Abstract
Invasive candidiasis in patients who are immunocompromised or in intensive care units (ICUs) presents both diagnostic and therapeutic problems. We previously described antibodies that were directed against Candida albicans cell wall fragments (CW), periodate-treated CW (CWIO4), phosphopeptidomannan (PPM), and β(1-3) glucan. In this study, circulating fungal antigens [mannan and β(1-3) glucan] and immunoglobulin G (IgG) subclass antibodies to these cell wall antigens (anti-CW) were analyzed in patients with systemic candidiasis. Sera were collected from 14 patients on two or three consecutive occasions, starting on the day when candidiasis was culture proven. The sera were analyzed by enzyme-linked immunosorbent assay. The control groups consisted of lactating mothers (n = 9) (group I) who had breast milk that was positive for C. albicans and also had acute inflammation of the nipples, and age-matched blood donors (n = 10) (group II). Within the first 3 weeks of Candida infection all of the patients were positive for β(1-3) glucan by the Gluspecy test, but no patients were positive for mannan in the less-sensitive Pastorex Candida test. The controls were negative for both β(1-3) glucan (<20 pg/ml) and mannan (<2.5 ng/ml). IgG1 anti-CW and IgG2 anti-PPM antibodies were the most discriminatory antibodies. The ratio of IgG1 anti-CW to IgG2 anti-PPM was significantly lower in nonsurviving patients than in the other patients within the first week of candidiasis (P = 0.019). The IgG2 levels of anti-CWIO4 and antiglucan antibodies correlated strongly (r = 0.681; P < 0.0001), and the absence of these antibodies was associated with increased levels of β(1-3) glucan. Increased levels of IgG1 anti-CW or IgG2 anti-PPM antibodies (titer of ≥3 logs) or of a combination of the two antibodies (log sum, ≥5) showed 92% sensitivity, 100% specificity, and positive predictive values. In conclusion, β(1-3) glucan and the two subclass antibodies appear to be early specific markers for the laboratory diagnosis of candidiasis. Furthermore, the kinetics of β(1-3) glucan appearance in serum may assist in evaluating the therapeutic efficacy of antifungal treatments.
For decades the incidence of invasive Candida infections has been rising, particularly in immunocompromised patients. The Candida genus is the fourth most common group of microorganisms recovered from the bloodstream of patients in the United States, and the incidence is also rising in Europe (23, 32). Leukemic and transplant patients with general defects in one or more immune defense mechanisms, as well as surgical patients in intensive care units (ICUs), frequently suffer from candidiasis. The infection is usually of endogenous origin (31). Difficulties in establishing a specific and early diagnosis of Candida infection is one of the reasons for the high mortality rate among these patients, so great efforts have been directed towards finding more rapid diagnostic methods (1, 5).
The major Candida albicans antigen is the highly branched and complex mannan component of the cell wall (20). Although methods for the detection of mannan in serum have been used for immunodiagnosis of systemic Candida infection with very high positive predictive values, it is estimated that <50% of cases of candidiasis are detected (3). The glucan of C. albicans cell wall consists of a β(1-3)(1-6) glucan heteropolymer (11), and C. albicans releases β(1-3) glucan into the culture medium during growth (18). The released glucan can be detected by a biochemical assay that uses amebocyte lysate coagulation factors from the horseshoe crab (21).
We recently reported that patients with systemic candidiasis have elevated levels of immunoglobulin G (IgG) antibodies to native cell wall fragments (CW) of C. albicans and to the phosphopeptidomannan (PPM) fraction of the cell wall, compared with healthy blood donors (15). Thus, the appearance of cell wall mannans and glucans in many Candida-infected patients, as well as the diversity of IgG antibody formation with respect to specificity, suggests the need for further investigation into the subclass distribution of these IgG antibodies.
The human IgG subclasses differ with respect to physical, chemical, and biological properties. IgG1, IgG2, and IgG3 activate complement, although IgG2 does so in a less efficient manner than the others. In contrast to IgG1, IgG3, and IgG4, the IgG2 antibody has no or low binding capacity to human mononuclear cells and neutrophils. The subclass distribution of the antibody response is influenced by the nature of the immunogen, the localization of entry into the body, and the age of the host. IgG1 and IgG3 antibodies are induced mainly by protein antigens, whereas IgG2 is raised mainly against polysaccharides (7, 16, 28).
In the present study, the IgM and IgG subclass antibody responses in serum to native CW and periodate-treated CW (CWIO4), PPM, and β(1-3)(1-6) glucan were studied using recently developed methods, and circulating levels of β(1-3) glucan and mannan were determined with commercially available kits. These analyses were conducted with patients with systemic candidiasis in order to elucidate the discriminatory power of these assays in cases of invasive candidiasis. Our results shed some light on the preconditions for the formation of C. albicans antigen-antibody complexes that have the capacity to circulate in the bloodstream.
MATERIALS AND METHODS
C. albicans.
The C. albicans serotype A strain (ATCC 64549) was grown in Sabouraud dextrose broth on a shaker (50 rpm) at 37°C for 24 h. The blastoconidium cells were obtained from the culture medium by centrifugation. The cells were washed three times in distilled water. The yeast cells expressed the antigenic factors 4, 5, and 6 (Candida Check; Iatron Laboratories, Tokyo, Japan) (15).
Antigens. (i) C. albicans CW.
Candida CWs were prepared by the method described earlier (15). Briefly, washed C. albicans yeast cells were shaken repeatedly together with glass beads. The supernatant fluid was collected, and the fragments were sedimented by centrifugation at 1,200 × g for 10 min.
(ii) CWIO4.
The Candida CWs were treated with sodium periodate to destroy the carbohydrate structures by oxidation, as described previously (15). Briefly, 0.15 M NaIO4 was added to the CW, which was suspended in 0.1 M sodium phosphate buffer (pH 6). The reaction was stopped after 3 h by the addition of 0.15 M Na2SO3, and the suspension was dialyzed against 0.1 M phosphate buffer (pH 6) followed by distilled water. The CWIO4 suspension was lyophilized.
(iii) PPM.
PPM was extracted as described earlier by Kondori et al. (15). Thus, the characteristics of the PPM antigen have been described in more detail previously (15). Briefly, freeze-dried C. albicans yeast cells were suspended in phosphate buffer (pH 7.0) and heat treated at 100°C for 2 h. The mildly extracted cell wall antigen was fractionated with cetavlon at pH 8.8 in the presence of boric acid. The precipitate was washed with sodium borate (pH 8.8) and dissolved in acetic acid with sodium acetate. After precipitation with ethanol the precipitate was washed with 2% acetic acid in ethanol and thereafter once more with ethanol. The water-soluble precipitate was lyophilized. This fraction was composed of approximately 90% carbohydrate, 10% protein, and 0.8% phosphate (15).
(iv) Glucan.
Glucan from Saccharomyces cerevisiae (Sigma Chemical Co., St. Louis, Mo.) was used. Glucan consists of β(1-3)(1-6)-linked glucose residues. This structure is also found in the glucan of C. albicans (12, 13). The S. cerevisiae glucan, which is insoluble in water, was solubilized at a concentration of 10 mg/ml in 0.3 M NaOH.
Study groups. (i) Candidiasis.
Serum samples were collected from 14 patients (age [mean ± standard deviation], 62 ± 12 years) on two or three consecutive occasions, starting on the day when candidiasis was culture proven (Table 1). All of the patients were treated with flucytosine during the serum collection period. The patients were classified according to the following laboratory and clinical criteria: (i) positive culture from normally sterile sites (blood, bile, and pericardial fluid); (ii) the presence of risk factors (cancer and chemotherapy, abdominal surgery, or the use of broad-spectrum antibiotics); and (iii) the presence of an infectious syndrome (fever) that did not respond to antibacterial therapy. Deaths that occurred within 2 months of the initiation of antimycotic treatment were regarded as being related to the fungal infection or a combination of the underlying disease and infection.
TABLE 1.
Underlying disease and culture parameters of patients with systemic candidiasis
| Patient no. | Gendera | Age (yr) | Hospital ward | Underlying condition(s) | No. of serum specimens | Source of Candida culture | Candida species | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 71 | ICU | Rectal neoplasms | 3 | Blood | C. albicans | Survival |
| 2 | F | 67 | Surgery | Pancreaticoduodenectomy | 3 | Blood | C. albicans | Survival |
| 3 | F | 40 | Neurology | Aneurysm | 3 | Blood | Survival | |
| 4 | M | 74 | ICU | Aortic aneurysm | 3 | Blood | C. albicans | Survival |
| 5 | M | 61 | ICU | Diabetes mellitus hemorrhagic pancreatitis | 3 | Blood | C. albicans | Survival |
| 6 | F | 56 | ICU | Candida septicemia | 3 | Blood | C. albicans | Survival |
| 7 | F | 74 | ICU | Diabetes mellitus | 3 | Blood | C. albicans | Deathb |
| 8c | F | 50 | Surgery | Non-Hodgkin's lymphoma | 3 | Blood | C. albicans | Death |
| 9d | F | 47 | ICU | Neoplasm, intestinal obstruction | 3 | Blood | C. albicans | Death |
| 10 | M | 66 | Transplantation unit | Diabetic angiopathics and nephropathies | 3 | Abdominal | C. albicans | Death |
| 11 | M | 66 | Thorax | Atrial fibrillation | 3 | Blood | C. albicans | Death |
| 12 | F | 82 | Transplantation unit | Gallbladder neoplasms | 3 | Blood | C. tropicalis | Survival |
| 13 | M | 64 | Surgery | Diabetic coma | 2 | Blood | C. glabrata | Survival |
| 14d | F | 46 | Transplantation unit | Hepatitis B and hepatitis C | 3 | Bile | C. glabrata | Survival |
Abbreviations: M, male; F, female.
Died within 2 months of initiation of antimycotic treatment.
Chemotherapy and cytotoxic treatment.
Chemotherapy.
(ii) Controls.
Two control groups were included in this study. One group (group I) comprised nine lactating mothers with superficial C. albicans infection of the nipples (age [mean ± standard deviation], 31 ± 5 years). Milk samples from these women were positive for C. albicans, and all suffered from pain and inflammation of the nipples. The other group consisted of 10 healthy blood donors (7 men and 3 women; age [mean ± standard deviation], 60 ± 4 years) (group II).
The study protocol was approved by the local ethics committee, Göteborg, Sweden (approval no. S131-00), and all patients gave informed consent.
IgG subclass antibody analysis.
Microplate wells (Immunoplate; Nunc, Roskilde, Denmark) were coated with 100 μl of 50-μg/ml CW or CWIO4. In the cases of glucan and PPM, concentrations of 20 and 5 μg/ml, respectively, were used. The antigens were diluted in 50 mM Na2CO3 buffer, pH 9.3. The plates were incubated at room temperature (RT) for 2 h and then kept at 4°C overnight. The plates were rinsed with phosphate buffered saline (PBS), 100 μl of blocking buffer (BF; 1% bovine serum albumin, 0.05% Tween 20 in PBS) was added to each well, and the plates were incubated for 1 h at RT. The plates were rinsed once with 0.05% Tween 20 in PBS (PBS-T). Human sera, which were diluted in 10-fold serial steps (1/100 to 1/10,000) in BF, were added to the wells (100 μl) and incubated for 2 h at RT. The plates were rinsed three times with PBS-T between incubation steps. Murine monoclonal antibodies to IgG1 (JL512), IgG2 (GOM1), IgG3 (ZG4), and IgG4 (RJ4) (Immunotech, Marseille, France) were diluted in BF and added to the wells (100 μl). The plates were incubated at RT for 2 h, and 100 μl of biotinylated rabbit anti-mouse IgG [F(ab)2; Dako, Glostrup, Denmark] diluted 1/5,000 in BF was added. After incubation at RT overnight, 100 μl of a 1/10,000 dilution of alkaline phosphatase-conjugated extravidin (Sigma) were added, and the plates were incubated at RT for 60 min. para-Nitrophenylphosphate (100 μl of 1 mg/ml; Sigma) in diethanolamine buffer (pH 9.8) was added to each well, and the absorbance was read at 405 nm, i.e., after 30 min of incubation when a suitable color had developed in the positive standard serum (see below).
Two serum samples were included as standards in each assay: one with a high antibody titer (positive standard, pooled sera from patients) and the other with a low antibody titer (pooled sera from healthy individuals). A graph was plotted for each serum sample and the standards. The serum antibody titer was defined as the log of the dilution that gave an absorbance value of 0.15 above the background value. When the log value of the positive standard varied by more than 10% compared to the mean value based on 10 separate runs, the titers of the patient samples were adjusted accordingly. The interassay variation, which was expressed as the coefficient of variation (CV) of three serum samples, was analyzed on three separate occasions and found to be <8%, irrespective of the IgG subclass or antigen used.
Glucan determination.
The β(1-3) glucan concentration in serum was determined using the Gluspecy kit (Seikagaku, Tokyo, Japan). All of the glassware used was heated to 180°C overnight to inactivate any contaminants. Serum samples were diluted 1/10 in pyrogen-free water and heat inactivated at 75°C for 10 min to inactivate inhibitory factors that might be present in the serum (26). The assay was run according to the manufacturer's manual, including the azo-coupling procedure for increasing the sensitivity of the assay. The absorbance of the samples was read at 560 nm, and a standard curve was plotted. The β(1-3) glucan concentration was determined for each sample, and a cutoff value for a positive glucan level was set at 20 pg/ml, which was approximately three times the mean level in healthy blood donors (glucan level [mean ± standard deviation], 7.0 ± 1.5 pg/ml).
Mannan determination.
Mannans in the serum were assayed using a commercially available latex agglutination test (Candida Pastorex; Sanofi Diagnostics Pasteur) according to the manufacturer's instructions.
Statistical analysis.
The data were analyzed by the method of Kruskal-Wallis to avoid random significance when comparing several groups. Correlations were analyzed by Spearman's rank correlation test.
RESULTS
Circulating antigens.
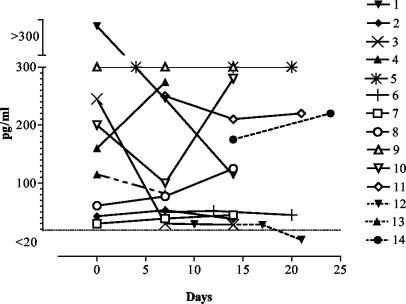
Analysis of β(1-3) glucan in the serum of patients with candidiasis showed that 37 of 38 samples were positive (levels >20 pg/ml) (Fig. 1). However, the sample that was negative for β(1-3) glucan was collected 3 weeks after the initial cultivation of C. tropicalis from one patient (patient 12) (Fig. 1). Out of the five patients (patients 7 to 11) who subsequently died, four showed glucan concentrations of at least 120 pg/ml (Fig. 1). Only one (patient 7) had glucan levels of <100 pg/ml in all samples. The remaining nine surviving patients had glucan levels of <100 pg/ml in their last serum samples, with the exception of patients 1, 4, 5, and 14. However, one of these patients (patient 1) showed glucan levels that dropped from more than 300 to 110 pg/ml, which was probably an indication of recovery. The patient with the lowest glucan level (patient 12) (Table 1) was infected with C. tropicalis.
FIG. 1.
Glucan concentrations in serum from patients with systemic candidiasis. Kinetics of the glucan concentration for each patient with respect to the time at which Candida was isolated (day 0) is shown. Samples with glucan concentrations of ≥20 pg/ml were considered to be positive. The numbers after the symbols refer to the patient number in Table 1. Open symbols represent nonsurvivors, and patients whose symbols are connected with a broken line were infected with Candida species other than C. albicans.
The β(1-3) glucan concentrations in the serum samples of patients with superficial fungal infections and from healthy blood donors were all <20 pg/ml. Thus, the concentrations of β(1-3) glucan in serum samples of patients with systemic candidiasis were significantly elevated compared to those of the controls.
The sera of the patients and controls were also analyzed for mannan using the Pastorex Candida assay. In this instance, the results for all of the patients and controls were negative.
IgG subclass antibodies against C. albicans. (i) IgG antibodies to CW
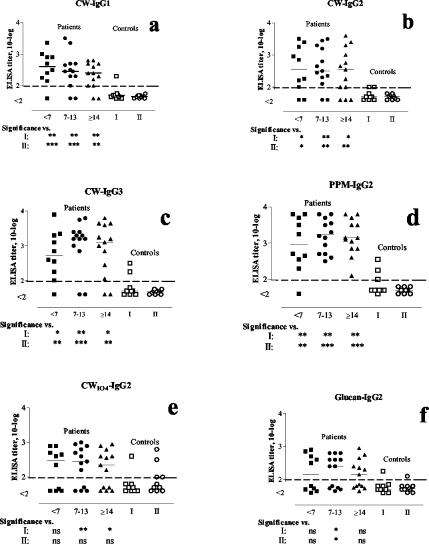
IgG-subclass antibody analysis using C. albicans CW as the coating antigen showed that IgG1, IgG2, and IgG3 antibody levels were significantly elevated in patients with invasive Candida infections compared to the results seen with the control groups (Fig. 2a to c). The majority of the samples from patients with C. albicans infections showed positive anti-CW IgG1, IgG2, and IgG3 titers. However, all of the samples from two (patients 12 and 14) (Table 1) of the three patients who were infected with C. tropicalis or C. glabrata were negative for IgG1 antibody to C. albicans CW (Fig. 3a). Only one of the women with superficial C. albicans infection of the nipples (group I) was positive for IgG1 antibodies to CW (Fig. 2a).
FIG. 2.
Serum IgG1, IgG2, and IgG3 antibodies to C. albicans cell wall antigens in patients with systemic candidiasis and controls (group I and II). Antibody levels were analyzed by enzyme-linked immunosorbent assay (<2 log; no detectable antibody activity in serum diluted 1:100). The numbers <7, 7 to 13, and ≥14 indicate less than 7 days, between 7 and 13 days, and 14 days or more, respectively, after the presumed onset of infection (cultivation of Candida from the blood). Symbols: *, P < 0.05; **, P < 0.01; ***, P < 0.001).
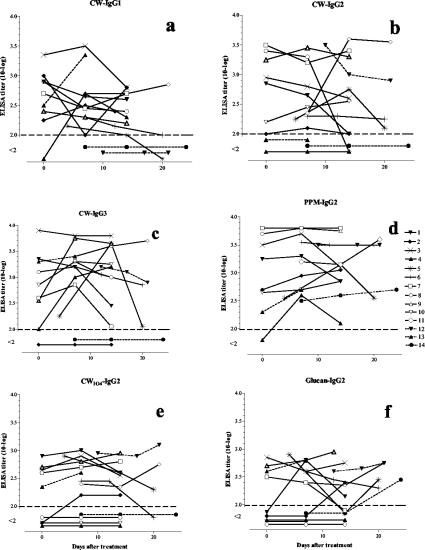
FIG. 3.
IgG1, IgG2, and IgG3 antibodies to C. albicans CW in patients with systemic candidiasis at different time points after the isolation of Candida. Antibody levels were analyzed by enzyme-linked immunosorbent assay. Dashed lines connect the symbols for patients with non-C. albicans Candida infections, and open symbols indicate patients who died within 2 months after initiation of antifungal treatment.
Three patients were negative in all of the tests for IgG2 antibodies to CW (Fig. 3b). Two of these patients were infected with C. glabrata (Fig. 3b; Table 1). Two out of nine patients in control group I showed borderline-positive titers for this antibody (Fig. 2b).
One of the two patients who were negative for IgG3 antibodies to CW was infected with C. glabrata (Fig. 3c). This patient did not show any anti-CW response (Fig. 3a to c). All of the healthy blood donors were negative for IgG3 antibodies, while two women in group I were positive. No significant differences (P > 0.05) were found between the patients and the controls regarding IgG4 and IgM antibodies to CW, with only small variations in the titers (not shown).
(ii) IgG1, IgG3, and IgG4 antibodies to CWIO4, glucan, and PPM.
No discriminatory differences were found between the patients and the controls with respect to IgG1, IgG3, and IgG4 antibodies to CWIO4, glucan, and PPM (data not shown).
(iii) IgG2 antibodies to CWIO4.
For the two last sampling points (≥7 to 13 and ≥14 days) the levels of IgG2 antibodies to CWIO4 were significantly higher than those in control group I (Fig. 2e). Four of the patients were negative for these antibodies, and one of these did not survive (Fig. 3e). In addition, all four patients displayed increased glucan values with time (P = 0.0037; Fisher's exact test).
(iv) IgG2 antibodies to PPM.
Levels of IgG2 antibodies to PPM were significantly increased in the candidiasis patients compared to those in the controls (Fig. 2d). All of the patients were positive for IgG2 antibody to PPM (Fig. 3d). Only one serum sample, which was taken within the first week, tested negative. This very early serum sample was also negative in all of the other antibody tests, except for the IgG3 anti-CW assay, in which the titer reached the limit of detection. Note that patient 14 (Table 1), despite immunosuppressive treatment, was positive at all time points for IgG2 antibody to PPM and at the last time point was positive for IgG2 antiglucan. In addition, C. glabrata was isolated from this patient. Three of the controls in group I were positive for IgG2 antibodies to PPM, whereas all of the healthy blood donors were negative (Fig. 2d). The ratios of the levels of IgG1 anti-CW to IgG2 anti-PPM were significantly lower in nonsurviving patients than in the other patients, especially within the first week (<7 days) of cultivation of Candida from blood samples (P = 0.019; data not shown).
(v) IgG2 antibodies to glucan.
The levels of IgG2 antibodies to glucan were also significantly higher in the candidiasis patients than in the controls, except in the case of serum samples taken at <7 days (Fig. 2f). Three patients, all of whom were infected with C. albicans, tested negative in all of the samples (Fig. 3f). These were the same patients that were found negative for IgG anti-CWIO4 (Fig. 3e). One of the controls in group I and another in group II were positive for IgG anti-CWIO4 (Fig. 2f).
Statistical analysis. (i) Correlation between different antibodies.
A strong correlation was found between the levels of IgG2 anti-CW and IgG2 anti-PPM antibodies, and somewhat less of a correlation was found between the levels of IgG2 anti-CW and IgG2 anti-CWIO4, which possibly reflects the fact that PPM is the predominating antigen in CW (Table 2). Furthermore, a strong correlation was established between the levels of IgG2 anti-CWIO4 and IgG2 antiglucan antibodies (r = 0.681, P < 0.0001; data not shown), which may indicate that glucan is a major component of CWIO4. No correlation was observed between the levels of IgG1 anti-CW and the IgG2 anti-CWIO4, anti-PPM, and antiglucan antibodies (Table 2).
TABLE 2.
The correlation coeffient (r)a between IgG2 antibodies against CWIO4, PPM, and glucan with IgG1, IgG2, IgG3, and IgM antibodies against CW in serum from patients with systemic candidiasis
| Anti-CW antibody |
r (P) versus IgG2 antibody against:
|
||
|---|---|---|---|
| CWIO4 | PPM | Glucan | |
| IgG1 | NSb | NS | NS |
| IgG2 | 0.495 (0.0010) | 0.681 (<0.0001) | NS |
| IgG3 | 0.365 (0.0191) | 0.310 (0.0484) | 0.410 (0.0077) |
| IgM | NS | 0.336 (0.0317) | NS |
Correlation calculated by Spearman's rank correlation test.
NS, not significant.
No significant correlation was found between the serum glucan concentration and the level of any IgG subclass. However, the glucan concentrations correlated with the presence of IgM antibodies to CW (r = 0.5384; P = 0.0004).
(ii) Sensitivity, specificity, and positive and negative predictive values.
The sensitivity of the IgG2 antibody detection test for CW increased from 85 to 98% when PPM was used (Table 3). The sensitivities of the IgG1 and IgG3 antibody detection tests for CW were almost the same (80 and 85%, respectively). The lowest specificity (26%) was found for IgM antibodies to CW, which means that these antibodies have no diagnostic value.
TABLE 3.
Sensitivity, specificity, and predictive values for the detection of IgG subclass antibodies in patients with systemic candidiasisa
| Antibody | Sensitivity (%) | Specificity (%) | Positive predic- tive value (%) | Negative predic- tive value (%) |
|---|---|---|---|---|
| CW | ||||
| IgG1 | 80 | 95 | 97 | 69 |
| IgG2 | 85 | 89 | 94 | 74 |
| IgG3 | 85 | 89 | 95 | 74 |
| IgM | 90 | 26 | 74 | 56 |
| PPM IgG2 | 98 | 95 | 93 | 95 |
| CWIO4 IgG2 | 66 | 74 | 85 | 50 |
| Glucan IgG2 | 61 | 89 | 93 | 52 |
Results were calculated per serum sample based on the analysis of 41 serum samples from 14 patients with systemic candidiasis and 19 serum samples from controls.
It is important to be able to distinguish patients with systemic candidiasis from those with other infections as early as possible after the onset of clinical symptoms. For this reason, the discriminatory power of a combination of the most sensitive test (IgG2 anti-PPM) and the most specific test (IgG1 anti-CW antibody) was analyzed (Table 4). A positive serum sample was defined as either a titer of 3 logs for any of the two antibodies or a log sum of at least 5. Whereas the sensitivity of the combined test was 70% within the first week (<7 days) of culture-proven candidiasis, it increased to 92% during the second week. The specificity and positive predictive value were 100% for all sampling occasions. Although the glucan concentration per se identified all of the candidiasis cases, the combined tests for glucan and IgG2 anti-PPM and IgG1 anti-CW antibodies provided strong support for the idea of an invasive Candida infection.
TABLE 4.
Sensitivity, specificity, and predictive values for combined detection of IgG1 anti-CW and IgG2 anti-PPM antibodies in patients with systemic candidiasis at different time points after culture-proven candidiasis
| Parametera | Combination of IgG1 anti-CW and IgG2 anti-PPMb
|
||
|---|---|---|---|
| <7 days | 7-13 days | >14 days | |
| Sensitivity | 70 | 93 | 85 |
| Specificity | 100 | 100 | 100 |
| Positive predictive value | 100 | 100 | 100 |
| Negative predictive value | 86 | 95 | 90 |
The results were calculated per serum sample from patients with systemic candidiasis (one sample) (see footnote b) within the time points indicated and 19 serum samples from controls.
In order to be positive, the individual titers of the two antibodies had to be ≥3 logs, or the combined titers had to be ≥5 logs.
DISCUSSION
The diagnosis of invasive candidiasis is both clinically and microbiologically difficult (9). To solve this problem, diagnostic methods that are based on the detection of marker substances as well as antibodies to Candida in the serum have been used (4, 6, 21, 25). Some of these techniques are commercially available, but their clinical usefulness is controversial. In the present study, glucan detection together with assays for IgG1 anti-CW and IgG2 anti-PPM antibodies were found to be reliable and early markers of systemic candidiasis. All of the patients were positive for glucan within the first 2 weeks of systemic candidiasis, whereas the glucan concentrations in all of the controls were lower than the cutoff value (<20 pg/ml). Similarly, patients with high titers (≥3 logs) of IgG1 anti-CW or IgG2 anti-PPM or with high combined titers (≥5 logs) of these antibodies showed 92% sensitivity and 100% specificity.
β(1-3) glucan is a structural component of the cell wall of C. albicans, as in all of the medically important fungi. The report by Obayashi et al. (21), in which glucan determination was found to be a promising method for the identification of patients with deep mycosis, is in agreement with our results. Obayashi et al. (21) recorded that 37 of 41 episodes of fungal infection showed positive results, and all of the episodes of nonfungal infections showed glucan concentrations that were lower than the cutoff value (sensitivity and specificity of 90 and 100%, respectively) (21). In our study, the glucan concentrations were determined in three consecutive serum samples from patients who were receiving treatment with flucytosine. Since 80% of the patients who died showed glucan concentrations of >120 pg/ml, whereas only 33% of the survivors showed values of this magnitude, increasing or persistently high glucan levels (in spite of antifungal treatment) appear to be indicators of more severe outcome. Obayashi et al. observed a similar relationship between the concentration of glucan and the development of infection and noted that the plasma β(1-3) glucan concentration tended to decrease as the antifungal therapy brought about clinical improvement (22). Thus, the success of antifungal treatment in patients with systemic candidiasis may be monitored by consecutive analyses of blood glucan levels.
The Pastorex Candida test was used for the detection of circulating mannan. However, none of our patients showed detectable mannan levels (≥2.5 ng/ml). The sensitivity of this test has been reported to range between 0 and 25% (6, 8, 17, 19). In accordance with our results Gutierrez et al. reported that all their patients with candidemia were negative for the Pastorex Candida test (6). It should also be noted that even if the levels of circulating mannan were fivefold higher than those of glucan, the former would not reach the detection level of Pastorex Candida mannan (>2.5 ng/ml). The low sensitivity of this test and differences in the patient groups may be an explanation for the reduced detectability of this antigen (10).
We recently reported that IgG antibodies to native CW and PPM distinguished patients with systemic candidiasis from healthy blood donors (15). In the present study, a more-detailed analysis of the antibodies to C. albicans CW showed that the IgG2 subclass antibody response predominated and that certain responses of IgG1 and IgG3 subclasses could be measured. The dominating IgG subclass in the overall anti-CW response to the cell wall antigens differed between individual patients (Fig. 3). The protein that is present in the native CW may be the major antigen that is involved in the IgG1 and IgG3 antibody responses, since these IgG subclasses are mainly induced by protein antigens (7, 28).
The response to polysaccharide antigens, such as dextran (α1-6 glucose polysaccharide), is predominated by antibodies of the IgG2 subclass (33). Consequently, the observed predominance of IgG2 subclass antibodies to C. albicans CW is anticipated, since the cell wall is composed mainly of carbohydrates. Keller et al. showed that normal human serum contained high levels of IgG antibodies that were directed against the glucan components of the nonencapsulated Cryptococcus neoformans cell wall. In that study, the antibody activity could be blocked completely by the addition of glucan from S. cerevisiae. Those IgG antibodies were of the IgG2 subclass (14). IgG2 antibodies to PPM, which is 90% polysaccharide, were significantly higher in patients with systemic candidiasis than in the controls. Only one serum sample from the candidiasis patient group was negative with regard to this antibody (Fig. 3d). This serum sample was collected from a patient at the onset of infection.
Our results show the potential value of antibodies in the laboratory assessment of patients with systemic candidiasis. However, IgG subclass antibody analysis confirms only the systemic candidiasis, not the species involved in infection. No consistent difference was found in antibody responses to the C. albicans cell wall antigens between patients who were infected with C. albicans and those who were infected with other Candida species. This is not surprising, since mannans of different Candida species have similar structures and share common cross-reactive epitopes (29).
The degree of reduced immunocompetence in the present patient group may not be so extensive, since several surgical patients were included. However, the few transplant and lymphoma patients showed clear-cut antibody titers, although these were restricted with respect to IgG subclasses and antigen (Fig. 3). It was shown recently that immunocompromised patients had increased antimannan antibody titers, which were observed shortly after the first clinical sign of invasive candidiasis (30). Further studies are needed to confirm that the determination of IgG1 anti-CW and IgG2 anti-PPM antibodies, in combination with β(1-3) glucan increases the specificity of diagnosis in immunocompromised patient groups.
All except one of the patients with systemic candidiasis were positive for antibodies to CW and PPM on the first day of culture-proven infection. The kinetics of particularly the IgG2 anti-CW and anti-PPM antibody titers (Fig. 3) showed that half or more of the antibody responses almost had reached their peak levels at the first time point of the analysis. These results, together with the insignificant IgM titers for any of the antigens tested, suggest that these patients had been previously exposed to Candida.
In this study blood culture (or in two cases bile and abdominal fluid) was used as the “gold standard” for systemic candidiasis in order to evaluate our antibody tests. It should be stressed, however, that although it may appear as though blood culture and Gluspecy provide a definite diagnosis of systemic candidiasis it is known that approximately only half of the systemic candidiasis patients become blood culture positive and that Gluspecy may become positive in patients with fever of unknown origin or patients who are receiving hemodialysis. Therefore, laboratory tests based on a positive Gluspecy result and positive antibody levels (single or in combination [Table 4]) should increase the accuracy of the diagnosis of systemic candidiasis in the absence of a positive blood culture.
The patients who died had significantly lower ratios of IgG1 anti-CW/IgG2 anti-PPM antibodies in the first week after the isolation of Candida. It has been suggested that the high concentrations of IgG2 antibodies to Pseudomonas aeruginosa in cystic fibrosis patients block the binding of IgG1 or IgG3, thereby preventing bacteria from being properly phagocytosed and contributing to the poor outcome for these patients (24). The poor binding of IgG2 to Fc receptors on macrophages and neutrophils may enable fungi to escape phagocytosis. The predominance of the IgG2 subclass antibody response may also reduce complement activation and subsequent killing mechanisms, since IgG2 fixes complement poorly. In addition, IgG2 antibodies may also minimize the inflammatory effects of the formed immune complexes (27). Moreover, in a recent study on experimental disseminated candidiasis in mice and a C. albicans vaccine, it was suggested that antibodies to C. albicans mannoprotein could block the protective potential of other antibodies (anti-β glucan antibodies) (2). However, the subclasses of the IgG antibodies were not determined.
Early diagnosis of systemic candidiasis is an important factor in decreasing patient mortality. Thus, the highly positive predictive values of the glucan and IgG subclass antibody analyses at the onset of systemic candidiasis are useful when initiating early antifungal treatment. Our results suggest that measurements of the IgG2 anti-PPM and IgG1 anti-CW antibody levels in combination with glucan determinations will contribute to the early diagnosis of systemic candidiasis.
REFERENCES
- 1.Armstrong, D. 1989. Problems in management of opportunistic fungal diseases. Rev. Infect. Dis. 11(Suppl. 7):S1591-S1599. [DOI] [PubMed] [Google Scholar]
- 2.Bromuro, C., A. Torosantucci, P. Chiani, S. Conti, L. Polonelli, and A. Cassone. 2002. Interplay between protective and inhibitory antibodies dictates the outcome of experimentally disseminated candidiasis in recipients of a Candida albicans vaccine. Infect. Immun. 70:5462-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Repentigny, L., L. Kaufman, G. T. Cole, D. Kruse, J. P. Latge, and R. C. Matthews. 1994. Immunodiagnosis of invasive fungal infections. J. Med. Vet. Mycol 32(Suppl. 1):239-252. [DOI] [PubMed] [Google Scholar]
- 4.de Repentigny, L., R. J. Kuykendall, F. W. Chandler, J. R. Broderson, and E. Reiss. 1984. Comparison of serum mannan, arabinitol, and mannose in experimental disseminated candidiasis. J. Clin. Microbiol. 19:804-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez, J., and J. Liebana. 1993. Immunological methods for the detection of structural components and metabolites of bacteria and fungi in blood. Ann. Biol. Clin. 51:83-90. [PubMed] [Google Scholar]
- 6.Gutierrez, J., C. Maroto, G. Piedrola, E. Martin, and J. A. Perez. 1993. Circulating Candida antigens and antibodies: useful markers of candidemia. J. Clin. Microbiol. 31:2550-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammarstrom, L., M. Granstrom, V. Oxelius, M. A. Persson, and C. I. Smith. 1984. IgG subclass distribution of antibodies against S. aureus teichoic acid and alpha-toxin in normal and immunodeficient donors. Clin. Exp. Immunol. 55:593-601. [PMC free article] [PubMed] [Google Scholar]
- 8.Ibanez-Nolla, J., J. M. Torres-Rodriguez, M. Nolla, M. A. Leon, R. Mendez, G. Soria, R. M. Diaz, and J. Marrugat. 2001. The utility of serology in diagnosing candidosis in non-neutropenic critically ill patients. Mycoses 44:47-53. [DOI] [PubMed] [Google Scholar]
- 9.Jones, J. M. 1990. Laboratory diagnosis of invasive candidiasis. Clin. Microbiol. Rev. 3:32-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, J. M. 1980. Quantitation of antibody against cell wall mannan and a major cytoplasmic antigen of Candida in rabbits, mice, and humans. Infect. Immun. 30:78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapteyn, J. C., G. J. Dijkgraaf, R. C. Montijn, and F. M. Klis. 1995. Glucosylation of cell wall proteins in regenerating spheroplasts of Candida albicans. FEMS Microbiol. Lett. 128:271-277. [DOI] [PubMed] [Google Scholar]
- 12.Kapteyn, J. C., L. L. Hoyer, J. E. Hecht, W. H. Muller, A. Andel, A. J. Verkleij, M. Makarow, H. Van Den Ende, and F. M. Klis. 2000. The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol. Microbiol. 35:601-611. [DOI] [PubMed] [Google Scholar]
- 13.Kapteyn, J. C., R. C. Montijn, G. J. Dijkgraaf, H. Van den Ende, and F. M. Klis. 1995. Covalent association of β-1,3-glucan with β-1,6-glucosylated mannoproteins in cell walls of Candida albicans. J. Bacteriol. 177:3788-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller, R. G., G. S. Pfrommer, and T. R. Kozel. 1994. Occurrences, specificities, and functions of ubiquitous antibodies in human serum that are reactive with the Cryptococcus neoformans cell wall. Infect. Immun. 62:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondori, N., L. Edebo, and I. Mattsby-Baltzer. 2003. Candida albicans cell wall antigens for serological diagnosis of candidemia. Med. Mycol. 41:21-30. [DOI] [PubMed] [Google Scholar]
- 16.Mattsby-Baltzer, I., L. Edebo, B. Jarvholm, B. Lavenius, and T. Soderstrom. 1990. Subclass distribution of IgG and IgA antibody response to Pseudomonas pseudoalcaligenes in humans exposed to infected metal-working fluid. J. Allergy Clin. Immunol. 86:231-238. [DOI] [PubMed] [Google Scholar]
- 17.Mitsutake, K., T. Miyazaki, T. Tashiro, Y. Yamamoto, H. Kakeya, T. Otsubo, S. Kawamura, M. A. Hossain, T. Noda, Y. Hirakata, and S. Kohno. 1996. Enolase antigen, mannan antigen, Cand-Tec antigen, and β-glucan in patients with candidemia. J. Clin. Microbiol. 34:1918-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki, T., S. Kohno, K. Mitsutake, S. Maesaki, K. Tanaka, and K. Hara. 1995. (1→3)-beta-D-glucan in culture fluid of fungi activates factor G, a Limulus coagulation factor. J. Clin. Lab. Anal. 9:334-339. [DOI] [PubMed] [Google Scholar]
- 19.Mori, T., and M. Matsumura. 1999. Clinical evaluation of diagnostic methods using plasma and/or serum for three mycoses: aspergillosis, candidosis, and pneumocystosis. Nippon Ishinkin Gakkai Zasshi 40:223-230. [DOI] [PubMed] [Google Scholar]
- 20.Nelson, R. D., N. Shibata, R. P. Podzorski, and M. J. Herron. 1991. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin. Microbiol. Rev. 4:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obayashi, T., M. Yoshida, T. Mori, H. Goto, A. Yasuoka, H. Iwasaki, H. Teshima, S. Kohno, A. Horiuchi, A. Ito, et al. 1995. Plasma (1→3)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17-20. [DOI] [PubMed] [Google Scholar]
- 22.Obayashi, T., M. Yoshida, H. Tamura, J. Aketagawa, S. Tanaka, and T. Kawai. 1992. Determination of plasma (1→3)-beta-D-glucan: a new diagnostic aid to deep mycosis. J. Med. Vet. Mycol. 30:275-280. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn. Microbiol. Infect. Dis. 31:327-332. [DOI] [PubMed] [Google Scholar]
- 24.Pressler, T., S. S. Pedersen, F. Espersen, N. Hoiby, and C. Koch. 1990. IgG subclass antibodies to Pseudomonas aeruginosa in sera from patients with chronic Ps. aeruginosa infection investigated by ELISA. Clin. Exp. Immunol. 81:428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiss, E., T. Obayashi, K. Orle, M. Yoshida, and R. M. Zancope-Oliveira. 2000. Non-culture based diagnostic tests for mycotic infections. Med. Mycol. 38:147-159. [PubMed] [Google Scholar]
- 26.Ronholm, E., H. Tomasdottir, J. Runeborg, I. Mattsby-Baltzer, M. Olausson, A. Aneman, and A. Bengtsson. 2000. Gastro-intestinal complement activation during human liver transplantation: impact on postoperative liver function. Acta Anaesthesiol. Scand. 44:850-857. [DOI] [PubMed] [Google Scholar]
- 27.Schumaker, V. N., M. A. Calcott, H. L. Spiegelberg, and H. J. Muller-Eberhard. 1976. Ultracentifuge studies of the binding of IgG of different subclasses to the Clq subunit of the first component of complement. Biochemistry 15:5175-5181. [DOI] [PubMed] [Google Scholar]
- 28.Shakib, F., and D. R. Stanworth. 1980. Human IgG subclasses in health and disease. Part I. Ric. Clin. Lab. 10:463-479. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki, S. 1997. Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr. Top. Med. Mycol. 8:57-70. [PubMed] [Google Scholar]
- 30.van Deventer, A. J., W. H. Goessens, J. H. van Zeijl, J. W. Mouton, M. F. Michel, and H. A. Verbrugh. 1996. Kinetics of anti-mannan antibodies useful in confirming invasive candidiasis in immunocompromised patients. Microbiol. Immunol. 40:125-131. [DOI] [PubMed] [Google Scholar]
- 31.Verduyn Lunel, F. M., J. F. Meis, and A. Voss. 1999. Nosocomial fungal infections: candidemia. Diagn. Microbiol. Infect. Dis. 34:213-220. [DOI] [PubMed] [Google Scholar]
- 32.Vincent, J. L., D. J. Bihari, P. M. Suter, H. A. Bruining, J. White, M. H. Nicolas-Chanoin, M. Wolff, R. C. Spencer, M. Hemmer, et al. 1995. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA 274:639-644. [PubMed] [Google Scholar]
- 33.Yount, W. J., M. M. Dorner, H. G. Kunkel, and E. A. Kabat. 1968. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J. Exp. Med. 127:633-646. [DOI] [PMC free article] [PubMed] [Google Scholar]