Many behaviors and physiological processes are influenced by internal recurrent daily rhythms, which likely represent an adaptation to the Earth’s rotation around the Sun and the recurrent 24-h light-dark cycles in the external environment. These circadian rhythms are an important regulator of many key biological processes that influence cellular metabolic pathways and organ function (1,2). The results from a series of studies have demonstrated the importance of normal circadian action for maintaining health in people and the disruption of circadian rhythm, which can have adverse effects on metabolic function. For example, experimentally induced sleep restriction and/or circadian misalignment, generated by inducing recurrent 28-h sleep-wake cycles, decrease insulin sensitivity and glucose tolerance (3–6). Data from epidemiological studies suggest that long-term alteration in sleep pattern increases the risk of obesity and metabolic diseases. The prevalence of obesity, hypertension, hypertriglyceridemia, and the metabolic syndrome are greater in shift workers than day workers, and short sleep duration is associated with an increased risk of obesity and diabetes (7,8).
Circadian rhythms are generated by a transcriptional autoregulatory feedback loop that involves core clock genes. CLOCK (circadian locomotor output cycles protein kaput) and BMAL1 (brain and muscle ARNT-like 1) proteins form a heterodimer complex that binds to E-boxes, which drive the transcription of Period (PER1, 2, and 3) and Cryptochrome (CRY1 and 2), which in turn produce a negative feedback loop by suppressing CLOCK:BMAL1-mediated transcriptional activity (1,2). In mammals, neurons in the hypothalamic suprachiasmatic nucleus act as a master pacemaker and synchronize the daily oscillations in peripheral tissues throughout the body (1,9). Data from studies conducted in rodent models show that circadian clock genes function both centrally in the suprachiasmatic nucleus and peripherally in key metabolic organs, including the liver, skeletal muscle, pancreatic islets, and adipose tissue (1,2) (Fig. 1). Clock genes are involved in regulating glucose metabolism in the liver. Gluconeogenesis is impaired in both ClockΔ19 mutant and Bmal1 knockout (KO) mice (10), and hepatic glucose export is also dysregulated in liver-specific Bmal1 KO mice (11). In contrast, CRY1 inhibits fasting-induced gluconeogenic enzyme expression in the liver, so overexpression of CRY1 improves glucose tolerance and hepatic insulin sensitivity in diabetic mice (12). In skeletal muscle, CLOCK and BMAL1 are essential for the maintenance of normal mitochondrial biogenesis and respiratory function (13). In pancreatic islets, CLOCK and BMAL1 help regulate glucose-stimulated insulin secretion, and both ClockΔ19 mutant and pancreas-specific Bmal1 KO mice have impaired glucose tolerance because of β-cell dysfunction (14). In adipose tissue, BMAL1 and PER2 regulate adipocyte differentiation, de novo lipogenesis, and fatty acid oxidation (15,16).
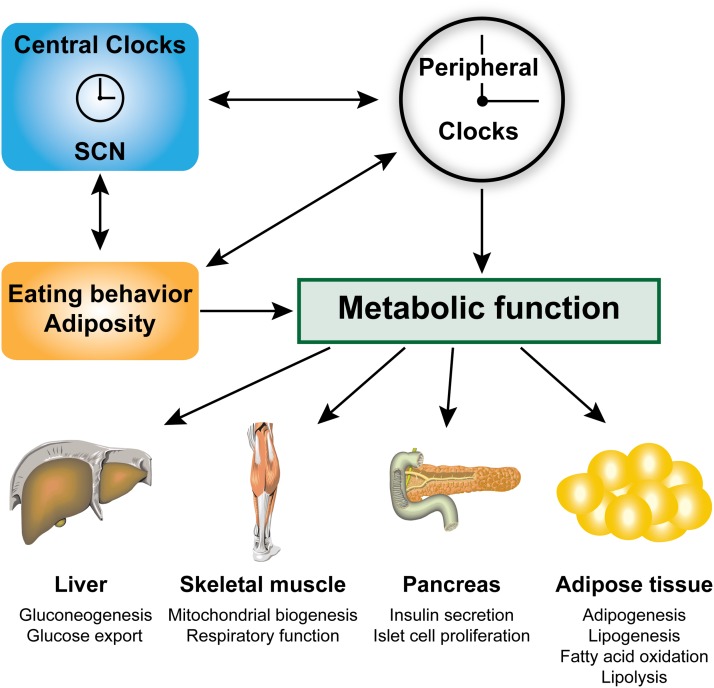
FIG. 1.
Interactive regulation of food intake and metabolic function by circadian clock genes. Central and peripheral clocks interact with each other to regulate food intake and specific metabolic pathways in key organ systems (1,2,9). Disruption of central or peripheral circadian rhythms can cause an increase in food intake and obesity, which in turn can affect central and peripheral circadian rhythm activity and directly impair metabolic function. Individual organs have their own clocks that directly affect metabolic pathways. The study by Shostak et al. (17) in this issue of Diabetes has identified a new function of clock genes in the regulation of lipolytic activity in white adipose tissue.
In this issue of Diabetes, Shostak et al. (17) present findings that demonstrate a new and important function of clock genes in regulating lipolytic activity in white adipose tissue. The investigators conducted a series of elegant experiments in wild-type (WT) mice and genetic mouse models (ClockΔ19 mutant, Bmal1 KO, and Per2::Luciferase knock-in mice) that demonstrate 1) 24-h serum free fatty acids (FFAs) and glycerol concentrations, which provide an index of adipose tissue lipolytic activity, are lower in WT than ClockΔ19 mutant and Bmal1 KO mice; 2) serum FFAs and glycerol concentrations and lipolytic activity in fat pad explants follow a circadian pattern in WT mice, which is abolished in ClockΔ19 mutant and Bmal1 KO mice; 3) adipose tissue obtained from different depots display an endogenous and sustained circadian rhythm manifested as autonomous bioluminescent rhythm in Per2::Luciferase knock-in mice in fad pad explants obtained from epididymal, perirenal, peritoneal, subcutaneous white adipose tissue, and intrascapular brown adipose tissue; 4) gene expression of the major proteins that hydrolyze adipose tissue triglycerides, adipose triglyceride lipase (Atgl), and hormone-sensitive lipase (Hsl), exhibit circadian variations in WT mice, which are abolished in ClockΔ19 mutant and Bmal1 KO mice; 5) CLOCK/BMAL1 regulate Atgl and Hsl transcription in adipose tissue by binding to the E-boxes in the Atgl and Hsl genes; and 7) the normal increase in adipose tissue lipolytic activity that occurs in response to food restriction is blunted in ClockΔ19 mutant mice, so these animals rely much more on liver glycogen than do WT mice as an energy source during fasting.
These results demonstrate that adipose tissue clock genes regulate the hydrolysis of adipose tissue triglycerides and provide a rhythmic release of FFAs and glycerol from adipocytes. Moreover, this circadian function has important physiological consequences because its disruption decreases overall daily lipolytic activity and blunts the lipolytic response to fasting. Adipose tissue is the body’s major fuel reserve. Therefore, the mobilization of adipose triglycerides and the release of FFAs and glycerol into the bloodstream are critical for survival during periods of food deprivation and for physical function during prolonged physical activity. Accordingly, alterations in adipose tissue clock function could have serious adverse consequences during fasting and endurance exercise. However, it is also possible that localized adipose tissue clock disruption and downregulation of lipolytic activity have beneficial metabolic effects if energy intake and adiposity are not increased because experimentally increasing circulating FFAs causes hepatic (18) and skeletal muscle (19) insulin resistance, whereas experimentally decreasing serum FFA concentrations improves insulin sensitivity (20).
An additional key finding from the study by Shostak et al. (17) is that ClockΔ19 mutant mice had greater food intake, body weight, and percent body fat than WT mice. Unfortunately, these effects confound the interpretation of the data from their study because it is possible that altered feeding patterns and increased adiposity affect circadian oscillations in adipose tissue lipolytic activity. A weight gain–matched control group is needed to fully resolve this issue. The increase in body weight and fat mass was likely caused by hyperphagia and by not a decrease in adipose tissue lipolytic activity. Body weight and body fat reflect the balance between energy intake and energy expenditure. Impaired lipolytic rate alone should not cause an accumulation of body fat without a concomitant positive energy balance. Therefore, these data suggest circadian rhythms are involved in the drive to eat, and they provide a potential mechanism responsible for weight gain and obesity associated with sleep deprivation and working at night.
The findings of Shostak et al. (17) add to our understanding of the molecular and physiological connection between circadian rhythm and adipose tissue metabolism. Additional studies conducted in adipose tissue–specific (and organ-specific) KO or transgenic mice, in conjunction with diet-matched control animals, are needed to help unravel the complex effects of clock rhythms in individual organs. The extraordinary diverse and profound effects of circadian rhythm disruption on eating behavior and multiorgan metabolic function make them particularly important to understand the potential link between central and peripheral clocks in the pathogenesis of obesity and metabolic dysfunction in people. These studies could lead to novel targets for treating obesity and its metabolic complications.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants DK 37948, DK 56341 (Nutrition Obesity Research Center), DK020579 (Diabetes Research Center), and UL1 RR024992 (Clinical and Translational Science Award).
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 2195.
REFERENCES
- 1.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 2012;35:445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res 2010;106:447–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009;106:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59:2126–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 2012;4:129ra143 [DOI] [PMC free article] [PubMed]
- 6.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009;94:3242–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 2001;58:747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev 2009;10(Suppl. 2):37–45 [DOI] [PMC free article] [PubMed]
- 9.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci 2001;2:521–526 [DOI] [PubMed] [Google Scholar]
- 10.Rudic RD, McNamara P, Curtis AM, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2004;2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 2008;105:15172–15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang EE, Liu Y, Dentin R, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 2010;16:1152–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews JL, Zhang X, McCarthy JJ, et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA 2010;107:19090–19095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010;466:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimba S, Ishii N, Ohta Y, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA 2005;102:12071–12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimaldi B, Bellet MM, Katada S, et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab 2010;12:509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shostak A, Meyer-Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 2013;62:2195–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boden G, Cheung P, Stein TP, Kresge K, Mozzoli M. FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am J Physiol Endocrinol Metab 2002;283:E12–E19 [DOI] [PubMed] [Google Scholar]
- 19.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 1996;97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santomauro AT, Boden G, Silva ME, et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 1999;48:1836–1841 [DOI] [PubMed] [Google Scholar]