Abstract
Diabetes is associated with persistent inflammation and defective tissue repair responses. The hypothesis of this study was that interleukin (IL)-1β is part of a proinflammatory positive feedback loop that sustains a persistent proinflammatory wound macrophage phenotype that contributes to impaired healing in diabetes. Macrophages isolated from wounds in diabetic humans and mice exhibited a proinflammatory phenotype, including expression and secretion of IL-1β. The diabetic wound environment appears to be sufficient to induce these inflammatory phenomena because in vitro studies demonstrated that conditioned medium of both mouse and human wounds upregulates expression of proinflammatory genes and downregulates expression of prohealing factors in cultured macrophages. Furthermore, inhibiting the IL-1β pathway using a neutralizing antibody and macrophages from IL-1 receptor knockout mice blocked the conditioned medium–induced upregulation of proinflammatory genes and downregulation of prohealing factors. Importantly, inhibiting the IL-1β pathway in wounds of diabetic mice using a neutralizing antibody induced a switch from proinflammatory to healing-associated macrophage phenotypes, increased levels of wound growth factors, and improved healing of these wounds. Our findings indicate that targeting the IL-1β pathway represents a new therapeutic approach for improving the healing of diabetic wounds.
Chronic wounds associated with diabetes, venous insufficiency, or pressure represent a major health problem, with millions of patients afflicted and the associated treatment costing billions of dollars per year (1). Despite the socioeconomic impact of chronic wounds, the underlying causes of impaired healing are not well-understood and effective treatments remain elusive. A common characteristic of these poorly healing wounds is a persistent inflammatory response, with prolonged accumulation of macrophages and elevated levels of proinflammatory cytokines (2–5). Translational research of the dysregulation of inflammation associated with impaired healing in diabetes should provide insight into the development of new therapeutic approaches.
During normal wound healing in mice, inflammatory cells such as macrophages promote healing indirectly by killing pathogens and clearing the wound of damaged tissue, but also promote healing directly by producing growth factors that induce angiogenesis, collagen deposition, and wound closure (6–9). In contrast, during impaired healing of diabetic mice, wounds exhibit prolonged accumulation of macrophage associated with elevated levels of proinflammatory cytokines and proteases and reduced levels of various growth factors, all of which mimic chronic wounds in humans (10–12). We recently demonstrated that in wounds of diabetic mice, macrophages exhibit a sustained proinflammatory phenotype with an impaired upregulation of healing-associated factors that is observed in nondiabetic mice as healing progresses (13). However, the underlying causes of the dysregulation of macrophage in diabetic wounds remain to be elucidated.
Multiple factors can influence macrophage phenotype and the actual phenotypes expressed in chronic wounds are likely determined by the balance of the proinflammatory and anti-inflammatory stimuli present in the wound environment. The proinflammatory environment observed in diabetic wounds has the potential to sustain a proinflammatory macrophage phenotype, which, in turn, would contribute to sustaining the proinflammatory environment. In fact, hyperglycemia is known to induce expression of interleukin (IL)-1β in a number of different cell types, including macrophages (14–16), and IL-1β, in turn, is known to induce a proinflammatory macrophage phenotype in part by inducing itself (17). Thus, the IL-1β pathway may be part of a positive feedback loop that sustains inflammation in chronic wounds and contributes to impaired healing. However, little is known about the actual role of IL-1β in diabetic wounds.
The central hypothesis of this study is that sustained activity of the IL-1β pathway in diabetic wounds contributes to impaired healing of these wounds. The results of this study demonstrate that sustained IL-1β expression in wounds of diabetic humans and mice is associated with a proinflammatory macrophage phenotype, and that inhibiting the IL-1β pathway in wounds of diabetic mice induces the switch from proinflammatory to healing-associated macrophage phenotypes and improves healing of these wounds.
RESEARCH DESIGN AND METHODS
Human subjects.
Five patients (two male and three female) with chronic wounds provided informed consent. Patients ranged in age from 54 to 70 years, had type 2 diabetes diagnoses, and had nonhealing wounds on either the sacral region or the lower limb lasting at least 3 months. Biopsy specimens were taken from debridement tissue that was removed from the center of the wound. All procedures involving human subjects were approved by the Institutional Review Board at the University of Illinois at Chicago according to the Declaration of Helsinki principles.
Animals.
Diabetic db/db mice, nondiabetic db/+ controls, IL-1 receptor 1 knockout mice, and C57Bl/6 wild-type controls were obtained from Jackson Laboratories. Experiments were performed on 12- to 16-week-old mice. All procedures involving animals were approved by the Animal Care Committee at the University of Illinois at Chicago.
Excisional wounding and treatment.
Mice were anesthetized with isoflurane and their dorsum was shaved and cleaned with betadine and then alcohol swab. Four 8-mm excisional wounds were made on the back of each mouse with a dermal biopsy punch and wounds were covered with Tegaderm (3M) to keep the wounds moist and to maintain consistency with treatment of human wounds. For some mice, an IL-1β–neutralizing antibody (R&D Systems) was administered as a single total dose of 20 μg per wound by intradermal injection at four equally spaced sites around the periphery of each wound; this treatment was applied 3 days postinjury to allow the initial inflammatory response to proceed normally. Controls were treated with nonspecific rat IgG.
Cell isolation.
Cells were dissociated from human chronic wound biopsy specimens and from mouse excisional wounds using an enzymatic digest with collagenase I, collagenase XI, and hyaluronidase (13). Neutrophils, T cells, and B cells were marked for depletion by incubating cells for 15 min with fluorescein isothiocyanate (FITC)-conjugated anti-Ly6G (1A8), anti-CD3 (17A2), and anti-CD19 (6D5) for mouse cells and with FITC-conjugated anti-CD15 (HI98), anti-CD3 (UCHT1), and anti-CD19 (HIB19) for human cells (1:10; Biolegend); these cells were depleted from the total cell population using anti-FITC magnetic beads and by following the manufacturer’s instructions (Miltenyi Biotec). The remaining myeloid cells, primarily cells of the monocyte/macrophage lineage, were then isolated using CD11b magnetic beads by following the manufacturer’s instructions. Previous studies indicated that >90% of the cells thus isolated were positive for monocyte/macrophage markers Ly6C or F4/80, or both, by flow cytometry (13). These cells were then either incubated overnight to measure cytokine release or stored at −80°C for later RNA analysis.
RNA analysis.
Total RNA was isolated from human or mouse cells using the RNeasy kit (Qiagen). cDNA was synthesized from 1 μg RNA using the Thermoscript RT-PCR System (Invitrogen). Real-time PCR was performed in a 7500Fast System (Applied Biosystems) using TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assay primer/probe sets (Applied Biosystems; Supplementary Table 1). All reactions were performed in triplicate, and cycle threshold values were averaged over triplicates. Relative gene expression was determined using the 2−ΔΔCT method, with GAPDH as the endogenous control gene.
Immunofluorescence.
Cryosections (10-μm-thick) were cut from human chronic wound biopsy specimens, fixed in cold acetone, and blocked with buffer containing 3% BSA. Sections were incubated overnight with primary antibodies against CD68 (Y1/82A, 1:100; Biolegend) and IL-1β (CRM56, 1:100; EBioscience). Sections were then incubated with FITC-conjugated and tetramethyl rhodamine isothyocyanate–conjugated isotype specific secondary antibodies (1:200; Invitrogen). Negative controls included no primary antibody or isotype-specific control antibodies (IgG1 and IgG2b; Biolegend) along with secondary antibodies. To visualize nuclei, slides were mounted with medium containing DAPI (Vector Laboratories). Digital images were obtained using a Nikon Instruments Eclipse 80i microscope with a 40×/0.75 objective, a DS-Fi1 digital camera, and NIS Elements software.
Wound healing assays.
For mouse wounds, healing was assessed on day 10 postinjury. Re-epithelialization and granulation tissue thickness were measured by morphometric analysis of cryosections taken from the center of the wound (found by serial sectioning through the entire wound) and stained with hematoxylin and eosin. Digital images were obtained using a Nikon Instruments 80i microscope with a 2×/0.06 objective and a DS-QI1 digital camera, and were analyzed using NIS Elements image analysis software. The percentage of re-epithelialization [(distance traversed by epithelium over wound from wound edge/distance between wound edges) × 100] was calculated for two sections per wound and was averaged over sections to provide a representative value for each wound (9,13). Average granulation thickness was measured in the same sections by dividing the wound bed area by wound length.
For angiogenesis, new blood vessels were identified by immunohistochemical staining for CD31 (390, 1:100; BD Biosciences) using our published procedure (9). Collagen deposition was assessed using Masson trichrome staining (IMEB, San Marcos, CA). For each assay, digital images were first obtained covering the wound bed (2–3 fields using a 20×/0.50 objective). The percent area stained in each image was then quantified by counting the number of clearly stained pixels above a threshold intensity and normalizing to the total number of pixels. The software allowed the observer to exclude staining identified as artifact and areas deemed to be outside the wound bed. For both trichrome and CD31 staining, two sections per wound were analyzed and data ere averaged over sections to provide a representative value for each wound.
Cell culture.
To generate cultures of human macrophage, peripheral blood mononuclear cells from normal volunteers (Zen-Bio) were plated in RPMI supplemented with 10% FBS, 2 mmol/L L-glutamine, 1% penicillin/streptomycin, and 20 ng/mL recombinant human macrophage colony-stimulating factor (Peprotech). After 7 days in culture, cells were stimulated for 18 h with interferon (IFN)-γ and tumor necrosis factor (TNF)-α (20 ng/mL each; Peprotech) or 20% human chronic wound–conditioned medium. Human wound–conditioned medium was generated by incubating chronic wound biopsy specimens in Dulbecco's modified Eagle's medium (DMEM) plus 10% FBS (1 mL/100 mg tissue) for 2 h at 37°C.
Bone marrow–derived mouse macrophages were cultured from wild-type C57Bl/6 mice and IL-1 receptor 1 knockout mice as described (18). Briefly, bone marrow cells were flushed from femurs and tibias and cultured in DMEM supplemented with 10% FBS, 10% L-929 cell–conditioned medium (source of mouse macrophage colony-stimulating factor), 2 mmol/L L-glutamine, and 1% penicillin/streptomycin at 10% CO2 and 37°C. Macrophages were then stimulated for 18 h with IFN-γ and TNF-α (20 ng/mL each; R&D Systems) or 20% mouse wound–conditioned medium with IL-1β–blocking antibody or control IgG (see figures for details). Mouse wound–conditioned medium was generated by incubating excised wounds in DMEM plus 10% FBS (1 mL/100 mg tissue) for 2 h at 37°C.
ELISA.
Mouse wounds were homogenized in cold PBS (10 μL of PBS per mg wound tissue) supplemented with protease inhibitor cocktail (Sigma) using a dounce homogenizer and then sonicated and centrifuged. Supernatants were used for ELISA of IL-1β, IL-6, IL-10, transforming growth factor (TGF)-β1, TNF-α (eBioscience), and IGF-1 (R&D Systems). For cell culture studies, culture medium was centrifuged and supernatants were used for ELISA assays. When wound-conditioned medium was used as a cell culture supplement, cytokine release was measured as the difference between levels achieved in wells with cultured cells and levels in blank wells that contained identical medium composition but no cells.
Statistics.
Values are reported as means ± SD. Measurements of macrophage gene expression, cytokine, and growth factor levels, re-epithelialization, granulation tissue thickness, trichrome staining, and CD31 staining data were compared using ANOVA. The Student-Newman-Keuls post hoc test was used when ANOVAs demonstrated significance. Differences between groups were considered significant if P ≤ 0.05.
RESULTS
Proinflammatory macrophage phenotype in diabetic human wounds.
Although macrophages are known to populate chronic wounds in diabetic patients (2), little is known about their phenotype. We isolated macrophages from biopsy specimens of chronic wounds in type 2 diabetic patients and phenotyped these cells by real-time PCR. Compared with nonactivated blood monocyte–derived macrophages, chronic wound macrophages expressed high levels of the proinflammatory molecules IL-1β, matrix metalloproteinase (MMP)-9, and TNF-α (Fig. 1A–D), and low levels of the healing-associated phenotype markers CD206, IGF-1, TGF-β, and IL-10 (Fig. 1E–H). In addition, immunofluorescence analysis of chronic wound biopsy cryosections showed that IL-1β protein colocalized with the macrophage marker CD68, indicating that chronic wound macrophages produce IL-1β protein (Fig. 1M–R and Supplementary Fig. 1). Overall, the phenotype of chronic wound macrophages was remarkably similar to that of “classically activated” blood monocyte–derived macrophage stimulated with IFN-γ and TNF-α in vitro, although IL-6 expression was lower in wound macrophage than in classically activated macrophages.
FIG. 1.
Macrophages isolated from chronic wounds in diabetic patients exhibit a proinflammatory phenotype that may be induced by the wound environment. Macrophages were isolated from chronic wound biopsy specimens and expression of proinflammatory markers IL-1β, MMP-9, TNF-α, and IL-6 (A–D) and healing-associated/anti-inflammatory markers CD206, IGF-1, TGF-β, and IL-10 (E–H) were assessed by real-time PCR. For comparison, blood-derived macrophages from healthy volunteers were left nonstimulated (Non), stimulated with TNF-α and IFN-γ (classically activated [CA]), or stimulated with chronic wound–conditioned medium (WCM). For the in vitro experiments, release of IL-1β, TNF-α, IGF-1, and TGF-β (I–L) into cell culture medium was measured by ELISA. In addition, chronic wound biopsy cryosections were immunostained for IL-1β and the macrophage marker CD68, with images taken at 20× (M–O; scale bar = 100 μm) and at 40× (P–R; scale bar = 50 μm); location of 40× images shown in box on 20× images. Nuclei stained with DAPI. For all graphs, bars = mean ± SD and n = 5 for both in vivo and in vitro experiments. *Mean value significantly different from that for nonstimulated controls, P < 0.05. diff, difference.
FIG. 2.
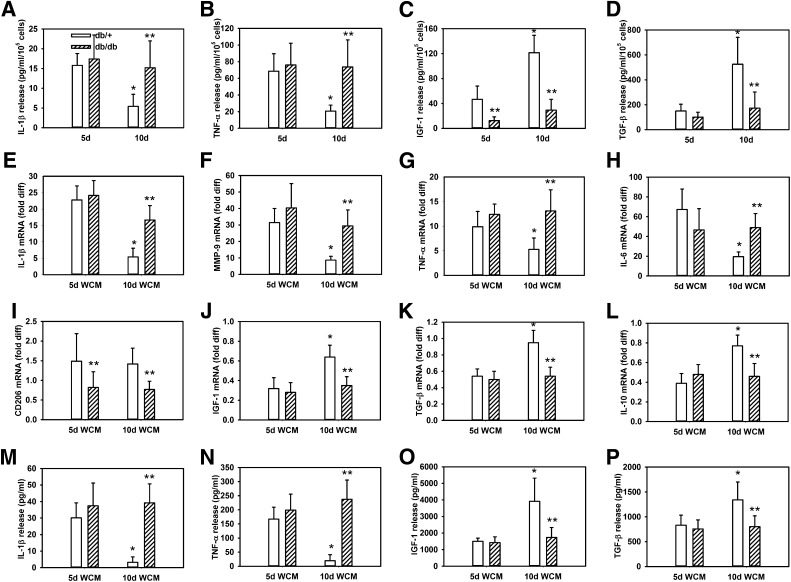
Macrophages isolated from wounds in diabetic mice exhibit a persistent proinflammatory phenotype that may be induced by the wound environment. Macrophages were isolated from wounds of nondiabetic (db/+) and diabetic (db/db) mice on days 5 and 10 postinjury, cultured overnight, and release of (A–D) IL-1β, TNF-α, IGF-1, and TGF-β was measured using ELISA. Also, bone marrow–derived macrophages from wild-type mice were cultured with conditioned medium (WCM) of day 5 (5d) or day 10 (10d) wounds from nondiabetic db/+ or diabetic db/db mice; expression of proinflammatory markers (E–H) IL-1β, MMP-9, TNF-α, and IL-6 and healing-associated/anti-inflammatory markers (I–L) CD206, IGF-1, TGF-β, and IL-10 were assessed by real-time PCR. In addition, release of (M–P) IL-1β, TNF-α, IGF-1, and TGF-β was measured using ELISA. For all graphs, bars = mean ± SD. For in vivo experiments, n = 6–7 mice for each strain and time point. For in vitro experiments, a separate set of bone marrow–derived macrophages (each harvested from a different mouse) was used for each of two experiments and wound-conditioned medium was generated from three mice per experiment, with n = 6 for each strain and time point. *Mean value significantly different from that for same strain on day 5 postinjury. **Mean value for db/db significantly different from that for db/+ at same time point, P < 0.05. diff, difference.
To provide insight into whether the chronic wound environment can induce the proinflammatory wound macrophage phenotype observed, we cultured nonactivated blood monocyte–derived macrophages with conditioned medium of chronic wounds. Compared with nonactivated blood monocyte–derived macrophages, chronic wound–conditioned medium increased expression of proinflammatory markers IL-1β, MMP-9, and TNF-α (Fig. 1A–D), and decreased expression of nonprohealing markers IGF-1, TGF-β, and IL-10 (Fig. 1E–H). Expression of IL-6 and CD206 were not significantly altered by wound-conditioned medium. Selected cytokines also were assessed at the protein level; chronic wound–conditioned medium increased release of IL-1β and TNF-α into the cell culture medium and decreased release of IGF-1 and TGF-β (Fig. 1I–L). Thus, chronic wound–conditioned medium induced a proinflammatory macrophage phenotype similar to that of classically activated macrophages.
Sustained proinflammatory macrophage phenotype in diabetic mouse wounds.
We reported previously that whereas wound macrophages in nondiabetic mice exhibit a switch from proinflammatory to prohealing phenotypes from days 5 to 10 postinjury, diabetic mice exhibit a persistent proinflammatory phenotype similar to that observed in the human chronic wound macrophages in the current study. To determine whether the differences observed in macrophage phenotype between db/+ and db/db mice translated to changes in protein secretion by these cells, we isolated cells from wounds of db/+ and db/db mice and measured release of proinflammatory, anti-inflammatory, and prohealing cytokines. In db/+ mice, macrophage release of IL-1β and TNF-α was high on day 5 postinjury but decreased on day 10 (Fig. 2A and B). In contrast, macrophage release of the prohealing growth factors IGF-1 and TGF-β were low on day 5 postinjury but increased on day 10 (Fig. 2C and D). These data are consistent with downregulation of the proinflammatory phenotype and upregulation of a healing-associated phenotype of wound macrophages as wound healing progresses in nondiabetic mice (13). However, in db/db mice, macrophage release of IL-1β and TNF-α was sustained at high levels on both day 5 and day 10 postinjury, and the level on day 10 was significantly higher than that produced by db/+ wound macrophages (Fig. 2A and B). In addition, macrophage release of IGF-1 and TGF-β was maintained at low levels on days 5 and 10 postinjury, which were significantly lower than levels produced by db/+ wound macrophage on day 10 (Fig. 2C and D). These data support the notion that wound macrophages in diabetic mice exhibit a persistent proinflammatory phenotype and fail to upregulate healing-associated factors.
To determine whether the wound environment in diabetic mice can induce the proinflammatory wound macrophage phenotype observed, we cultured bone marrow–derived macrophage from nondiabetic mice with day 5 or day 10 wound–conditioned medium from nondiabetic db/+ or diabetic db/db mice. Conditioned medium from day 5 wounds of both db/+ and db/db mice induced a proinflammatory phenotype of cultured mouse bone marrow–derived macrophage with upregulation of IL-1β, MMP-9, TNF-α, and IL-6 (Fig. 2E–H) and downregulation of IGF-1, TGF-β, and IL-10 compared with nonactivated control macrophages (Fig. 2I–L). Conditioned medium from day 10 wounds of db/+ mice induced significantly less upregulation of proinflammatory genes and no longer downregulated healing-associated factors (Fig. 2E–L). In contrast, conditioned medium from day 10 wounds of db/db mice induced significantly higher proinflammatory gene expression and significantly lower healing-associated gene expression than conditioned medium from db/+ wounds (Fig. 2E–L). One exception to this pattern was that expression of CD206 was not significantly altered by conditioned medium from db/+ wounds but was significantly reduced by conditioned medium from db/db compared with db/+ wounds. Selected cytokines also were assessed at the protein level and the differences in mRNA expression were paralleled by differences in protein levels. For example, IL-1β and TNF-α secretion was higher and IGF-1 and TGF-β secretion was lower in macrophages stimulated with day 10 db/db wound–conditioned medium compared with cells stimulated with day 10 db/+ wound–conditioned medium (Fig. 2M–P). Taken together, these in vitro data indicate that the diabetic wound environment is sufficient to induce a proinflammatory macrophage phenotype and to suppress the healing-associated phenotype.
Blocking IL-1β activity downregulates proinflammatory macrophage phenotype and upregulates prohealing factors in cultured cells.
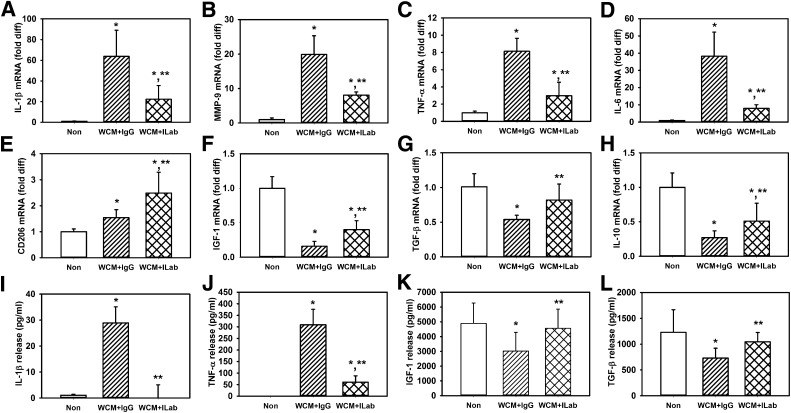
IL-1β is a potent proinflammatory cytokine and is known to induce itself in macrophages and produce a proinflammatory phenotype (17). To determine whether IL-1β in the wound environment thus contributes to such a proinflammatory positive feedback loop, we blocked IL-1β signaling in cultured macrophage treated with wound-conditioned medium using an IL-1β neutralizing antibody or using macrophages isolated from IL-1R1 knockout mice. The IL-1β–blocking antibody inhibited the induction of proinflammatory markers IL-1β, MMP-9, TNF-α, and IL-6 by day 10 db/db wound–conditioned medium (Fig. 3A–D). Interestingly, the blocking antibody also increased expression of healing-associated markers CD206, IGF-1, TGF-β, and IL-10 (Fig. 3E–H). Differences in protein secretion of selected cytokines, namely IL-1β, TNF-α, IGF-1, and TGF-β, paralleled the changes in mRNA levels (Fig. 3I–L). IL-1β in the culture medium were undetectable when the neutralizing antibody was present; this finding could be the result of interference of the blocking antibody with detection by the ELISA assay as well as a potential reduction in the amount released by the macrophages (however, see data regarding IL-1 receptor 1–null macrophages).
FIG. 3.
IL-1β–neutralizing antibody downregulates diabetic wound–conditioned medium–induced proinflammatory phenotype and upregulates healing-associated phenotype in cultured macrophages. Bone marrow–derived macrophages from wild-type mice left nonstimulated (Non) or stimulated with day 10 db/db wound-conditioned medium (WCM) along with control IgG or stimulated with IL-1β–neutralizing antibody (ILab); expression of proinflammatory markers (A–D) IL-1β, MMP-9, TNF-α, and IL-6 and healing-associated/anti-inflammatory markers (E–H) CD206, IGF-1, TGF-β, and IL-10 were measured by real-time PCR. In addition, release of (I–L) IL-1β, TNF-α, IGF-1, and TGF-β was measured using ELISA. For all graphs, bars = mean ± SD. For these experiments, a separate set of bone marrow–derived macrophages (each harvested from a different mouse) was used for each of two experiments and wound-conditioned medium was generated from three mice per experiment, with n = 6 for each condition. *Mean value significantly different from that for nonstimulated controls. **Mean value significantly different from that for conditioned medium plus IgG-treated samples, P < 0.05. diff, difference.
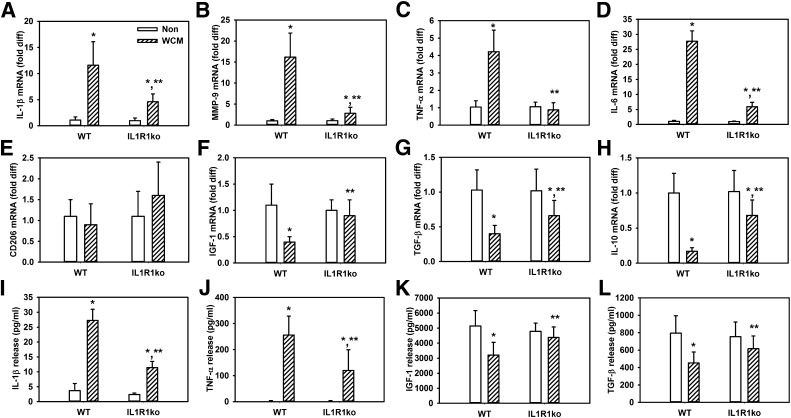
The IL-1β neutralizing data were corroborated in experiments using macrophages cultured from IL-1 receptor 1 knockout mice; wound-conditioned medium–treated IL-1 receptor 1 knockout macrophage exhibited blunted upregulation of IL-1β, MMP-9, TNF-α, and IL-6 expression (Fig. 4A–D) and maintained a higher level expression in the healing-associated markers IGF-1, TGF-β, and IL-10 (Fig. 4E–H) compared with wild-type macrophages. In contrast, there was no significant difference in expression of CD206 between wild-type and IL-1 receptor 1 knockout macrophages. Again, the mRNA data were consistent with protein secretion data, which showed that IL-1β and TNF-α release was reduced and release of IGF-1 and TGF-β was higher in wound-conditioned medium–treated IL-1 receptor 1 knockout macrophages compared with wild-type macrophages (Fig. 4I–L). These data indicate that IL-1 receptor 1 knockout macrophage are less sensitive to wound-conditioned medium–induced upregulation of proinflammatory markers and downregulation of healing-associated markers than wild-type macrophages and are consistent with the hypothesis that IL-1β in the diabetic wound environment induces a proinflammatory macrophage phenotype.
FIG. 4.
Cultured macrophages from IL-1 receptor 1 knockout (IL1R1ko) mice are less sensitive to diabetic wound–conditioned medium than macrophages from wild-type mice. Bone marrow–derived macrophages from wild-type (WT) and IL1R1ko mice stimulated with day 10 db/db wound–conditioned medium, expression of proinflammatory markers (A–D) IL-1β, MMP-9, TNF-α, and IL-6, and healing-associated/anti-inflammatory markers (E–H) CD206, IGF-1, TGF-β, and IL-10 were measured by real-time PCR. In addition, release of (I–L) IL-1β, TNF-α, IGF-1, and TGF-β was measured using ELISA. For all graphs, bars = mean ± SD. For these experiments, a separate set of bone marrow–derived macrophages (each harvested from a different mouse) was used for each of two experiments and wound-conditioned medium was generated from three mice per experiment, with n = 6 for each condition. *Mean value significantly different from that for nonstimulated controls of same strain. **Mean value significantly different from that for wound-conditioned medium–treated wild-type macrophages, P < 0.05. diff, difference.
Blocking IL-1β activity downregulates proinflammatory macrophage phenotype in wounds, upregulates prohealing phenotype, and improves healing in db/db mice.
We next performed in vivo experiments to determine whether treating wounds in db/db mice with an IL-1β–neutralizing antibody could downregulate the proinflammatory wound macrophage phenotype, upregulate a healing-associated phenotype, and improve healing of these wounds. Mice whose wounds were treated with blocking antibody showed no change in blood glucose (459 ± 92 mg/dL) compared with mice treated with control IgG (439 ± 106 mg/dL).
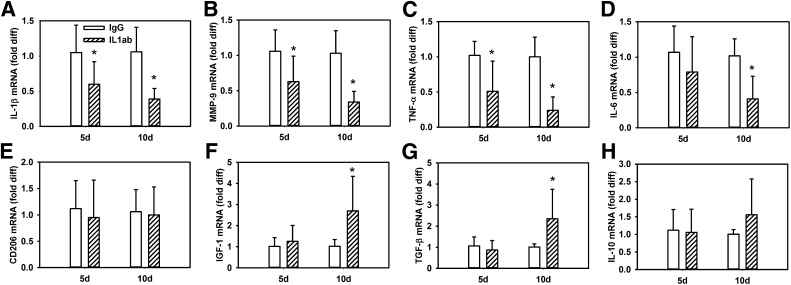
Macrophages isolated on days 5 and 10 postinjury from wounds treated with the IL-1β–neutralizing antibody exhibited progressively lower levels of expression of proinflammatory genes IL-1β, MMP-9, TNF-α, and IL-6 over time compared with macrophages isolated from IgG-treated wounds (Fig. 5A–D). Interestingly, macrophages did not yet show upregulation of healing-associated factors IGF-1, TGF-β, or IL-10 on day 5 postinjury but did show upregulation of these factors on day 10 postinjury compared with macrophages isolated from IgG-treated wounds (Fig. 5E–H). In contrast, CD206 did not show upregulation at either time point. Thus, treatment with the IL-1β–blocking antibody downregulated the proinflammatory macrophage phenotype by day 5 postinjury and upregulated a healing-associated phenotype by day 10 in wounds of diabetic mice.
FIG. 5.
IL-1β–neutralizing antibody downregulates proinflammatory macrophage phenotype and upregulates healing-associated phenotype in wounds of diabetic mice. Wounds in db/db mice were treated with control IgG or IL-1β–blocking antibody (IL1ab) on day 3 postinjury and macrophages were isolated on day 5 (d5) and day 10 (d10) postinjury. Expression of proinflammatory markers (A–D) IL-1β, MMP-9, TNF-α, and IL-6 and healing-associated/anti-inflammatory markers (E–H) CD206, IGF-1, TGF-β, and IL-10 were measured by real-time PCR. For all graphs, bars = mean ± SD, n = 6. *Mean value significantly different from that for IgG-treated mice at same time point, P < 0.05. diff, difference.
Wounds treated with the IL-1β–neutralizing antibody also showed accelerated re-epithelialization as well as granulation tissue formation assessed in hematoxylin and eosin–stained cryosections on day 10 postinjury (Fig. 6A–D). Wounds treated with blocking antibody also showed increased collagen deposition assessed in trichrome-stained cryosections (Fig. 6E). The improved healing was not associated with increased CD31 staining, indicating little to no effect on angiogenesis (Fig. 6F). Furthermore, wounds treated with the blocking antibody showed reduced levels of IL-1β and TNF-α and increased levels of IGF-1 and TGF-β (Fig. 6G–J), paralleling the changes in wound macrophage phenotype and indicating a more prohealing cytokine environment.
FIG. 6.
IL-1β–neutralizing antibody improves healing of wounds in diabetic mice. Images showing cryosections stained with trichrome for (A) control IgG-treated and (B) IL-1β–neutralizing antibody–treated wounds on day 10 postinjury. Note the increased re-epithelialization and granulation tissue in the IL-1β antibody (IL1ab)–treated wounds. Scale bar = 0.5 mm. Arrows indicate ends of migrating epithelial tongues. gt, granulation tissue; mm, deep muscle. The muscle layer observed underneath the wound in some sections is likely a part of the deep tissue collected that helps to maintain the integrity of the fragile wounds, particularly in untreated and IgG-treated diabetic mice. For wounds in db/+, untreated db/db, IgG-treated db/db, and IL1ab-treated db/db mice on day 10 postinjury, quantification of (C, D) re-epithelialization and granulation tissue thickness measured in hematoxylin and eosin–stained cryosections, (E) trichrome staining measured as percent area stained blue for collagen, and (F) CD31 staining measured as percent area stained for this endothelial cell marker. In addition, wounds from each group of mice were homogenized and levels of (G–J) IL-1β, TNF-α, IGF-1, and TGF-β measured using ELISA. For all graphs, bars = mean ± SD, n = 6–8. *Mean value significantly different from that for wounds in db/+ mice. **Mean value significantly different from that for control IgG-treated wounds.
DISCUSSION
Chronic wounds are common in diabetic patients and the associated morbidity and mortality are high (19–21). Chronic wounds are typically associated with a persistent inflammatory response that involves accumulation of macrophages (2–5); however, little is known about the regulation and function of these cells. The major novel finding of this study is that the proinflammatory cytokine IL-1β is part of a key regulatory pathway in this chronic inflammatory response. Our studies indicate that IL-1β participates in a proinflammatory positive feedback loop that sustains the proinflammatory macrophage phenotype observed in poorly healing wounds of both humans and mice and blocks the induction of a healing-associated macrophage phenotype observed during normal healing. Importantly, inhibiting IL-1β in vivo downregulates the proinflammatory macrophage phenotype and upregulates expression of prohealing factors in wounds of diabetic mice and improves healing of these wounds.
IL-1β is a potent proinflammatory cytokine that plays a role in the pathophysiology of many inflammatory diseases, including diabetes (17,22). Recent studies have shown that blocking IL-1β systemically can improve glycemic control and β-cell function in mice and humans (23,24). In our studies, we applied the IL-1β–blocking antibody locally to wounds and observed no changes in blood glucose levels. Thus, the downregulation of the proinflammatory macrophage phenotype and improved wound healing observed are likely attributable to direct effects in the wound. Our data thus indicate that targeting the IL-1β pathway in chronic wounds may provide a novel approach for improving wound healing in diabetic patients.
IL-1 appears to play homeostatic roles in noninjured skin as well as roles in skin inflammation. IL-1 produced by keratinocytes is thought to play a role in epithelial-mesenchymal signaling required for normal skin homeostasis (25). In addition, IL-1 is thought to contribute to skin inflammation in autoinflammatory diseases and skin diseases such as psoriasis and scleroderma (26–28). During skin wound healing in healthy mice, levels of keratinocyte-derived chemokine and macrophage inhibitory protein-1α were reduced by IL-1 receptor antagonist treatment (29). However, inflammatory and healing responses were not altered significantly in IL-1 receptor knockout mice in one study (30), although these mice exhibited reduced fibrosis in skin wounds in another study (31), suggesting that IL-1 may play ancillary roles in normal healing. In contrast, IL-1β levels are sustained at high levels in chronic wounds of humans and in wounds of diabetic mice (3,5,11,13,32,33), and our findings demonstrate that these elevated levels of IL-1β promote the proinflammatory wound environment and impaired healing associated with diabetes.
The phenotype adopted by macrophages during tissue repair may be influenced both by cell lineage and by the microenvironment of the macrophage during the healing process (34,35). For example, after ischemic damage to the heart, a switch from proinflammatory and proteolytic Ly6Chi monocytes (Mo) to noninflammatory prohealing Ly6Clo Mo/macrophage appeared to be the result of separate waves of Mo infiltration, indicating that cell lineage may play an important role in determining cell phenotype during tissue repair (36). In contrast, after toxin-induced injury to skeletal muscle, the switch from Ly6Chi Mo to Ly6Clo Mo/macrophage appeared to be the result of differentiation of the Ly6Chi subset within the muscle, indicating environmental control of this phenotype switch (37). Although we have not yet investigated the role of cell lineage in the phenotypes of wound macrophages observed in our studies, data from both our in vitro and our in vivo experiments support a role for the wound environment in regulating macrophage phenotype and, in particular, sustaining a proinflammatory phenotype in diabetic wound macrophage. For our studies, we compared the phenotypes of macrophages isolated from human and mouse wounds to the phenotypes of cultured blood monocyte–derived (human) or bone marrow–derived (mouse) macrophages. These cultured macrophages may exhibit different phenotypes than tissue macrophages and may respond differently to changes in the cytokine environment. However, this limitation should not detract from our finding that blocking IL-1β in wounds of diabetic mice in vivo or in the modeled wound environment in vitro downregulates the proinflammatory macrophage phenotype and promotes a healing-associated phenotype.
Macrophages are critical orchestrators in the healing of many tissues, including skin, skeletal and cardiac muscle, and liver (9,37–39). In addition to their roles in host defense, macrophages promote tissue repair by clearing the wound of damaged tissue and by producing growth factors that regulate the activity of other cells required for healing. Despite the positive role of macrophages in promoting wound healing in nondiabetic mice, the prolonged presence of macrophages in wounds of diabetic mice (33,40) and chronic wounds of humans (2,4) suggests the potential for macrophage dysfunction to contribute to impaired healing. In fact, macrophages isolated from chronic venous ulcers (41) exhibit a similar proinflammatory phenotype to that observed for macrophages isolated from diabetic wounds in the current study. This proinflammatory phenotype in chronic venous ulcers was attributed to phagocytosis of erythrocytes that had been extravasated because of underlying venous hypertension and subsequent iron overloading. Because this series of events is likely specific to chronic venous ulcers, other pathways likely induce the proinflammatory phenotype in diabetic wounds. Our data indicate that the IL-1β plays a key role in sustaining the proinflammatory macrophage phenotype and in impairing the healing of diabetic wounds. Our data further suggest that the IL-1β pathway may be a therapeutic target for improving the healing of these wounds.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (R01GM092850 to T.J.K.).
No potential conflicts of interest relevant to this article were reported.
R.E.M. contributed to the study design, researched data, and wrote the manuscript. M.M.F. researched data and reviewed and edited the manuscript. W.J.E. contributed to the study design and reviewed and edited manuscript. T.J.K. designed the study and wrote the manuscript. T.J.K. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the Wound Healing Society 22nd Annual Meeting, Atlanta, Georgia, 19–22 April 2012.
The authors thank Drs. Giamila Fantuzzi and Luisa DiPietro, University of Illinois at Chicago, for critical comments on a previous draft of this article, and Ariel Johnson, University of Illinois at Chicago, for technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1450/-/DC1.
REFERENCES
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998;111:850–857 [DOI] [PubMed] [Google Scholar]
- 3.Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996;4:411–420 [DOI] [PubMed] [Google Scholar]
- 4.Rosner K, Ross C, Karlsmark T, Petersen AA, Gottrup F, Vejlsgaard GL. Immunohistochemical characterization of the cutaneous cellular infiltrate in different areas of chronic leg ulcers. APMIS 1995;103:293–299 [DOI] [PubMed] [Google Scholar]
- 5.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen 2000;8:13–25 [DOI] [PubMed] [Google Scholar]
- 6.Goren I, Allmann N, Yogev N, et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 2009;175:132–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 1975;78:71–100 [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010;184:3964–3977 [DOI] [PubMed] [Google Scholar]
- 9.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 2009;175:2454–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006;23:594–608 [DOI] [PubMed] [Google Scholar]
- 11.Goren I, Kämpfer H, Podda M, Pfeilschifter J, Frank S. Leptin and wound inflammation in diabetic ob/ob mice: differential regulation of neutrophil and macrophage influx and a potential role for the scab as a sink for inflammatory cells and mediators. Diabetes 2003;52:2821–2832 [DOI] [PubMed] [Google Scholar]
- 12.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 1990;136:1235–1246 [PMC free article] [PubMed] [Google Scholar]
- 13.Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011;56:256–264 [DOI] [PubMed] [Google Scholar]
- 14.Koenen TB, Stienstra R, van Tits LJ, et al. Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1beta transcription in human adipose tissue. Diabetes 2011;60:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasu MR, Devaraj S, Jialal I. High glucose induces IL-1beta expression in human monocytes: mechanistic insights. Am J Physiol Endocrinol Metab 2007;293:E337–E346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010;11:136–140 [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519–550 [DOI] [PubMed] [Google Scholar]
- 18.Bryer SC, Fantuzzi G, Van Rooijen N, Koh TJ. Urokinase-type plasminogen activator plays essential roles in macrophage chemotaxis and skeletal muscle regeneration. J Immunol 2008;180:1179–1188 [DOI] [PubMed] [Google Scholar]
- 19.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361:1545–1551 [DOI] [PubMed] [Google Scholar]
- 20.Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–387 [DOI] [PubMed] [Google Scholar]
- 21.White R, McIntosh C. Topical therapies for diabetic foot ulcers: standard treatments. J Wound Care 2008;17:426–, 428–432. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol 2004;4:378–385 [DOI] [PubMed] [Google Scholar]
- 23.Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. Treatment with an Interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine 2008;44:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 25.Chong HC, Tan MJ, Philippe V, et al. Regulation of epithelial-mesenchymal IL-1 signaling by PPARbeta/delta is essential for skin homeostasis and wound healing. J Cell Biol 2009;184:817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artlett CM, Sassi-Gaha S, Rieger JL, Boesteanu AC, Feghali-Bostwick CA, Katsikis PD. The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum 2011;63:3563–3574 [DOI] [PubMed] [Google Scholar]
- 27.Feldmeyer L, Werner S, French LE, Beer HD. Interleukin-1, inflammasomes and the skin. Eur J Cell Biol 2010;89:638–644 [DOI] [PubMed] [Google Scholar]
- 28.Dombrowski Y, Peric M, Koglin S, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 2011;3:82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y, Liang D, Li X, et al. The role of interleukin-1 in wound biology. Part II: In vivo and human translational studies. Anesth Analg 2010;111:1534–1542 [DOI] [PubMed] [Google Scholar]
- 30.Graves DT, Nooh N, Gillen T, et al. IL-1 plays a critical role in oral, but not dermal, wound healing. J Immunol 2001;167:5316–5320 [DOI] [PubMed] [Google Scholar]
- 31.Thomay AA, Daley JM, Sabo E, et al. Disruption of interleukin-1 signaling improves the quality of wound healing. Am J Pathol 2009;174:2129–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trengove NJ, Langton SR, Stacey MC. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen 1996;4:234–239 [DOI] [PubMed] [Google Scholar]
- 33.Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 2000;115:245–253 [DOI] [PubMed] [Google Scholar]
- 34.Cortez-Retamozo V, Etzrodt M, Pittet MJ. Regulation of macrophage and dendritic cell responses by their lineage precursors. J Innate Immun 2012;4:411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weidenbusch M, Anders HJ. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. J Innate Immun 2012;4:463–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 2007;204:1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 2005;115:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 2007;170:818–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goren I, Müller E, Schiefelbein D, et al. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol 2007;127:2259–2267 [DOI] [PubMed] [Google Scholar]
- 41.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 2011;121:985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.