Abstract
Ras guanyl nucleotide releasing proteins (RasGRPs) are guanine nucleotide exchange factors that activate Ras and Rap. We recently reported that xrasgrp2, which is a homolog of the human rasgrp2, plays a role in vasculogenesis and/or angiogenesis during early development of Xenopus embryos. However, the function of RasGRP2 in human vascular endothelium remains unknown. Therefore we aimed to analyze the function of human RasGRP2 in vascular endothelial cells. RasGRP2 overexpression did not increase Ras activation. However, it slightly increased Ras expression and increased proliferation in ECV304 cells. Furthermore, RasGRP2 overexpression increased Rap1 activation and cell–matrix adhesion in ECV304 cells. These data demonstrate that RasGRP2 increases cell viability and cell–matrix adhesion through increased Ras expression and Rap1 activation, respectively, in endothelial cells.
Keywords: Rap1, Ras, RasGRP2, cell proliferation, cell–matrix adhesion, endothelial cells
Introduction
Ras guanyl nucleotide releasing proteins (RasGRPs) are guanine nucleotide exchange factors (GEFs) that activate Ras and Rap and share the Ras exchange motif, CDC25 domain, potential calcium-binding EF-hands, and diacylglycerol (DAG)-binding C1 domain.1,2 Because RasGRP2 cannot bind DAG, it fails to translocate to membranes in vivo in response to DAG analogs.3 RasGRP2, also called CalDAG-GEFI, plays a role in controlling T-cell and platelet adhesion through Rap1 activation,4,5 and is involved in integrin-independent neutrophil chemotaxis.6 RasGRP2 has been reported to be associated with Huntington disease and immune-mediated thrombocytopenia and thrombosis.7,8
We recently constructed aggregates from animal cap cells that were co-treated with activin and angiopoietin-2 and that expressed the vascular endothelial markers X-msr, Xtie2, and Xegfl7.9,10 Using microarray analysis and expression pattern analysis, we identified a novel vascular-expressed gene xrasgrp2 in Xenopus embryos; this gene is a homolog of the human rasgrp2 gene.11 XRasGRP2 overexpression resulted in induction of ectopic vascular formation, and its knockdown delayed vascular development.12 These results suggest that XRasGRP2 plays a role in vasculogenesis and/or angiogenesis during early development of Xenopus embryos.
Rap1 is a member of the Ras family of small GTPases. Its GDP-GTP cycle is regulated by GEFs including C3G, RasGRPs, Epacs, PDZ-GEFs and DOCK4, and GTPase-activating proteins (GAPs) including RapGAPs and SPA-1 family.13 In endothelial cells, Rap1 activated by GEFs plays an important role in angiogenesis.14,15 Recently, we showed that human umbilical artery endothelial cells and human umbilical vein endothelial cells express RasGRP2.16 However, the effect of RasGRP2 on endothelial cells remains poorly understood.
In the present study, we investigated the effect of RasGRP2 in vascular endothelial-like ECV304 cells. We found that RasGRP2 affects cell viability and cell–matrix adhesion in endothelial cells.
Results
To investigate the effect of RasGRP2 in endothelial cells, we established RasGRP2 stably transfected ECV304 (ECV304rasgrp2) cells. As a result of performed RT-PCR analysis, ECV304rasgrp2 overexpressed rasgrp2 mRNA (Fig. 1A). Similarly, as a result of performed western blot analysis, ECV304rasgrp2 overexpressed RasGRP2 protein (Fig. 1B). Additionally, there was the RasGRP2 expression of ECV304rasgrp2 cells more than 100 times than that of ECV304mock cells (Fig. 1C). In microscopy, ECV304rasgrp2 cells did not result in a dramatic morphological change (Fig. 1D).
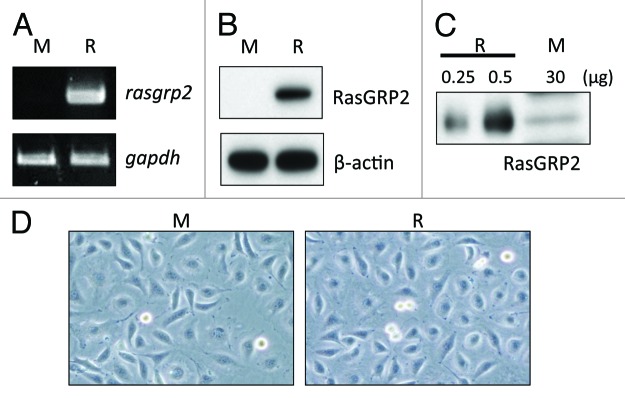
Figure 1. RasGRP2 expression in ECV304rasgrp2 cells and ECV304mock cells. (A) Rasgrp2 mRNA assessed by RT-PCR. Gapdh expression served as a quantitative control. (B and C) RasGRP2 protein assessed by western blot analysis. Equal protein loading was verified using an anti-β-actin antibody. (D) Cells were photographed using a differential-phase microscope with 100× magnification. M, ECV304mock; R, ECV304rasgrp2.
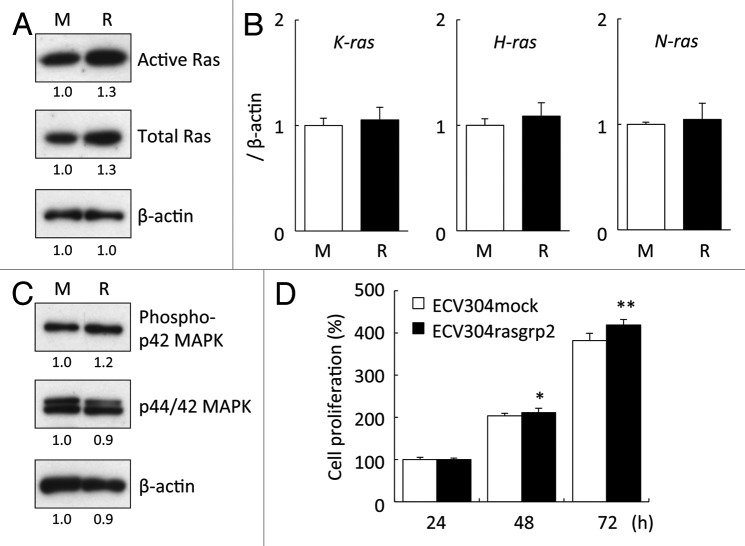
We analyzed Ras activation by RasGRP2 by using a pull-down method based on Raf-1-RBD. This result showed that Ras was activated 1.3 times in ECV304rasgrp2 cells. However, the expression of Ras protein was slightly increased in ECV304rasgrp2 cells, indicating that there was no difference in the active Ras/total Ras ratio between ECVmock cells and ECVrasgrp2 cells (Fig. 2A). Other RasGRP2 overexpressing clones showed similar effects (data not shown). Interestingly, quantitative real-time RT-PCR revealed that RasGRP2 did not affect the mRNA expression of K-, H-, and N-Ras (Fig. 2B).
Figure 2. Effect of RasGRP2 overexpression on Ras expression and cell proliferation. (A) Ras activation assessed by Ras activity assay. Upper panel, active form of Ras; middle panel, total Ras. (B) K-, H-, and N-ras mRNA assessed using real-time RT-PCR. (C) Phosphorylation of p44/42 MAPK protein assessed by western blot analysis. (D) Cell proliferation was determined by WST-8 assay. Data are shown as the mean ± SD (n = 6) *P < 0.05, **P < 0.01 vs. ECV304mock.
Furthermore, we analyzed the function of p42 MAPK, which is a downstream signal for Ras. This result showed that the phosphorylation of p42 MAPK is increased in ECV304rasgrp2 cells (Fig. 2C). Ras is central in a network controlling cell proliferation and cell survival.1 To investigate the cell proliferation in both ECV304mock and ECV304rasgrp2 cells, we performed WST-8 assay. Cell proliferation significantly increased in ECV304rasgrp2 cells, suggesting that the Ras-MAPK signaling is activated in ECV304rasgrp2 cells (Fig. 2D).
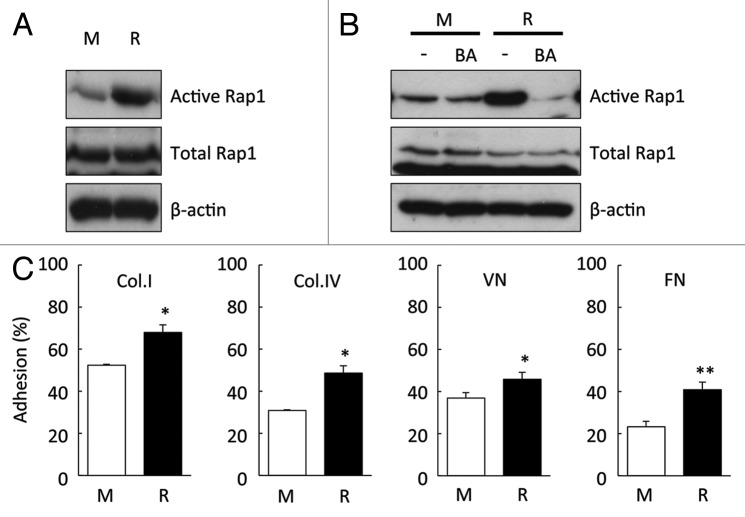
We analyzed Rap1 activation by RasGRP2 using a pull-down method based on RalGDS-RBD. Rap1 activation was substantially increased in ECV304rasgrp2 cells (Fig. 3A). To investigate whether Rap1 is activated by RasGRP2, cells were pre-treated with BAPTA-AM which is an intracellular calcium chelator. RasGRP2 has potential calcium-binding EF-hands that may be involved in Rap1 activation.4,5 The increase in Rap1 activation in ECV304rasgrp2 cells was suppressed by BAPTA-AM pre-treatment. This suppression, albeit to a lesser extent, was also observed in ECVmock cells, which have low levels of endogenous RasGRP2 (Fig. 3B).
Figure 3. Effect of RasGRP2 overexpression on Rap1 activation and cell adhesion. (A) Rap1 activation assessed by Rap activity assay. Upper panel, active form of Rap1; middle panel, total Rap1. (B) Rap1 activation assessed by Rap activity assay with or without 10 μM BAPTA-AM (BA, intracellular calcium chelator) for 30 min. (C) Cell adhesion assessed by adhesion assay. Data are shown as the mean ± SD (n = 3) *P < 0.05, **P < 0.01 vs. ECV304mock.
Rap is a major activator of integrins increasing cell–matrix adhesion.1,13 To investigate the role of RasGRP2 in the regulation of cell adhesion, we used adhesion assays using extracellular matrix components, such as collagen I, collagen IV, fibronectin, and vitronectin. RasGRP2 overexpression significantly increased the adhesion of ECV304 cells to extracellular matrix components (Fig. 3C).
Discussion
Among the RasGRPs, RasGRP2 activates Rap selectively, and not Ras.1,2 Ras plays an important role in cell proliferation by activating ERK which is a downstream signal molecule; in contrast, Rap is a major activator of integrins.1,13 Carmona et al. demonstrated that Rap1 did not affect cell proliferation in endothelial cells.14 Indeed, our results demonstrated that RasGRP2 overexpression increased Rap1 activation and not Ras activation. However, RasGRP2 slightly increased Ras expression and increased proliferation in ECV304 cells (Fig. 2A). This slight increase may result from promotion of translation or suppression of proteolysis, but not from an increase in mRNA levels.
Rap1 plays an important role in cell–cell and cell–matrix adhesion in endothelial cells.14,15,17-19 Similarly, it is known that RhoA, a member of the Rho family of small GTPase, is involved in cell adhesions.20,21 Although we analyzed RhoA activation by RasGRP2 by using a pull-down assay, the activation was not detected in both ECV304mock and ECV304rasgrp2 cells (data not shown). This result suggested that RasGRP2 activates Rap1 mainly in cell adhesions.
With regard to cell–cell adhesion, Fukuhara et al. demonstrated that Epac-Rap1 signaling promotes decreased cell permeability via VE-cadherin.17 However, our data demonstrated that RasGRP2 overexpression did not decrease cell permeability (data not shown). This discrepancy may occur because ECV304 cells have abundant expression of N-cadherin but low expression of VE-cadherin.22,23 It regulates the polarity of migrating neurons in the developing mouse brain cortex through the control of N-cadherin,24 while Rap1 is required for cell–cell adhesion mediated by not only VE-cadherin but also E-cadherin.25,26 On the other hand, Vuchak et al. demonstrated that inhibition of Rap1 activity by Rap1GAP impairs cell–matrix adhesion without affecting cell–cell adhesion in colon carcinoma cells, and suggested that this effect is mediated by localized expression of Rap1GAP.27 Furthermore, it has been reported that sustained Rap1 activity may affect cell–cell adhesion adversely.28,29 RasGRP2 may not participate in cell–cell adhesion. These relationships are complicated and further study, including Rap1GAP activity, would be necessary.
With regard to cell–matrix adhesion, Carmona et al. demonstrated that inhibition of Rap1 activity by overexpression of Rap1GAP1 or knockdown of Rap1a or Rap1b reduced adhesion to collagen I and fibronectin via integrin in endothelial cells.14 In the present study, RasGRP2 overexpression increased cell–matrix adhesion in ECV304 cells (Fig. 3C). Activation of integrin is indispensable for cell–matrix adhesion, and cell–matrix adhesion induces outside-in signaling, which affects the phosphorylation of Akt and FAK in endothelial cells.14 These are the molecules which are important in the maintenance of endothelial cells,30 and RasGRP2 may increase apoptotic resistance through them.
In this study, we performed overexpression analysis of RasGRP2. To establish the functional role of Rasgrp2, we intend to test the knockdown or loss-of-function experiments as confirmatory evidence.
In conclusion, these data demonstrate that RasGRP2 increases cell viability and cell–matrix adhesion through increased Ras expression and Rap1 activation, respectively, in endothelial cells (Fig. 4). The findings of this study may be helpful in the study of angiogenesis in endothelial cells.
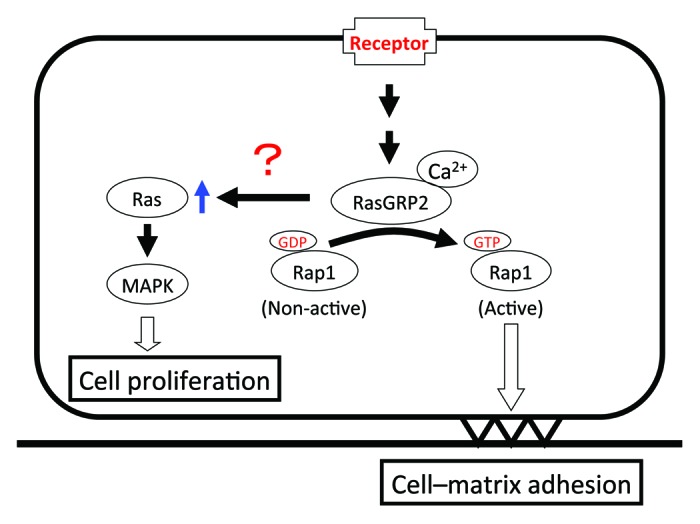
Figure 4. Proposed model for RasGRP2-mediated response.
Materials and Methods
Vector construction and transfection
The DNA fragment of rasgrp2 was amplified from human placenta cDNA (Clontech) by using the Expand Long Template kit (Roche), digested with HindIII and EcoRI, and cloned into the HindIII/EcoRI sites of the pcDNA3.1 vector (Clontech). ECV304 cells were transfected with the rasgrp2 vector and its mock vector using Fugene HD (Roche). After 24 h, cells were selected using G418 (Roche), and stably transfected cells were established.
Cell cultures
Stably transfected ECV304 cells were grown in Medium 199 (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Equitech-Bio) in the presence of 1 mg/mL G418 under standard cell culture conditions (humidified atmosphere, 5% CO2, 37 °C). Cells (8 × 104 cells/mL) were then seeded in various plates or culture dishes (BD Biosciences) and incubated for 36 h before the start of all experiments except the cell proliferation and adhesion assay. To investigate the specific effects of RasGRP2, cells were pre-incubated with or without 10 μM BAPTA-AM (AAT Bioquest) for 30 min.
Reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from transfected ECV304 cells using ISOGEN II (Nippon Gene), and cDNA was synthesized using random primers and reverse transcriptase. Rasgrp2 and glyceraldehyde-3-phosphate dehydrogenase (gapdh) cDNA fragments were amplified from the cDNA mix. The primers used were as follows: rasgrp2, 5′-CAG GCA ACT ATG GCA ACT AC-3′ and 5′-CCT GAT CCA GCT TGG GTT TG-3′; gapdh, 5′-GCT GCA TTC GCC CTC TTA ATG G-3′ and 5′-CAG TCT TGG ATG AGA AAG GTG-3′.
Preparation of cell lysate and western blot analysis
Cell lysates were prepared as previously described,31 dissolved in LDS sample buffer (Invitrogen) containing 10% sample reducing agent (Invitrogen), boiled for 10 min at 70 °C, separated by SDS-PAGE, and then electro-transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore). Membranes were blocked for 30 min by using the PVDF blocking reagent for Can Get Signal® (Toyobo). After washing with PBS containing 0.05% Tween 20 (PBS-T), the membranes were incubated with rabbit anti-RasGRP2 antibody (GeneTex), mouse anti-β-actin antibody, rabbit anti-Rap1 antibody (Santa Cruz), mouse anti-pan-Ras antibody (Cell Biolabs), rabbit anti-phospho-p44/42 MAPK antibody, or rabbit anti-p44/42 MAPK antibody (Cell Signaling) in Can Get Signal® Solution 1 (Toyobo) for 1 h. Subsequently, the membranes were washed thrice with PBS-T and incubated with anti-rabbit IgG antibody (GeneTex) or anti-mouse IgG antibody (DakoCytomation) in Can Get Signal® Solution 2 (Toyobo) for 1 h. After five additional washes with PBS-T, immunoreactive proteins were detected using ECL Prime Western Blotting Detection Reagents and Amersham hyperfilmTM ECL (GE Healthcare).
Ras and Rap1 activity assay
Ras activation was examined using the pan-Ras activation assay kit (Cell Biolabs). This assay kit is a pull-down method based on the specific binding of agarose beads containing the Ras-binding domain of Raf-1 (Raf1-RBD) to the active, GTP-bound form of Ras. Briefly, cell lysates (50 μg) and Raf1-RBD (6 μg) agarose beads were incubated for 1 h at 4 °C with gentle rotation. The beads were then washed thrice with excess lysis buffer, and bound proteins were eluted in LDS sample buffer. Rap1 activation was examined using RalGDS-RBDGST (Jena Bioscience) and Cosmogel® GST-Accept (Nacalai Tesque). Briefly, cell lysates (150 μg) and RalGDS-RBDGST (2 μg) were incubated for 1 h at 4 °C with gentle rotation. After adding 20 μg of Cosmogel® GST-Accept, the samples were incubated for 30 min at 4 °C with gentle rotation. The beads were then washed thrice with excess lysis buffer, and then the bound proteins were eluted in LDS sample buffer.
Cell proliferation
After cells (2 × 104 cells/mL) were incubated for 24 to 72 h, 10 µL/well of WST-8 solution (Dojindo Laboratories) was added, and the cells were incubated for 2 h. Absorbance was then measured at 450 nm and 650 nm by using a microplate reader (Spectra Max 190; Molecular Devices). The net difference (A450 − A650) was used as a measure of cell proliferation.
Real-time RT-PCR
Real-time RT-PCR was performed using a Smart Cycler® II System (Takara), as previously described.31 The primers used were as follows: K-ras, 5′-GAC TGA ATA TAA ACT TGT GGT AGT TGG AG-3′ and 5′-TCC TCT TGA CCT GCT GTG TCG-3′; H-ras, 5′-TTT GAG GAC ATC CAC CAG TAC A-3′ and 5′-GCC GAG ATT CCA CAG TGC-3′; N-ras, 5′- CAG AGG CAG TGG AGC TTG A-3′ and 5′-GCT TTT CCC AAC ACC ACC T-3′; and β-actin, 5′-TCC ACC TCC AGC AGA TGT GG-3′ and 5′-GCA TTT GCG GTG GAC GAT-3′.
Adhesion assay
A 96-well plate was coated with 2.5 μg/mL human vitronectin (PeproTech) in Medium 199 for 1 h at 37 °C, and plates coated with collagen I, collagen IV, or fibronectin were purchased from BD Biosciences. Cells were seeded at 12 000 cells/well in the coated plates for 15 min at 37 °C. The non-adherent cells were removed by washing twice with PBS (+), thereafter, the adherent cells were fixed in 4% paraformaldehyde. The cells were then stained with 0.5% crystal violet for 30 min, the dye was eluted with 1% SDS, and the absorption at 550 nm was measured using a microplate reader. Equal amount of cells was seeded in non-coated plates for 3 h at 37 °C and defined as 100% adhesion.
Statistical analysis
All experiments were performed in duplicate and repeated at least two or three times; each experiment yielded essentially identical results. Data are expressed as the mean ± standard deviation (SD). The significance of differences between group means was determined using a one-way analysis of variance, t test, or the Welch test. P < 0.05 was defined as significant.
Glossary
Abbreviations:
- RasGRPs
Ras guanyl nucleotide releasing proteins
- GEFs
guanine nucleotide exchange factors
- DAG
diacylglycerol
- GAPs
GTPase-activating proteins
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/24082
References
- 1.Raaijmakers JH, Bos JL. Specificity in Ras and Rap signaling. J Biol Chem. 2009;284:10995–9. doi: 10.1074/jbc.R800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone JC. Regulation and Function of the RasGRP Family of Ras Activators in Blood Cells. Genes Cancer. 2011;2:320–34. doi: 10.1177/1947601911408082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JE, Goulding RE, Ding Z, Partovi A, Anthony KV, Beaulieu N, et al. Differential membrane binding and diacylglycerol recognition by C1 domains of RasGRPs. Biochem J. 2007;406:223–36. doi: 10.1042/BJ20070294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghandour H, Cullere X, Alvarez A, Luscinskas FW, Mayadas TN. Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion. Blood. 2007;110:3682–90. doi: 10.1182/blood-2007-03-077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cifuni SM, Wagner DD, Bergmeier W. CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin alphaIIbbeta3 in platelets. Blood. 2008;112:1696–703. doi: 10.1182/blood-2008-02-139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbo C, Duerschmied D, Goerge T, Hattori H, Sakai J, Cifuni SM, et al. Integrin-independent role of CalDAG-GEFI in neutrophil chemotaxis. J Leukoc Biol. 2010;88:313–9. doi: 10.1189/jlb.0110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crittenden JR, Dunn DE, Merali FI, Woodman B, Yim M, Borkowska AE, et al. CalDAG-GEFI down-regulation in the striatum as a neuroprotective change in Huntington’s disease. Hum Mol Genet. 2010;19:1756–65. doi: 10.1093/hmg/ddq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolla M, Stefanini L, André P, Ouellette TD, Reilly MP, McKenzie SE, et al. CalDAG-GEFI deficiency protects mice in a novel model of Fcγ RIIA-mediated thrombosis and thrombocytopenia. Blood. 2011;118:1113–20. doi: 10.1182/blood-2011-03-342352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagamine K, Furue M, Fukui A, Asashima M. Induction of cells expressing vascular endothelium markers from undifferentiated Xenopus presumptive ectoderm by co-treatment with activin and angiopoietin-2. Zoolog Sci. 2005;22:755–61. doi: 10.2108/zsj.22.755. [DOI] [PubMed] [Google Scholar]
- 10.Nagamine K, Furue M, Fukui A, Matsuda A, Hori T, Asashima M. Blood cell and vessel formation following transplantation of activin-treated explants in Xenopus. Biol Pharm Bull. 2007;30:1856–9. doi: 10.1248/bpb.30.1856. [DOI] [PubMed] [Google Scholar]
- 11.Nagamine K, Matsuda A, Asashima M, Hori T. XRASGRP2 expression during early development of Xenopus embryos. Biochem Biophys Res Commun. 2008;372:886–91. doi: 10.1016/j.bbrc.2008.05.159. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Takahashi S, Haramoto Y, Onuma Y, Nagamine K, Okabayashi K, et al. XRASGRP2 is essential for blood vessel formation during Xenopus development. Int J Dev Biol. 2010;54:609–15. doi: 10.1387/ijdb.092929ks. [DOI] [PubMed] [Google Scholar]
- 13.Hattori M, Minato N. Rap1 GTPase: functions, regulation, and malignancy. J Biochem. 2003;134:479–84. doi: 10.1093/jb/mvg180. [DOI] [PubMed] [Google Scholar]
- 14.Carmona G, Göttig S, Orlandi A, Scheele J, Bäuerle T, Jugold M, et al. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood. 2009;113:488–97. doi: 10.1182/blood-2008-02-138438. [DOI] [PubMed] [Google Scholar]
- 15.Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, et al. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin αvβ₃. Blood. 2011;118:2015–26. doi: 10.1182/blood-2011-04-349282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagamine K, Matsuda A, Hori T. Identification of the gene regulatory region in human rasgrp2 gene in vascular endothelial cells. Biol Pharm Bull. 2010;33:1138–42. doi: 10.1248/bpb.33.1138. [DOI] [PubMed] [Google Scholar]
- 17.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–46. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J Cell Physiol. 2011;226:2052–62. doi: 10.1002/jcp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J Exp Med. 2006;203:2703–14. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res. 2007;313:3616–23. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshioka K, Yoshida K, Cui H, Wakayama T, Takuwa N, Okamoto Y, et al. Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat Med. 2012;18:1560–9. doi: 10.1038/nm.2928. [DOI] [PubMed] [Google Scholar]
- 22.Sandoval R, Malik AB, Naqvi T, Mehta D, Tiruppathi C. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2001;280:L239–47. doi: 10.1152/ajplung.2001.280.2.L239. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto A, Onodera H, Mori A, Nagayama S, Yonenaga Y, Tachibana T. Tumour plasticity and extravascular circulation in ECV304 human bladder carcinoma cells. Anticancer Res. 2006;26(1A):59–69. [PubMed] [Google Scholar]
- 24.Jossin Y. Polarization of migrating cortical neurons by Rap1 and N-cadherin: Revisiting the model for the Reelin signaling pathway. Small GTPases. 2011;2:322–8. doi: 10.4161/sgtp.18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–32. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- 26.Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, et al. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuchak LA, Tsygankova OM, Meinkoth JL. Rap1GAP impairs cell-matrix adhesion in the absence of effects on cell-cell adhesion. Cell Adh Migr. 2011;5:323–31. doi: 10.4161/cam.5.4.17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, et al. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci. 2005;118:4765–83. doi: 10.1242/jcs.02584. [DOI] [PubMed] [Google Scholar]
- 29.Asuri S, Yan J, Paranavitana NC, Quilliam LA. E-cadherin dis-engagement activates the Rap1 GTPase. J Cell Biochem. 2008;105:1027–37. doi: 10.1002/jcb.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Q, Rounds S. Focal adhesion kinase and endothelial cell apoptosis. Microvasc Res. 2012;83:56–63. doi: 10.1016/j.mvr.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takino J, Yamagishi S, Takeuchi M. Glycer-AGEs-RAGE signaling enhances the angiogenic potential of hepatocellular carcinoma by upregulating VEGF expression. World J Gastroenterol. 2012;18:1781–8. doi: 10.3748/wjg.v18.i15.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]