is needed in integrin outside-in signaling to build concrete cell adhesion and spreading. This study indicates need of more molecular studies on this interesting feature of integrin signaling.17 The inside-out signaling is told to be responsible for integrin-ligand interaction which again promotes formation of integrin clusters, focal adhesion sites and subsequent outside-in signaling. The interplay between both directions of integrin signaling is continued in a synchronized fashion which regulates the several physiological behaviors of cell. This synchrony is lost in pathological condition and found to be associated with numerous human disorders including development and metastasis cancer (Fig. 1).
Figure 1. Integrins regulate different cellular activities like cell migration-invasion, cell proliferation, cell survival-apoptosis, attachment of cell with ECM, cell cycle and proliferation, etc., and thereby the sequential events of metastasis.
Metastasis
Basics of metastasis
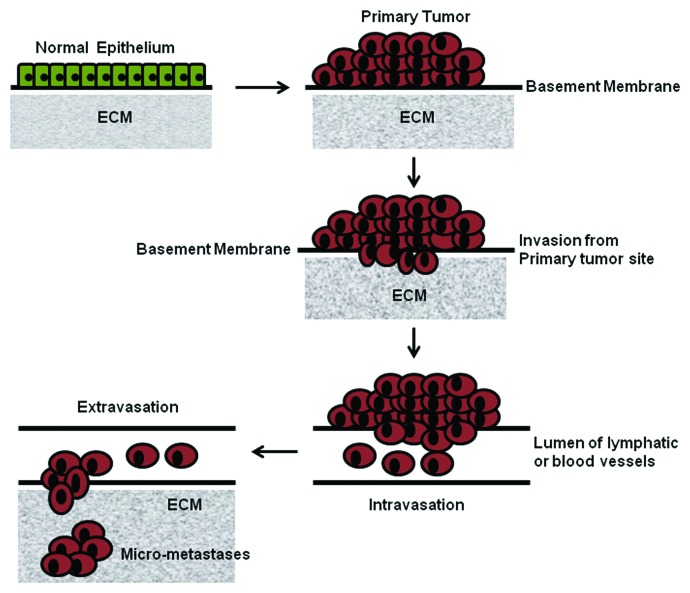
Metastasis, the series of actions through which cancer cells disseminate from the primary site of tumor, propagate, and form secondary tumors at distant sites, is a serious clinical problem as it is a disseminated disease. Probably this phenomenon makes the difference between cancer and the other diseases. Metastasis is often impossible to eradicate successfully, which results in the death of most cancer patients. Metastasis results from a complex network of sequential molecular events involving many steps. All of those are interlinked through a series of interactions and invasive processes as well as responses to specialized stimuli. Until now, in spite of its clinical significance, it remains incompletely understood. Some of the principle molecular players in the major steps of the metastatic cascade are tumor angiogenesis, disaggregation of cells from the primary tumor mass regulated by group of cadherins and catenins,18,19 invasion and migration of the cells through the basement membrane and extracellular matrix surrounding the tumor epithelium, and subsequent invasion of the BM of the endothelium of local blood vessels. This is mediated through integrins and proteases, including urokinase form of plasminogen activator (uPA), matrix metalloproteinases (MMPs),20,21 cathepsins, and intravasation of the tumor cells into the blood vessels prior to hematogeneous dissemination to distant sites. Another important criterion of this process is adhesion of the circulating tumor cells to the endothelial cell lining at the capillary bed of the target organ site. This occurs through adhesive interactions between cancer cells and endothelial cells involving some cell adhesion proteins like integrins, selectins and members of the immunoglobulin superfamily.22 The next important phase is invasion of the tumor cells through the endothelial cell layer, neighboring basement membrane (this process is called extravasation), and target organ tissue. Then there is step of development of secondary tumor foci at the distant organ. In this section we will discuss some intricate features of each steps of tumor metastasis (Fig. 2).
Figure 2. Some of the vital events of the metastatic cascade.
Sequential events of metastasis
Invasion from primary tumor site
Initiation of metastatic journey of cancer cells occurs through separation of some cancer cells from the mass of primary tumor and their protrusion through tumor-associated stroma and thereafter into the adjacent normal tissue parenchyma. The first strong barrier for the cancer cells invasion is the basement membrane. The basement membrane is a form of extracellular matrix that plays critical role in maintaining architecture of epithelial tissues, partially by separating the epithelial and stromal compartments. The basement membrane is also found to store embedded growth factor molecules that can be liberated by carcinoma-secreted proteases. Moreover, the BM also plays crucial roles in signal transduction events within carcinoma cells via pathways initiated by integrin-mediated cell-matrix adhesions, leading to alterations in cell polarity, proliferation, invasiveness, and survival.23 Budding evidence indicates that the minute tissue architecture of normal epithelium serves as an intrinsic barrier to invasiveness. This barrier must be overcome by incipient metastatic carcinoma cells before they can develop their invasive and malignant property. In the mammary gland, myoepithelial cells oppose invasion by helping to maintain BM integrity; indeed, co-implantation with myoepithelial cells reversed the invasiveness of breast carcinoma xenografts.24 Similarly, in ovarian carcinomas, the mesothelial cell layer that lines peritoneal and pleural organs serves as an obstacle to further dissemination that can be overcome by carcinoma cell-exerted, myosin-dependent traction forces that physically displace mesothelial cells.25 Moreover, modulation of ECM stiffness, achieved by altering collagen crosslinking, affects breast carcinoma progression via altered integrin signaling.26 At a cell-biological level, most types of carcinomas can invade as cohesive multicellular units through a process known “collective invasion”. Alternatively, individual tumor cells may invade via two distinct programs: the protease-, stress-fiber-, and integrin-dependent “mesenchymal invasion” program or the protease-, stress-fiber-, and integrin-independent, Rho/ROCK-dependent “amoeboid invasion” program.27 Indeed, differential expression of molecules that enable either mesenchymal or amoeboid invasion can be observed in signatures of local invasiveness derived from mammary carcinoma models.28 Tumor cells can apparently interconvert between these various invasion strategies in response to changing microenvironmental conditions. This has caused some to propose that robust suppression of single-cell invasion requires concomitant inhibition of the mesenchymal and amoeboid invasion programs.27 Indeed, certain regulators of invasion function as pleiotropically acting factors that simultaneously modulate components of both pathways. For example, microRNA (miRNA) miR-31 inhibits breast cancer invasion via concurrent suppression of key effectors of both the mesenchymal (such as integrin α5) and amoeboid (such as RhoA) invasion programs.29 The single-cell invasion pathways cited above are clearly incompatible with one critical element of epithelial tissue organization, specifically the E-cadherin-mediated intercellular junctions that knit together epithelial cell sheets and prevent dissociation of individual epithelial cells from their neighbors. In order to overcome this and other obstacles to invasion, carcinoma cells may appoint a cell-biological program known as epithelial mesenchymal transition (EMT), which is critical for multiple aspects of normal embryonic morphogenesis. The EMT program, which involves dissolution of adherens and tight junctions and a loss of cell polarity, dissociates the cells within epithelial cell sheets into individual cells that exhibit multiple mesenchymal attributes, including heightened invasiveness. EMT programs are orchestrated by a set of pleiotropically acting transcription factors, including Slug, Snail, Twist, ZEB1, and ZEB2, which organize entrance into a mesenchymal state by suppressing expression of epithelial markers and inducing expression of other markers associated with the mesenchymal state. Indeed, several of these transcription factors directly repress levels of E-cadherin, the keystone of the epithelial state. Certain miRNAs, notably those belonging to the miR-200 family, also regulate EMT programs. One important mechanism by which miR-200 promotes an epithelial phenotype involves its ability to post-transcriptionally suppress expression of the ZEB1 and ZEB2 EMT-inducing transcription factors. Acting in the opposite direction, ZEB1 and ZEB2 transcriptionally repress miR-200 family members, thereby establishing a double-negative-feedback loop that operates as a bistable switch, reinforcing the residence of cells in either the mesenchymal or epithelial state.30 Finally, loss of the BM barrier allows direct invasion by carcinoma cells of the stromal region. Active proteolysis, affected principally by matrix metalloproteinases (MMPs), drives this loss. In normal tissue, the activity of MMPs is carefully controlled via transcriptional and posttranslational mechanisms. Carcinoma cells have devised numerous means to derail the normal control of MMP activity which almost invariably leading to enhanced MMP function. Moreover, several integrin-mediated signals are induced, which leads to enhanced MMP activity and thereby accelerated cell migration-invasion.31-33 Reports have shown that during degradation of the BM and other ECM remain in between the path of invading tumor cells, MMP-expressing cells also secrete growth factors that remain there and thereby induce cancer cell proliferation.34 Once invading carcinoma cells have dissolved the basement membrane, they pass in the stroma, where they are threatened with several of tumor-associated stromal cells. The composition of these cells is directed by the maturity of tumor. With the progression of primary tumor, the stroma is developed to more responsive and it gets many of the new features of the stroma.35 The tumor cells invading into a stromal environment encounter different types of cells like fibroblasts and myofibroblasts, endothelial cells, adipocytes, and various bone marrow-derived cells such as mesenchymal stem cells, as well as macrophages and other immune cells.36 These stromal cells take part in further enhancing the aggressive features of carcinoma cells by inducing different types of signaling. Reports have showed that breast cancer invasiveness may be stimulated through the local adipocytes secreted interleukin-6 (IL-6).37 Moreover, by stimulating tumor-associated macrophages (TAMs), stromal CD4+ T lymphocytes promote mammary carcinoma invasion through activation of epidermal growth factor receptor (EGFR) signaling.38 This background knowledge suggests a both side interaction of carcinoma cells and stroma, where the cells induces formation of reactive stroma and the stroma enhances the malignant nature of the carcinoma cells. This indicates toward a feedback interaction between these two tissue components. Microarray analysis of stroma from breast cancer tissue have shown characteristics features capable of inducing invasive property of cell.39,40 Recent observations are accumulating knowledge showing relevance of stroma in making cancer more vulnerable.
Intravasation
Intravasation defines entry of invasive carcinoma cells into the lumen of lymphatic or blood vessels. Spread of carcinoma cells through lymphatic system is a common part of metastasis and used as prognostic marker of advancement of cancer, but spread of carcinoma cells through blood circulation system is the most prevalent mechanism of tumor cell dissemination.41 Intravasation needs changes of the cells in molecular level that facilitate the ability of carcinoma cells to move through the walls of microvessels made by pericyte and endothelial cell. In colon cancer the transcriptional modulator N-terminal enhancer of split are found to inhibit the intravasation of cells by impairing trans-endothelial invasion through Notch-dependent mechanisms.42 Again report shows cytokine-transforming growth factor-β (TGF-β) induces mammary carcinoma intravasation, by increasing carcinoma cell penetration of microvessel walls or increasing invasiveness.43 Perivascular TAMs are found to enhance intravasation process through a positive feedback loop. In this loop involve reciprocal secretion of epidermal growth factor (EGF) and colony stimulating factor-1 (CSF-1) by TAMs and cancer cells, respectively.44 Interestingly, the event of intravasation depends on formation and structural characteristics of surrounding blood vessels. Tumor cells induce formation of new blood vessels in its microenvironment through many vascular endothelial growth factors (VEGF)-regulated mechanisms. These vessels are generally fragile, and immature compared with the vasculatures present in normal tissue.45 Reports show that intravasation is also facilitated by interaction between adjacent endothelial cells that constitute microvasculatures and absence of pericyte coverage. In this connection this may be noted that cyclooxygenase-2 (COX-2), epiregulin (EREG), MMP-1, and MMP-2, which are found to stimulate formation of new defective vessels surrounding tumor cells, also induce intravasation.46
Self defense of carcinoma cells in circulation
After entering blood vessels the cells are able to spread in many directions through circulation. Recent techniques have made it possible to track those cells within the blood vessels of the patients.47 With these techniques the cells circulating between primary and metastatic site can be detected. To make an effective metastasis, circulating cells have to survive and reach an ambient environment. During the flow in circulation tumor cells face different adverse environmental stresses. One of those is absence of integrin-dependent attachment to the extracellular matrix (ECM), which is essential for cell survival. In absence of that the cells will undergo anoikis, a type of apoptosis induced by loss of anchorage. There are some metabolic and/or signaling pathways, like pentose phosphate pathway and regulation of glucose uptake, tyrosine kinase TrkB, which gives the cells protection from this type of death.48,49 There are several schools of thought regarding the lifetime of cancer cells in circulation. A report has proposed that breast cancer cells survive in circulation for several hours.50 Another hurdle to the circulating cells are their size. Typically the blood capillaries are about 8 μm in diameter, whereas diameters of carcinoma cells are generally between 20–30 μm. So, it may be considered that the cells are trapped within the capillaries during their first pass, which happens within minutes after intravasation. This indicates the tumor cells that escape these barriers spend much less time within circulation and their overcome death. Two other threats to circulating tumor cells are dynamic force of blood flow and patients own innate immunity. But tumor cells battle with these problems by forming emboli through interacting with platelets. A class of protein that helps in this interaction is called selectin.36
Launch at a distant site
It is seen though the circulating tumor cells disseminate to all directions of body, but there is strict specificity where they can settle. This sites are depends on the primary site of the tumor. A specific type of cancer is destined to form secondary tumor at a specific distant site. A very early report by Stephen Padget (1889) explained this theory as the “seed and soil hypothesis”. After several decades the same idea was resumed by other groups.51 Some proposals suggested that trapping is higher in organs that possess more frequent capillaries than other microvessels. For example, for colorectal cancer, the liver is a site for frequent trapping site, where metastatic cells are drained through portal veins.41 Another proposal says this site specificity is a predetermined, and mediated by specific combinations of attachment proteins present in the tumor cells and cells of that site.52
Extravasation
After settling within microvessels of distant site, circulating tumor cells starts to grow which eventually form a microcolony. This microcolony sometimes breaks the hosting walls of vessel and makes contact with surrounding tissue.53 In another way, cells penetrate endothelial and pericytic cell layer which separate tissue microenvironment and blood vessels. This step is called extravasation. In contrast with supporting factors of intravasation, in extravasation the TAM cells are not expected to be equally available and blood vessels are highly active and non-fragile.45,54 So these environmental factors build another barrier to the tumor cells, even after escaping from adverse situation within circulation. To overcome these physical barriers, tumor cells secrete some molecules like matrix metalloproteinases (MMP-1, MMP-2, MMP-3, and MMP-10) angiopoietin-like-4 (Angpt2 and Angptl4), as well as the pleiotropically acting factors EREG, COX-2, etc., which degrade those physical barriers and increases the permeability of distant microenvironment. These molecules facilitate the process of extravasations along with some specialized mechanisms like CCL-2-dependent recruitment of inflammatory monocyte in pulmonary metastasis.55-57
Micrometastases
The microenvironment of this distant site is usually far different from the primary site of the tumor, in terms of types of stromal cells, composition of ECM, available growth factors and cytokines, etc. So at first the cells have to adapt and survive the environment. Some groups have suggested that the tumor cells primarily adopt the situation by forming some premetastatic niche. According to this hypothesis, primary tumor releases signals by secreting lysyl oxidase (LOX), which induces host fibroblasts to secrete fibronectin (FN). A group has proposed that prior to arrival of the circulating tumor cells; FN in turn attracts VEGF receptor-1 positive hematopoetic progenitor cells from the bone marrow to that site, by interacting with integrin α4β1 expressed on those cells. This signaling event induces MMP-9 expression, secretion and activation which along with other sequestered proteins alters the microenvironment and acts as chemoattractants for the coming metastatic tumor cells.58
Colonization of tumor cells
It is seen that even after formation of stable microcolonies at distant site, cells cannot form mature metastatic colonies for long-term. Some groups indicate that formation of larger colony is hindered due to inadaptability of microenvironment.59 Some groups have indicated this dormancy is caused by inability to form integrin β1, focal adhesion kinase (FAK), Src complex and underlying signaling.60,61 It is reported that this process is sometime overcome by induction through molecules like osteopontin (OPN) or stromal cell-derived factor 1 (SDF-1). An alternative way suggests that micrometastases may proliferate continuously.59
In this way the term metastasis includes a series of interdependent physical and signaling events. Ultimately this flow of cellular activities form a mass of transformed cells at an organ other than the initially damaged organ and this makes cancer more devastating.
Integrin-Mediated Signaling and Metastasis
Metastasis is a multistep process which depends on regulation of some cellular properties like cell attachment-detachment, phenotypic changes of cell, e.g., epithelial to mesenchymal transformation, cell survival, cell migration and invasion, etc. These processes are regulated by different molecular events within the cells which are reflected in the regulation of metastasis. From that point of view cell adhesion molecules and growth factor receptors are the important regulators of metastasis. Integrins are the type of cell adhesion molecule that takes crucial part in regulation of metastasis.
Integrins are the important example of bidirectional receptor signaling. Inside-out signals induce conformational changes between the integrin headpiece and the cytoplasmic domains with exposure of neoepitopes, i.e., the ligand-induced binding sites. Attainment of high-affinity states is necessary for binding of outside-in signals which regulates cellular responses to environment. This bidirectional signaling is very important in cancer because it is seen that constitutive activation of integrins from endogenous stimuli mediates stronger binding to the ECM and therefore a more dynamic interaction of these adhesion receptors with their substrates. This is necessary for migration and metastasis studies correlating integrin expression levels in human tumors with pathological outcomes, such as patient survival and metastasis. Studies have identified several integrins that might have an important role in cancer progression. Tumor cell expression of the integrins αvβ3, αvβ5, α5β1, α6β4, α4β1, and αvβ6 is correlated with disease progression in various tumor types. Study on non-small cell lung cancer has showed importance of integrin expression development and progression of lung cancer. The group has suggested integrin α5, β1, and β3 expression as prognostic marker of overall survival in early stage of that type of cancer. They have also shown expression of those integrins as marker for lymph node metastasis and recurrence. Notably, integrins αvβ3, α5β1, and αvβ6, most of the times are found to be expressed at very low even undetectable levels in most adult epithelial cells. Whereas those can be highly overexpressed in some tumors.62 Keller and Brown showed frequent association of bone metastasis with advanced prostate cancer is determined by integrin-mediated interaction of metastatic cancer cells and bone microenvironment.63 A recent observation showed integrin αVβ3 is responsible for making the choice of attachment to the bone microenvironment and subsequent growth of tumor.64 These observations shows integrin as regulator of metastasis.
To colonize distant target organs, cancer cells must cut the connections from the primary tumor, gain access to blood vessels and survive within the vasculature exposed to shear forces which physically oppose cell attachment. Activated αVβ3 may rescue blood circulating cancer cells from shear-induced tumor cell arrest by binding to leukocytes and platelets to survive.65 Once localized to the metastatic environment, cancer cells of different tissue origins may utilize distinct integrin-ligand combinations to colonize the same target organ and receive local mitogenic stimuli. In most cancers the αvβ3 integrin is the prime initial receptor to support adhesion and migration to bone matrix. Moreover, overexpression of all three integrins, N-cadherins, and melanoma cell adhesion molecule (MCAM) in a melanoma tissue microarray66 was associated with a higher incidence of worse prognosis gastrointestinal metastasis, according to American Joint Committee on Cancer (AJCC) staging metastatic category (M1c), rather than development of more favorable subcutaneous and lymph node metastasis (AJCC M1a, M1b). In their activated state, several integrins recognize ligands, which are usually not bound when inactive. Such aberrant binding may favor colonization of certain microenvironments, such as the bone matrix via sialoprotein or interact with molecules which further enhance malignant behavior, such as osteopontin. Osteopontin is a secreted glycoprotein that is overexpressed in a number of different carcinomas and can promote adhesion, migration and metastasis by binding predominantly to the αvβ3 integrin and the CD44 antigen. This indicates, more aggressive cancers not only have the potency to express more osteopontin but also may be more responsive to this protein67,68
In addition to their ligation-dependent effects, some cases have shown that unligated integrins can positively or negatively influence tumor cell metastasis. How integrins affect tumor cell survival both in the ligated and unligated states may be a critical determinant of the efficacy of integrin-targeted therapeutic strategies in cancer. A recent report has shown that in integrin-mediated death-resistant tumor cells the unligated integrin αvβ3 significantly upregulates anchorage-independent tumor cell metastasis in vivo69 Growing evidences are showing integrin crosstalk with growth factor cytokines could have important implications for tumor metastasis and the acquisition of drug resistance70,71
Our lab has shown in cervical cancer cell SiHa, integrin α5β1-fibronectin interaction induces expression, appreciable activation of pro-MMP-9 and moderate change of pro-MMP-2 activity involving FAK, ILK, ERK, PI-3K, and NFκB signaling cascade. This ligand-integrin interaction was also found to accelerate cell migration. Another study from our lab showed, in the same cervical cancer cell line, interaction of integrin α5β1 with laminin also induced MMP-9 expression and activation involving FAK, PI-3K and ERK signaling.33,72 Interestingly we found induction of MMP-9 expression-activation and migration of breast cancer cell is regulated by FAK, where activation of FAK is induced by its association with α5β1 integrin.73 In addition to this several other groups have focused on importance of integrin α5β1 in development and/or progression of cancer. For instance, in pancreatic cancer model upregulation of integrin α5β1 was reported to be associated with radiation induced invasion of the cells.74 A very recent publication on cervical cancer system has reported direct association of integrin α5β1 with an important receptor tyrosine kinase, c-Met. They have shown this association activates c-Met in a hepatocyte growth factor/scatter factor (HGF/SF) independent pathway, subsequently which plays crucial role in angiogenesis.75 Studies on a pool of breast cancer tissue sample shows that integrin α5β1 and its downstream signaling cascade is the pathway through which angiopoietin-2 induces breast cancer cell invasion and thereby metastasis.76 A report by Murillo et. al. showed in a colon cancer model, inhibition of integrin α5β1 resulted in decreased cell adhesion and PI3K activation.77 Another report on colon cancer model reported unusual upregulation of the same integrin found to be linked with metastatic progression.78 In a hepatic cancer system inhibition of integrin α5β1 was reported to induce cell invasion involving ERK1/2 and p38 MAPK signaling cascades.79 In breast cancer cell line MCF-7, fibronectin-α5β1 interaction was found to increase expression and activation of both MMP-2 and MMP-9.19 Upregulation of both of these MMPs were found when human melanoma cell A375 was exposed to fibronectin.18 This interaction also reported to upregulate MMP-9 in human myeloid leukemia cells K562.80 Our group also showed in a highly invasive breast cancer cell line MDA-MB-231, fibronectin-α5β1 interaction upregulates MMP-9 expression and activity. In cervical cancer cell line SiHa, not only integrin α5β1 but integrin αvβ3 was found to be associated with MMP activity.81
Studies have shown expression of integrin αvβ3 expression to be a major determinant of breast cancer cell bone metastasis.82 Integrin αvβ3 is found as modulator of MMP-2 activation lymph node metastasis.83 Role of β3 and β5 integrins in growth and metastasis of murine mammary carcinomas is established by several groups.84 An interesting observation shows crucial involvement of platelet and osteoclast β3 integrins in bone metastasis.85 It was found that in blood flow breast cancer cells attachment is dependent on integrin αvβ3 activation status.86 Another integrin combination, integrin αvβ5 found to build crosstalk with epidermal growth factor receptor. This link between integrin and growth factor receptor promotes carcinoma cell metastasis. Cell invasion and metastasis was found to be stimulated by epidermal growth factor treatment. This was found to be retarded by blocking integrin αvβ5 suggesting inter-relation of integrin and growth factor receptors.87
Therapeutic Aspects
Altered expression of integrins in different types of cancer, in relation to that with tumor progression, tumor cell metastasis, and their ability to crosstalk with growth factor receptors, has made this protein a point of target for therapeutic interventions. Different groups have shown inhibitors of integrin have shown suppressive effect on the metastatic cascade. Integrin inhibitors are now in clinical trials, which include monoclonal antibody and RGD peptide analog.88 This section will focus on the strategies that use integrin as a tool to battle metastasis.
Approaches targeting αvβ3 and αvβ5
Integrin αvβ3 is upregulated in both tumor cells and angiogenic endothelial cells, which makes it an attractive therapeutic target. The first strategy of integrin deactivation was anti-integrin monoclonal antibody approach. Among those LM609 may be mentioned which showed appreciable anti-angiogenic activity in preclinical model experiments.89 An altered form of this molecule was developed with name of etaracizumab (MEDI-522) for treatment in humans.
This new molecule along with its anti-angiogenic effect also inhibited tumor growth with direct effect on tumor cells.90 Due to its high efficiency etaracizumab (MEDI-522) was subjected to clinical trial. At the initial phases of trial, vitaxin, the precursor of etaracizumab, showed appreciable suppression of angiogenic activities with low toxicity and disease stability in some patients with renal cancer and solid tumors. In later stages, it showed effectivity in advanced stages of melanoma.91-93
In xenograft tumor models anti-integrin αv monoclonal antibody (CnTo 95) showed anti-tumor and anti-angiogenic effect.94 In Phase I trial has shown nontoxic effect and found to be localized to tumors.95 Cilengitide is an interesting molecule, having ability to inhibit both αvβ3 and αvβ5 integrins.96 Currently cilengitide is under phase trials in lung cancer, prostate cancer and glioblastoma patients where it is showing extended survival with minimum side effects.97
These results were also supported by the observation by other groups showing effects of low concentrations of integrin antagonists in different cases.98-100
Targeting β1 integrins
Among integrin-antagonist strategies another crucial target is integrin-β1, precisely α5β1. This way have demonstrated appreciable efficacy in reducing tumor load in several preclinical studies. Anti-integrin-β1 inhibitory antibody substantially affected in vitro and in vivo growth of human breast cancer tumor cells.101 Volociximab is an anti-integrin α5β1 monoclonal antibody which blocks the function of integrin α5β1. Volociximab is found to inhibit angiogenesis and hinder tumor growth.102,103 Clinical efficacy and tolerance of volociximab was found satisfactory in phase I trial.104 This observation led this to phase II trial for solid tumor.105 A group has demonstrated a mechanism-based receptor binding model to describe to describe the pharmacokinetic and pharmacodynamic of volociximab in cancer patients.106
A different approach has built a non-RGD-based peptide inhibitor, ATn-161.This is an inhibitor of integrin α5β1. In vivo studies have shown its efficacy in blocking breast cancer growth and metastasis.107 A combination therapy of ATN-161with fluorouracil showed significant reduction of tumor burden and liver metastasis in a mouse model study of colon cancer metastasis to the liver.108 ATn-161 was found to be well tolerated in patients with advanced solid tumor.109
Other integrin targeted inhibitors
There are bunches of integrin-antagonists that have shown appreciable efficiency in preclinical studies, but those have to pass through the clinical trials. S247, an integrin αvβ3 antagonist inhibited breast cancer metastasis in a xenograft tumor model.110 The compound also decreased colon cancer metastasis, angiogenesis and increased survival.111 Another integrin αvβ3 antagonist, PsK1404, was found to inhibit breast and ovarian cancer bone metastases without hampering osteoclastic activity.112 Anti-metastatic effect was found upon treatment of two RGD-peptide-mimic, s137 and s247.113 Growth of subcutaneously injected human hepatocellular carcinoma cell was fund to be arrested when either ItGAV or ItGB3 was silenced using antisense approach.114 Another group has demonstrated that both in vivo and in vitro growth of human pharyngeal carcinoma cells were arrested by treatment of integrin αvβ6 inhibitor, 6.3G9 monoclonal antibody.115 In addition this monoclonal antibody was found to inhibit TGFβ signaling, indicating some of its efficiency involve crosstalk with TGFβ receptor mediated signaling. These compounds draw attention to move for clinical trial with these molecules, standardization of their combinations with other clinical parameters.
Conclusion
Both in physiological and pathological conditions, cell attachment to it surrounding extracellular matrix (ECM), plays a key role in inducing several biological activities like cell migration-invasion, cell survival-apoptosis, and cell proliferation. Integrin is one of the most important cell attachment molecules and it is found to be involved in regulating all of the above mentioned cellular activities. In spite of emphasis given on different targets of cancer research like growth factor related pathways, angiogenic regulators, and cancer immunology. Integrin biology truly demand its place in cancer research, since it is linked with all of these and many more pathways which regulate each and every step of cancer development and progression. As metastasis is a multistep process involving a number of integrin-regulated cellular activities, so this will be a meaningful approach to target different integrin molecules to regulate metastasis. The mode of treatment may be either combinatorial or single-agent treatment, which may be more firmly decided after more studies on integrins and metastasis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/23840
References
- 1.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–73. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, et al. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takagi J, Strokovich K, Springer TA, Walz T. Structure of integrin alpha5beta1 in complex with fibronectin. EMBO J. 2003;22:4607–15. doi: 10.1093/emboj/cdg445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–5. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 7.Hogervorst F, Kuikman I, von dem Borne AE, Sonnenberg A. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J. 1990;9:765–70. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 9.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 10.O’Toole TE, Mandelman D, Forsyth J, Shattil SJ, Plow EF, Ginsberg MH. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991;254:845–7. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- 11.O’Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, et al. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–59. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 13.Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–45. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 15.Calderwood DA, Yan B, de Pereda JM, Alvarez BG, Fujioka Y, Liddington RC, et al. The phosphotyrosine binding-like domain of talin activates integrins. J Biol Chem. 2002;277:21749–58. doi: 10.1074/jbc.M111996200. [DOI] [PubMed] [Google Scholar]
- 16.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, et al. Structural basis of integrin activation by talin. Cell. 2007;128:171–82. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Montanez E, Ussar S, Schifferer M, Bösl M, Zent R, Moser M, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–30. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis-Tuffin LJ, Rodriguez F, Giannini C, Scheithauer B, Necela BM, Sarkaria JN, et al. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS One. 2010;5:e13665. doi: 10.1371/journal.pone.0013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao YL, Shepard CR, Wells A. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer. 2010;9:179. doi: 10.1186/1476-4598-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–24. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 21.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 22.Gassmann P, Kang ML, Mees ST, Haier J. In vivo tumor cell adhesion in the pulmonary microvasculature is exclusively mediated by tumor cell--endothelial cell interaction. BMC Cancer. 2010;10:177. doi: 10.1186/1471-2407-10-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–9. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwanicki MP, Davidowitz RA, Ng MR, Besser A, Muranen T, Merritt M, et al. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011;1:144–57. doi: 10.1158/2159-8274.CD-11-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–94. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 29.Valastyan S, Weinberg RA. MicroRNAs: Crucial multi-tasking components in the complex circuitry of tumor metastasis. Cell Cycle. 2009;8:3506–12. doi: 10.4161/cc.8.21.9802. [DOI] [PubMed] [Google Scholar]
- 30.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Banerji A, Das S, Chatterjee A. Culture of human A375 melanoma cells in the presence of fibronectin causes expression of MMP-9 and activation of MMP-2 in culture supernatants. J Environ Pathol Toxicol Oncol. 2008;27:135–45. doi: 10.1615/JEnvironPatholToxicolOncol.v27.i2.60. [DOI] [PubMed] [Google Scholar]
- 32.Das S, Banerji A, Frei E, Chatterjee A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci. 2008;82:467–76. doi: 10.1016/j.lfs.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Maity G, Sen T, Chatterjee A. Laminin induces matrix metalloproteinase-9 expression and activation in human cervical cancer cell line (SiHa) J Cancer Res Clin Oncol. 2011;137:347–57. doi: 10.1007/s00432-010-0892-x. [DOI] [PubMed] [Google Scholar]
- 34.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–65. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 38.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–27. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 40.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell. 2011;19:125–37. doi: 10.1016/j.ccr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–96. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–56. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 45.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–27. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 46.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–70. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 47.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–13. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–9. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 50.Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–62. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 51.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 52.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–74. doi: 10.1016/S1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 53.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–2. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 54.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–9. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, et al. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009;69:7529–37. doi: 10.1158/0008-5472.CAN-08-4382. [DOI] [PubMed] [Google Scholar]
- 57.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 60.Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–50. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A. 2009;106:10290–5. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kren A, Baeriswyl V, Lehembre F, Wunderlin C, Strittmatter K, Antoniadis H, et al. Increased tumor cell dissemination and cellular senescence in the absence of beta1-integrin function. EMBO J. 2007;26:2832–42. doi: 10.1038/sj.emboj.7601738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91:718–29. doi: 10.1002/jcb.10662. [DOI] [PubMed] [Google Scholar]
- 64.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–43. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–8. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson-Hurst K, Becker D. The role of N-cadherin, MCAM and beta3 integrin in melanoma progression, proliferation, migration and invasion. Cancer Biol Ther. 2006;5:1375–82. doi: 10.4161/cbt.5.10.3241. [DOI] [PubMed] [Google Scholar]
- 67.Gupta A, Cao W, Chellaiah MA. Integrin αvβ3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-κB ligand signaling axis. Mol Cancer. 2012;11:66. doi: 10.1186/1476-4598-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Desai B, Rogers MJ, Chellaiah MA. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol Cancer. 2007;6:18. doi: 10.1186/1476-4598-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prévost N, et al. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15:1163–9. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67:61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 71.Grzesiak JJ, Smith KC, Burton DW, Deftos LJ, Bouvet M. Integrin-mediated laminin-1 adhesion upregulates CXCR4 and IL-8 expression in pancreatic cancer cells. Surgery. 2007;141:804–14. doi: 10.1016/j.surg.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maity G, Fahreen S, Banerji A, Roy Choudhury P, Sen T, Dutta A, et al. Fibronectin-integrin mediated signaling in human cervical cancer cells (SiHa) Mol Cell Biochem. 2010;336:65–74. doi: 10.1007/s11010-009-0256-5. [DOI] [PubMed] [Google Scholar]
- 73.Ganguly K, Sen T, Pal S, Biswas J, Chatterjee A. Studies on Focal Adhesion Kinase in human breast cancer cell MDA-MB-231. Advances in Biological Chemistry. 2012;2:29–42. doi: 10.4236/abc.2012.21004. [DOI] [Google Scholar]
- 74.Yao H, Zeng ZZ, Fay KS, Veine DM, Staszewski ED, Morgan M, et al. Role of α(5)β(1) Integrin Up-regulation in Radiation-Induced Invasion by Human Pancreatic Cancer Cells. Transl Oncol. 2011;4:282–92. doi: 10.1593/tlo.11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E. Ligand-independent activation of c-Met by fibronectin and α(5)β(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2011;30:1566–76. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imanishi Y, Hu B, Jarzynka MJ, Guo P, Elishaev E, Bar-Joseph I, et al. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007;67:4254–63. doi: 10.1158/0008-5472.CAN-06-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murillo CA, Rychahou PG, Evers BM. Inhibition of alpha5 integrin decreases PI3K activation and cell adhesion of human colon cancers. Surgery. 2004;136:143–9. doi: 10.1016/j.surg.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 78.Gong J, Wang D, Sun L, Zborowska E, Willson JK, Brattain MG. Role of alpha 5 beta 1 integrin in determining malignant properties of colon carcinoma cells. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1997;8:83–90. [PubMed] [Google Scholar]
- 79.Vellón L, Royo F, Matthiesen R, Torres-Fuenzalida J, Lorenti A, Parada LA. Functional blockade of α5β1 integrin induces scattering and genomic landscape remodeling of hepatic progenitor cells. BMC Cell Biol. 2010;11:81. doi: 10.1186/1471-2121-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dutta A, Sen T, Chatterjee A. Culture of K562 human myeloid leukemia cells in presence of fibronectin expresses and secretes MMP-9 in serum-free culture medium. Int J Clin Exp Pathol. 2010;3:288–302. [PMC free article] [PubMed] [Google Scholar]
- 81.Chattopadhyay N, Mitra A, Frei E, Chatterjee A. Human cervical tumor cell (SiHa) surface alphavbeta3 integrin receptor has associated matrix metalloproteinase (MMP-2) activity. J Cancer Res Clin Oncol. 2001;127:653–8. doi: 10.1007/s004320100271. [DOI] [PubMed] [Google Scholar]
- 82.Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res. 2005;25(1A):79–83. [PubMed] [Google Scholar]
- 83.Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, et al. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30–5. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 84.Taverna D, Crowley D, Connolly M, Bronson RT, Hynes RO. A direct test of potential roles for beta3 and beta5 integrins in growth and metastasis of murine mammary carcinomas. Cancer Res. 2005;65:10324–9. doi: 10.1158/0008-5472.CAN-04-4098. [DOI] [PubMed] [Google Scholar]
- 85.Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, et al. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100:14205–10. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Felding-Habermann B. Tumor cell-platelet interaction in metastatic disease. Haemostasis. 2001;31(Suppl 1):55–8. [PubMed] [Google Scholar]
- 87.Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD, et al. Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer Res. 2009;69:1383–91. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–17. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brooks PC, Strömblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–22. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mulgrew K, Kinneer K, Yao XT, Ward BK, Damschroder MM, Walsh B, et al. Direct targeting of alphavbeta3 integrin on tumor cells with a monoclonal antibody, Abegrin. Mol Cancer Ther. 2006;5:3122–9. doi: 10.1158/1535-7163.MCT-06-0356. [DOI] [PubMed] [Google Scholar]
- 91.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, et al. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–61. [PubMed] [Google Scholar]
- 92.Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, et al. Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against alphavbeta3 integrin receptor, in patients with advanced solid tumors. Invest New Drugs. 2008;26:35–43. doi: 10.1007/s10637-007-9077-0. [DOI] [PubMed] [Google Scholar]
- 93.McNeel DG, Eickhoff J, Lee FT, King DM, Alberti D, Thomas JP, et al. Phase I trial of a monoclonal antibody specific for alphavbeta3 integrin (MEDI-522) in patients with advanced malignancies, including an assessment of effect on tumor perfusion. Clin Cancer Res. 2005;11:7851–60. doi: 10.1158/1078-0432.CCR-05-0262. [DOI] [PubMed] [Google Scholar]
- 94.Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55. doi: 10.1038/nrc2543. [DOI] [PubMed] [Google Scholar]
- 95.Mullamitha SA, Ton NC, Parker GJ, Jackson A, Julyan PJ, Roberts C, et al. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2128–35. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 96.Smith JW, Ruggeri ZM, Kunicki TJ, Cheresh DA. Interaction of integrins alpha v beta 3 and glycoprotein IIb-IIIa with fibrinogen. Differential peptide recognition accounts for distinct binding sites. J Biol Chem. 1990;265:12267–71. [PubMed] [Google Scholar]
- 97.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–7. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Odekon LE, Frewin MB, Del Vecchio P, Saba TM, Gudewicz PW. Fibronectin fragments released from phorbol ester-stimulated pulmonary artery endothelial cell monolayers promote neutrophil chemotaxis. Immunology. 1991;74:114–20. [PMC free article] [PubMed] [Google Scholar]
- 99.Legler DF, Wiedle G, Ross FP, Imhof BA. Superactivation of integrin alphavbeta3 by low antagonist concentrations. J Cell Sci. 2001;114:1545–53. doi: 10.1242/jcs.114.8.1545. [DOI] [PubMed] [Google Scholar]
- 100.MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, et al. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001;48:151–7. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 101.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–35. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhaskar V, Zhang D, Fox M, Seto P, Wong MH, Wales PE, et al. A function blocking anti-mouse integrin alpha5beta1 antibody inhibits angiogenesis and impedes tumor growth in vivo. J Transl Med. 2007;5:61. doi: 10.1186/1479-5876-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhaskar V, Fox M, Breinberg D, Wong MH, Wales PE, Rhodes S, et al. Volociximab, a chimeric integrin alpha5beta1 antibody, inhibits the growth of VX2 tumors in rabbits. Invest New Drugs. 2008;26:7–12. doi: 10.1007/s10637-007-9078-z. [DOI] [PubMed] [Google Scholar]
- 104.Ricart AD, Tolcher AW, Liu G, Holen K, Schwartz G, Albertini M, et al. Volociximab, a chimeric monoclonal antibody that specifically binds alpha5beta1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res. 2008;14:7924–9. doi: 10.1158/1078-0432.CCR-08-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuwada SK. Drug evaluation: Volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr Opin Mol Ther. 2007;9:92–8. [PubMed] [Google Scholar]
- 106.Ng CM, Bai S, Takimoto CH, Tang MT, Tolcher AW. Mechanism-based receptor-binding model to describe the pharmacokinetic and pharmacodynamic of an anti-α5β1 integrin monoclonal antibody (volociximab) in cancer patients. Cancer Chemother Pharmacol. 2010;65:207–17. doi: 10.1007/s00280-009-1023-8. [DOI] [PubMed] [Google Scholar]
- 107.Khalili P, Arakelian A, Chen G, Plunkett ML, Beck I, Parry GC, et al. A non-RGD-based integrin binding peptide (ATN-161) blocks breast cancer growth and metastasis in vivo. Mol Cancer Ther. 2006;5:2271–80. doi: 10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 108.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry GC, Parikh AA, et al. Inhibition of integrin alpha5beta1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer. 2003;104:496–503. doi: 10.1002/ijc.10958. [DOI] [PubMed] [Google Scholar]
- 109.Cianfrocca ME, Kimmel KA, Gallo J, Cardoso T, Brown MM, Hudes G, et al. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br J Cancer. 2006;94:1621–6. doi: 10.1038/sj.bjc.6603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harms JF, Welch DR, Samant RS, Shevde LA, Miele ME, Babu GR, et al. A small molecule antagonist of the alpha(v)beta3 integrin suppresses MDA-MB-435 skeletal metastasis. Clin Exp Metastasis. 2004;21:119–28. doi: 10.1023/B:CLIN.0000024763.69809.64. [DOI] [PubMed] [Google Scholar]
- 111.Reinmuth N, Liu W, Ahmad SA, Fan F, Stoeltzing O, Parikh AA, et al. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–87. [PubMed] [Google Scholar]
- 112.Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, et al. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67:5821–30. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 113.Shannon KE, Keene JL, Settle SL, Duffin TD, Nickols MA, Westlin M, et al. Anti-metastatic properties of RGD-peptidomimetic agents S137 and S247. Clin Exp Metastasis. 2004;21:129–38. doi: 10.1023/B:CLIN.0000024764.93092.5f. [DOI] [PubMed] [Google Scholar]
- 114.Li J, Tan H, Dong X, Xu Z, Shi C, Han X, et al. Antisense integrin alphaV and beta3 gene therapy suppresses subcutaneously implanted hepatocellular carcinomas. Dig Liver Dis. 2007;39:557–65. doi: 10.1016/j.dld.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 115.Van Aarsen LA, Leone DR, Ho S, Dolinski BM, McCoon PE, LePage DJ, et al. Antibody-mediated blockade of integrin alpha v beta 6 inhibits tumor progression in vivo by a transforming growth factor-beta-regulated mechanism. Cancer Res. 2008;68:561–70. doi: 10.1158/0008-5472.CAN-07-2307. [DOI] [PubMed] [Google Scholar]