Abstract
An oral biofilm is a community of surface-attached microorganisms that coats the oral cavity, including the teeth, and provides a protective reservoir for oral microbial pathogens, which are the primary cause of persistent and chronic infectious diseases in patients with dry mouth or Sjögren's syndrome (SS). The purpose of this study was to establish an animal model for studying the initial adhesion of oral streptococci that cause biofilm formation in patients with dry mouth and SS in an attempt to decrease the influence of cariogenic organisms and their substrates. In nonobese diabetogenic (NOD) mice that spontaneously develop insulin-dependent diabetes mellitus (IDDM) and SS, we replaced major histocompatibility complex (MHC) class II (Ag7 Eg7) and class I Db with MHC class II (Ad Ed) and class I Dd from nondiabetic B10.D2 mice to produce an animal model that inhibited IDDM without affecting SS. The adhesion of oral streptococci, including Streptococcus mutans, onto tooth surfaces was then investigated and quantified in homologous recombinant N5 (NOD.B10.D2) and N9 (NOD.B10.D2) mice. We found that a higher number of oral streptococci adhered to the tooth surfaces of N5 (NOD.B10.D2) and N9 (NOD.B10.D2) mice than to those of the control C57BL/6 and B10.D2 mice. On the basis of our observation, we concluded that these mouse models might be useful as animal models of dry mouth and SS for in vivo biological studies of oral biofilm formation on the tooth surfaces.
Oral streptococci are present in large numbers in dental plaque, and several types interact with the enamel salivary pellicle to form a biofilm on tooth surfaces (9, 16, 17, 21, 29). Streptococci account for approximately 20% of the total number of salivary bacteria (24), with Streptococcus salivarius being the primary organism. Further, the densities of Streptococcus mutans and Streptococcus sanguis in saliva are more than 1 × 105 cells per ml. S. mutans is a pioneering organism that plays an important role in biofilm formation on tooth surfaces and is a primary causative agent of dental caries (9, 16, 21). The mechanical forces of salivary flow and tongue movement tend to dislodge and expel bacteria from tooth surfaces and the oral cavity (3, 5, 6), and their importance in controlling microbial colonization in the oral cavity has been well demonstrated in individuals with diabetes mellitus, Sjögren's syndrome (SS), and dry mouth, who suffer from a rapid overgrowth of biofilm and rampant caries, making them highly susceptible to oral infections (1-2, 6). Thus, attempts to investigate the initial adhesion by oral streptococci, including S. mutans, in mouse models are likely to aid in the understanding and prevention of oral infectious diseases caused by the components of oral biofilm.
Previous studies of S. mutans infections in the oral cavities of mice have been performed by feeding the animals diets containing sucrose in the presence of glucans (13, 15, 30, 43). Since the adherence of S. mutans to the tooth surface may depend on the balance between physical adherence and synthesis of insoluble glucans in a natural environment, that infection method may be inappropriate for investigation of natural biofilm formation associated with streptococci, including S. mutans (18, 39).
The nonobese diabetogenic (NOD) mouse strain is currently the best available model for the study of insulin-dependent type 1 diabetes mellitus (IDDM) and SS (11, 31), both of which develop spontaneously and are characterized by lymphatic infiltration of the pancreas and salivary glands. Oral changes are prominent features of these diseases, which are manifested by dry mouth and hyposalivation (6, 7, 37). NOD mice are also used as an animal model for the study of oral infectious diseases associated with systemic diseases such as diabetes and SS or dry mouth.
The unique major histocompatibility complex (MHC) class II genes (I-Ag7, no expression of I-E) represent dominant susceptibility factors and mediate activated T cells during the development of diabetes in NOD mice (11, 22, 25, 36, 41, 42). In the NOD model of SS, histopathological analyses of the salivary glands in MHC-congenic strains of NOD mice have indicated that the I-Ag7 region is not required for lymphocytic infiltration (26, 31). Further, replacement of the NOD MHC class I Kd region with another haplotype, MHC class I Kwm7, as well as replacement of the MHC class II Ag7 Eg7 and class I Dd regions with the corresponding region from the other MHC haplotype, has been shown to prevent diabetes (12). However, replacement with MHC class I K does not completely prevent development of insulitis. In another report, NOD mice pretreated nasally by using peptides restricted with MHC class I Kd showed a delayed onset of spontaneous IDDM, though insulitis could not be prevented by the induction of tolerance (23).
In the present study, we attempted to establish an animal model for oral infectious diseases such as dental caries by focusing on replacement of the MHC class II and class I D region but not the class I K region in nondiabetic NOD mice by outcrossing B10.D2 mice (Kd, I-Ad, and Dd) with NOD mice (Kd, I-Ag7, and Db) because the MHC class I K region in B10.D2 mice is identical with that in NOD mice (12). The present backcrossed and intercrossed NOD mice with the MHC class II and MHC class I D region replaced with that from B10.D2 mice developed SS, however, not diabetes. We then attempted to determine whether these mice would be useful as animal models for a sucrose-free study of the initial adhesion of oral streptococci on tooth surfaces in humans.
MATERIALS AND METHODS
Bacterial strains and culture conditions. The Streptococcus strains used in this study were S. mutans MT8148, S. sanguis ATCC 10556, S. sobrinus 6715, S. salivarius ATCC 9759, and S. mitis ATCC 6249. All bacteria were grown in an atmosphere of H2 and CO2 (GasPack; Becton Dickinson and Co., Franklin Lakes, N.J.) in brain heart infusion broth (Difco Laboratory, Detroit, Mich.) at 37°C overnight and were then harvested and washed twice with sterile phosphate-buffered saline (PBS).
Animals and assessment of diabetes and saliva.
NOD/LtJ and B10.D2 mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and Japan SLC (Shizuoka, Japan), respectively, and maintained in accordance with the guidelines of the National Institute of Infectious Diseases. Clinical onset of diabetes in NOD mice was determined by the presence of glucose in urine and blood. Urine was tested weekly using Uristix reagent strips (Bayer Medical Ltd., Newbury, United Kingdom) and confirmed to be positive by blood glucose measurements. After being anesthetized, the mice were injected with a cocktail of isoproterenol (0.20 μg/100 gm of body weight) and pilocarpine (0. 05 μg/100 gm) (Sigma Chemical, St. Louis, Mo.) in PBS as a secretagogue. Following the intraperitoneal injection, saliva was collected from each mouse by using a micropipette for 15 min and was stored at −80°C.
Bacterial sampling and CFU counting.
All oral streptococci were cultured in brain heart infusion broth overnight and were then washed twice with sterile PBS. Chlorhexidine (0.2%)-soaked sterile cotton swabs were used for disinfecting the oral cavities of the mice, including the lower incisor teeth, which were immediately washed with sterile PBS. Oral streptococci were introduced into the oral cavities at a final concentration of 7 × 109 CFU in 250 μl of PBS in all females at 4 or 8 months of age for 2.5 min, after which the mice were not provided food or drinking water. Following inoculation, samples were collected from the labial surfaces of the lower incisor teeth with a sterile cotton ball and then dipped into 2 ml of PBS. The samples in PBS were sonicated by ultrasonic dispersion (power output, 60 W) for 10 seconds and were then poured onto Mitis-Salivarius agar plates containing 0.02 M bacitracin (MSB) by using an EDDY JET spiral system (Gunze Sangyo, Inc., Tokyo, Japan). CFU were determined after 48 h of anaerobic incubation at 37°C.
Generation of backcross mice.
To generate the backcross generation, NOD mice were mated with B10.D2 mice to produce (NOD × B10.D2)F1 mice, and then heterozygous F1 mice were mated with NOD mice to produce first-generation backcross (BC1) mice. BC1, BC2, BC3, and BC7 heterozygous mice were then mated with NOD mice to produce BC2, BC3, BC4, and BC8 mice, respectively, and BC4 and BC8 heterozygous mice were intercrossed to produce N5 and N9 MHC-recombinant NOD mice. After an outcross of the NOD strain to the B10.D2 strain, repetitive backcrossing with NOD mice was performed, with breeder selections based on genomic PCRs and/or simple sequence length polymorphism (SSLP) analysis with microsatellite markers (MapPairs; Research Genetics, Huntsville, Ala.), as shown in Table 1. The PCRs (25 μl) were done using a PTC-200 (MJ Research, Watertown, Mass.) for 40 cycles (94°C, 15 seconds; 58°C, 45 seconds; 72°C, 5 min, after an initial denaturation at 94°C for 3 min) and were then analyzed on a 3% agarose gel. An SSLP analysis of the B10.D2 allele from the backcross generation identified the mice as homozygous for allelic variants characteristic of NOD mice with replacements of the MHC region with that from B10.D2 mice at all of the Idd17 linkage markers (Table 1). Typing of these markers without Idd17 confirmed the homozygous presence of the NOD-derived background genome at the identified Idd loci in the backcross generation (Table 1).
TABLE 1.
Linkage markers analyzed for homozygosity to NOD-derived Idd loci in NOD.B10.D2 congenic mice
| Idd locus/ chromosome | Linkage marker homozygous to NOD allele | Relative microsatellite sizea |
|---|---|---|
| Idd1/17 | D17Mit 198 | B10.D2 > NOD |
| D17Mit 195 | B10.D2 > NOD | |
| D17Mit 194 | B10.D2 = NOD | |
| D17Mit 173 | B10.D2 = NOD | |
| D17Mit 145 | B10.D2 = NOD | |
| D17Mit 82 | B10.D2 > NOD | |
| D17Mit 34 | B10.D2 > NOD | |
| D17Mit 28 | B10.D2 > NOD | |
| D17Mit 59 | B10.D2 = NOD | |
| D17Mit 62 | B10.D2 = NOD | |
| Idd2/9 | D9Mit 25 | B10.D2 = NOD |
| Idd3/3 | D3Mit 95 | B10.D2 < NOD |
| D3Mit 103 | B10.D2 = NOD | |
| D3Mit 206 | B10.D2 = NOD | |
| Idd4/11 | D11Mit 115 | B10.D2 < NOD |
| D11Mit 320 | B10.D2 < NOD | |
| Idd5/1 | D1Mit 46 | B10.D2 < NOD |
| Idd6/6 | D6Mit 15 | B10.D2 > NOD |
| D6Mit 339 | B10.D2 < NOD | |
| D6Mit 52 | B10.D2 > NOD | |
| Idd7/7 | D7Mit 20 | B10.D2 > NOD |
| Idd8, Idd12/14 | D14Mit 222 | B10.D2 > NOD |
| D14Mit 110 | B10.D2 = NOD | |
| Idd9, Idd11/14 | D4Mit 59 | B10.D2 < NOD |
| Idd10/3 | D3Mit 103 | B10.D2 = NOD |
| Idd13/2 | D2Mit 257 | B10.D2 < NOD |
| D2Mit 17 | B10.D2 = NOD | |
| Idd14/13 | D13Mit 61 | B10.D2 < NOD |
| Idd15/15 | D5Mit 48 | B10.D2 > NOD |
Microsatellite markers with the indicated allelic size variants were typed in backcross mice used for the intercross.
MHC serological typing.
Spleen cells were dissociated from NOD and MHC-recombinant N5 NOD and N9 NOD mice and incubated with monoclonal antibodies (mAbs) determined to react with MHC class I and class II molecules. Fluorescein isothiocyanate- and phycoerythrin-conjugated goat anti-mouse mAb H-2Kd (SF-1.1.1) reacted in cells from both NOD and B10.D2 mice, and mAb I-Ek (14-4-4S) and mAb H-2Dd (34-5-8S) reacted in cells from B10.D2 mice. mAb I-Ek was used for detection of I-Ed expression because it cross-reacted with the I-Ed molecule. After being incubated with these mAbs for 45 min at 4°C, the cells were washed and then analyzed by flow cytometry (fluorescence-activated cell sorting [FACS]) (Becton Dickinson).
Histology.
Pancreas specimens and submandibular glands were frozen in OCT compound. Tissue sections (5 μm) were stained with hematoxylin and eosin and were then examined for evidence of mononuclear cell inflammation. Histological observations and photomicrography were performed by using an Olympus BX50WI microscope (Olympus Inc., Tokyo, Japan).
Statistical analysis.
A Kaplan-Meier cumulative survival test was used to compare the incidence of diabetes. Comparative analyses were performed by analysis of variance. A P value of < 0.05 was considered statistically significant for two-tailed comparisons. All statistical analyses were performed using StatView software for the Macintosh operating system.
RESULTS
Generation of backcross and intercross of NOD.B10.D2 strain and assessment of diabetes and salivary flow rate.
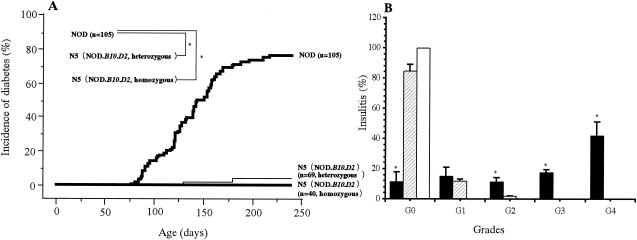
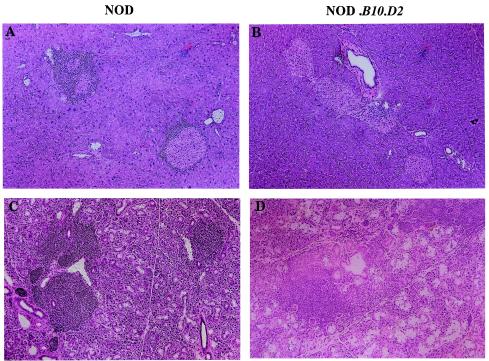
BC4 and BC8 heterozygous mice (Mhc: d/d at K, d/g7 at A and E, and d/b at D) were intercrossed to produce N5 (NOD.B10.D2) and N9 (NOD.B10.D2) generation mice. The N5 (NOD.B10.D2) and N9 (NOD.B10.D2) intercross animals were typed for the Mhc haplotypes and divided into three groups (NOD type, heterozygous, and homozygous). Further, the expression of MHC class I Kd, class II I-Ed, and class I Dd in the backcross and intercross N5 (NOD.B10.D2) mice was confirmed serologically by FACS analysis (Fig. 1). Mice that were homozygous for B10.D2 Mhc in the fourth and eighth backcross generations (BC4 and BC8) were used to establish the N5 (NOD.B10.D2) and N9 (NOD.B10.D2) strains and were then maintained by brother-sister mating. Replacement (homozygous recombination) of the MHC class II (A E) and class I D regions prevented the development of diabetes (78% of NOD type and 8% of N5 heterozygous strains developed diabetes). However, the incidence of insulitis in the N5 (NOD.B10.D2) strain (grade 0, 92%; grade 1, 8%; grade 2, 0%; grade 3, 0%; and grade 4, 0% at 6 months of age) was significantly different from that in the NOD type mice, and no insulitis was observed in F1 mice (Fig. 2B). Histological examination of the pancreas specimens showed only slight insulitis in the N5 (NOD.B10.D2) mice compared with that in the NOD colony (Fig. 3), while examination of the submandibular glands showed a strong infiltration of lymphocytes in the N5 (NOD.B10.D2) and N9 (NOD.B10.D2) strains (Fig. 3). Thus, replacement of the NOD MHC class II I-A and class I D regions with the B10-D2 MHC Class II I-A and Class I D regions prevented the development of diabetes and insulitis but not SS in N5 (NOD.B10.D2) and N9 (NOD.B10.D2) generation mice.
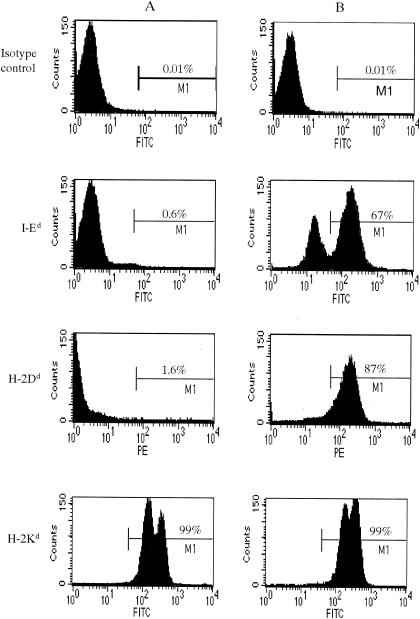
FIG. 1.
Serological typing of lymphocytes. Spleen cells from NOD (A) and N5 (NOD.B10.D2) (B) female mice at 4 months of age were examined for the expression of MHC class I H-2Kd, MHC class II I-Ed, and H-2Dd by using FACS analysis. NOD, MHC class II I-Ed, and H-2Dd antigens were expressed in the NOD mice. Each histogram shows the percentage of total spleen cells from NOD and N5 (NOD.B10.D2) mice. Histograms are representative of the results of three independent experiments with 10 mice in each group, with similar results obtained in each experiment. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
FIG. 2.
Cumulative incidence of diabetes and insulitis in NOD and new N5 (NOD.B10.D2) mice. (A) Mice homozygous for B10.D2 MHC avoided diabetes in comparison with the heterozygous and NOD types. (B) Insulitis scores were calculated by using hematoxylin and eosin staining of pancreas sections from F1 (□), N5 (▨), and NOD (▪) female mice at 4 months of age. The degree of lymphocyte infiltration was graded as follows: G0, no infiltrating cells in the islets; G1, infiltrating cells adjacent to the islets; G2, infiltrating cells occupying less than 25% of the islets; G3, infiltrating cells occupying 25 to 50% of the islets; G4, infiltrating cells occupying more than 50% of the islets. For example, the percentage of G1 insulitis = (number of G1)/(G0 + G1 + G2 + G3 + G4 + G4) × 100. Results are expressed as the means ± standard deviations (SDs) of the results for 12 mice per strain. *, P < 0.001 for the results for NOD mice versus those for N5 and F1 mice.
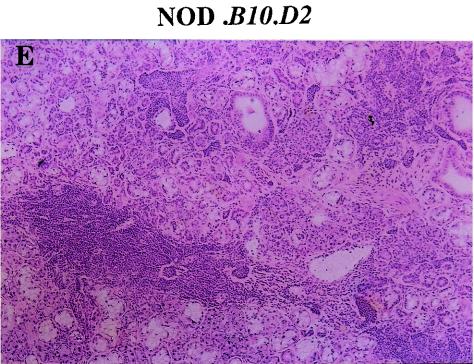
FIG. 3.
Histology of exocrine glands from NOD, N5 (NOD.B10D2), and N9 (NOD.B10D2) female mice at 4 months of age. (A) Massive infiltration by mononuclear cells in the pancreas islets of NOD mice; (B) no infiltrating cells in the pancreas islets of N5 (NOD.B10D2) mice; (C) massive infiltration by mononuclear cells in the submandibular glands of NOD mice; and (D) moderate to massive infiltration by mononuclear cells in the submandibular glands of N5 (NOD.B10D2) mice; (E) massive infiltration by mononuclear cells in the submandibular glands of N9 (NOD.B10D2) female mice. Tissues were stained with hematoxylin and eosin. Magnification, × 100.
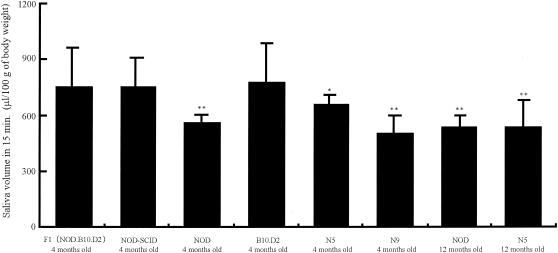
As shown in Fig. 4, the total volume of secreted saliva after stimulation by a secretagogue was significantly lower for NOD mice and N5 and N9 (NOD.B10.D2) mice at 4 and 12 months of age than for the F1 (NOD.B10.D2), NOD-scid, and B10.D2 mice at 4 months of age. However, there was no significant difference between NOD and N9 (NOD.B10.D2) mice at 4 or 12 months of age and only a slight difference between NOD and N5 (NOD.B10.D2) mice at 4 months of age.
FIG. 4.
Salivary flow rates for various mouse strains. The total volume of secreted saliva per 100 g of body weight for NOD, N5 (NOD.B10.D2), and N9 (NOD.B10.D2) female mice was reduced compared to that for NOD-scid, F1 (NOD.B10.D2), and B10D2 female mice at 4 months of age. Data are expressed as the means ± SDs of the results for 12 mice per strain. *, P < 0.05; **, P < 0.001.
To assess the incidence of SS, sialadentis was analyzed histologically for those mice that presented a decreasing volume of secreted saliva, as shown in Fig. 4. The incidence of SS with both decreasing saliva and sialadentis was 10 of 12 (83%), 10 of 12 (83%), 9 of 12 (75%), 10 of 12 (83%), and 10 of 12 (83%) in 4-month-old NOD, 12-month-old NOD, 12-month-old N5 (NOD.B10.D2), and 12-month-old N9 (NOD.B10.D2) mice, respectively. However, sialadentis was not observed in the control 12-month-old B10.D2 and F1 (NOD.B10.D2) mice.
Adhesion of oral streptococci to tooth surfaces.
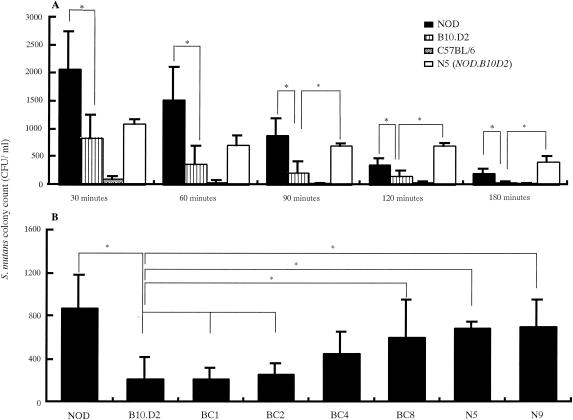
As shown in Fig. 5, S. mutans adhesion results revealed that bacteria adhered in higher numbers to the tooth surfaces of N5 (NOD.B10.D2) and N9 (NOD.B10.D2) mice than to those of the control B10.D2, NOD, and C57BL/6 mice at various time points. After the adhesion phase was extended from 90 to 180 min, there was a decrease in biofilm growth (Fig. 5A); however, a large number of bacteria remained on the tooth surfaces of the mice. S. mutans adhesion for N5 (NOD.B10.D2) mice (407.0 ± 93.0 CFU/ml) and NOD mice (203.0 ± 75.0 CFU/ml) was significantly higher than that for the control B10.D2 and C57BL/6 mice at 180 min after inoculation (P < 0.05) (Fig. 5A). Further, distinct differences among N5 (NOD.B10.D2), NOD, and B10.D2 mice were observed at 90 min after inoculation. Therefore, bacterial samplings in later experiments with streptococcal inoculation were performed at 90 min. Significantly greater numbers of S. mutans bacteria (879.0 ± 302.6, 600.4 ± 351.6, 691 ± 151, and 702.0 ± 205.5 CFU/ml) adhered to the tooth surfaces of NOD, BC8, N5 (NOD.B10.D2), and N9 (NOD.B10.D2) mice, respectively, than to those of B10.D2 (216.0 ± 203.0 CFU/ml), BC1 (206.0 ± 98.0 CFU/ml), and BC2 (256.0 ± 103.0 CFU/ml) mice (Fig. 5B).
FIG. 5.
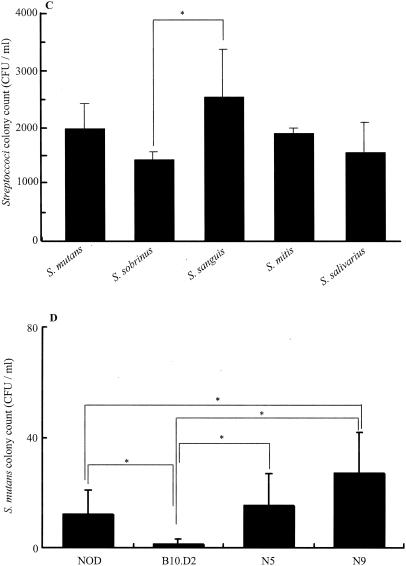
Adhesion of oral streptococci on tooth surfaces of mice. (A) Adhesion of S. mutans to the tooth surfaces of NOD, B10.D2, N5 (NOD.B10.D2), and C57BL/6 female mice at 4 months of age at different time points. CFU were determined in Mitis-Salivarius agar plates containing 0.02 M bacitracin after 48 h of anaerobic incubation at 37°C. (B) Adhesion of S. mutans for NOD, B10.D2 BC1, BC4, BC2, BC8, N5 (NOD.B10.D2), and N9 (NOD.B10.D2) female mice at 8 months of age 90 min after inoculation. CFU were determined in Mitis-Salivarius agar plates containing 0.02 M bacitracin after 48 h of anaerobic incubation at 37°C. (C) Adhesion of S. mutans, S. sobrinus, S. sanguis, S. mitis, and S. salivarius for N5 (NOD.B10.D2) female mice at 8 months of age 90 min after inoculation. CFU were determined in Mitis-Salivarius agar plates after 48 h of anaerobic incubation at 37°C. (D) Adhesion of S. mutans for NOD, B10.D2, N5 (NOD.B10.D2), and N9 (NOD.B10.D2) female mice at 8 months of age 24 h after inoculation. Data were obtained from three independent experiments with 15 mice in each strain, and values are expressed as the means ± SDs of the results. *, P < 0.05.
S. mutans, S. sanguis, S. sobrinus, S. salivarius, and S. mitis were also inoculated into the oral cavities of N5 (NOD.B10.D2) mice (Fig. 5C). The CFU of S. sanguis showed that it had the highest level of adhesion among these streptococci. Further, S. mutans and S. mitis were found in greater numbers than S. sobrinus and S. salivarius, though the differences were not statistically significant (Fig. 5C). After the adhesion phase was extended from 180 min to 24 h, there was a decrease in S. mutans adhesion on the tooth surfaces of the mice (Fig. 5D); however, a significant number of bacteria remained on the tooth surfaces of NOD (17.0 ± 12.0 CFU/ml), N5 (15.0 ± 11.2 CFU/ml), and N9 (31.0 ± 16.0 CFU/ml) mice compared to that for B10.D2 (1.0 ± 2.0 CFU/ml) mice (Fig. 5D).
DISCUSSION
Previous studies of labial salivary glands have suggested that MHC molecules are genetically associated with SS in humans (10, 23, 27). Class II HLA antigen is expressed on SS salivary gland epithelial cells but not in normal salivary gland cells (8, 20). Other results have also demonstrated that there is no single class II allele associated with primary SS among different ethnic groups (14), though there is no direct evidence that the MHC class II gene confers susceptibility to the development of primary SS. Recently, a report by Robinson et al. indicated that the unique NOD MHC class II I-Ag7 is not essential for exocrine tissue autoimmunity in NOD mice (31). In our experiments, we also found that recombination of the region containing MHC class II and class I D derived from B10.D2 mice could not prevent development of salivary gland lymphocytic infiltration in NOD mice. However, such a recombination was considered to be essentially responsible for the progression of lymphocytic infiltration in the pancreas. These findings suggest that immunoreactivity to the auto antigen in the N5 (NOD.B10.D2) and N9 (NOD.B10.D2) mice with replacements of the MHC class II (Ad Ed) and class I Dd regions, respectively, is associated with an immune function that has a role in inflammation of the pancreas islet rather than the development of SS and that separate genes contribute to the development of IDDM and SS in NOD mice.
It has also been reported that salivary components play important roles in controlling microbial colonization in the oral cavities of individuals with SS or dry mouth (37). SS may lead to qualitative and quantitative changes in the protective salivary films or pellicles that coat hard and soft tissues (5, 6), and the loss of enamel or cemental protective pellicles could result in an increase in dental caries and periodontitis (32). Further, an alteration in the mucosal pellicle may make oral soft tissues more susceptible to desiccation and environmental insult, leading to colonization by opportunistic microflora (21, 24). The S. mutans adhesion results seen in the present experiments demonstrated that a significant number of bacteria adhered to the tooth surfaces of NOD, N5 (NOD.B10.D2), and N9 (NOD.B10.D2) mice compared to that for C57BL/6 and B10.D2 mice (Fig. 5A, B and D). Further, the NOD background gene tended to increase the binding of S. mutans to tooth surfaces as the number of backcrosses increased (Fig. 5B). However, NOD mice that develop diabetes are not suitable for studies of long-term infection by oral bacteria, as their average life span is short compared with those of N5 (NOD.B10.D2) and N9 (NOD.B10.D2) mice.
The ability of oral streptococci to bind to salivary pellicle proteins on the tooth surface is of considerable etiological significance (28, 33), and S. mutans and S. sanguis are known to be primarily involved with the formation of bacterial flora on teeth. S. sanguis and S. mitis are early colonizers of the salivary pellicle, while S. mutans colonizes later; however, the ability of each to bind to salivary proteins and glycoproteins is strong and important in biofilm development (19, 40). The present results showed a tendency for an increase in affinity of S. sanguis, S. mitis, and S. mutans for the mouse tooth surfaces (Fig. 5C). The affinity of streptococci for the tooth surfaces of patients is considered to be closely related to dry mouth and SS (1-3, 4, 34). Therefore, the N5 (NOD.B10.D2) and N9 (NOD.B10.D2) mouse strains may be useful animal models, as they presented initial adherence activities of oral streptococci on their tooth surfaces similar to those for humans with dry mouths. They may also be suitable for studies of long-term infection by oral bacteria, as their average life span is long compared to that of NOD mice.
A problem facing in vivo oral biofilm research is the lack of a naturalistic, reproducible, longitudinal monitoring system that would permit the assessment of dry mouth and oral bacterial infection in the same animal throughout the duration of the study. Studies of S. mutans infections in mouse oral cavities have been performed by feeding the animals with powdered diet 301 and diet 2000, which contain unnatural amounts of sucrose (1 and 56%, respectively) (13, 38, 43). In other infection studies, mice were provided with either a 5% sucrose diet or a sucrose-free diet, in which the 56% sucrose in diet 2000 was replaced with wheat flour (15, 30). When these methods were used, S. mutans was found to produce a larger amount of insoluble glucan in the oral cavities of mice than in normal humans. However, continuous ingestion of food containing such excess amounts of sucrose is unusual (35). Therefore, an experimental system using a generated NOD.B10.D2 strain may be more useful than those previously reported for the initial adherence of streptococci on tooth surfaces without synthesis of insoluble glucan.
In the present study, the importance of salivary flow for controlling the initial adhesion of oral streptococci in the oral cavity was also demonstrated in the mouse models of SS and dry mouth. We believe that N5 (NOD.B10.D2) and N9 (NOD.B10.D2) mice, which have a high sensitivity for initial adhesion, may be useful for in vivo biological studies of oral biofilm formation on the tooth surfaces of patients with dry mouth or SS.
Acknowledgments
This work was supported in part by Grants in Aid for Developmental Scientific Research (13671991 and 1539057) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was also funded by a part of Ground Research for Space Utilization, promoted by NASDA and the Japan Space Forum.
REFERENCES
- 1.Almstahl, A., U. Kroneld, A. Tarkowski, and M. Wikstrom. 1999. Oral microbial flora in Sjögren's syndrome. J. Rheumatol. 26:110-114. [PubMed] [Google Scholar]
- 2.Almstahl, A., M. Wikstrom, and U. Kroneld. 2001. Microflora in oral ecosystems in primary Sjögren's syndrome. J. Rheumatol. 28:1007-1013. [PubMed] [Google Scholar]
- 3.Almstahl, A., and M. Wikstrom. 1999. Oral microflora in subjects with reduced salivary secretion. J. Dent. Res. 78:1410-1416. [DOI] [PubMed] [Google Scholar]
- 4.Baughan, L. W., F. J. Robertello, D. C. Sarrett, P. A. Denny, and P. C. Denny. 2000. Salivary mucin as related to oral Streptococcus mutans in elderly people. Oral Microbiol. Immunol. 15:10-14. [DOI] [PubMed] [Google Scholar]
- 5.Bergdah, M., and J. Bergdah. 2000. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J. Dent. Res. 79:1652-1658. [DOI] [PubMed] [Google Scholar]
- 6.Daniels, T., and P. Fox. 1992. Salivary and oral components of Sjögren's syndrome. Rheum. Dis. Clin. N. Am. 18:571-589. [PubMed] [Google Scholar]
- 7.David, V. S., D. C. Harold, S. V. Don, S. H. Matthew, C. R. Peter, D. R. Scott, A. F. Sara, H. L. Edward, and D. S. Leonard. 1996. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD. Ignull mice. J. Exp. Med. 184:2049-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox, R. I., T. Bumol, R. Fantozzi, R. Bone, and R. Schreibery. 1986. Expression of histocompatibility antigen HLA-DR by salivary gland epithelial cells in Sjögren's syndrome. Arthritis Rheum. 29:1105-1111. [DOI] [PubMed] [Google Scholar]
- 9.Hamada, S., and H. D. Slade. 1980. Biology, immunology and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harley, J., M. Reichlin, F. Arnett, E. L. Alexander, W. B. Bias, and T. Provost. 1986. Gene interaction at HLA-DQ enhances autoantibody production in primary Sjögren's syndrome. Science 232:1145-1147. [DOI] [PubMed] [Google Scholar]
- 11.Hattori, M., J. B. Buse, R. A Jackson, L. Glimcher, M. E. Dorf, M. Minami, S. Makino, K. Moriwaki, H. Kuzuya, H. Imura, W. M. Strauss, J. G. Seidman, and G. S. Eisenbarth. 1986. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science 231:733-735. [DOI] [PubMed] [Google Scholar]
- 12.Hattori, M., E. Yamato, N. Itoh, H. Senpuku, T. Fujisawa, M. Yoshino, M. Fukuda, E. Matsumoto, T. Toyonaga, I. Nakagawa, M. Petruzzelli, A. McMurray, H. Weiner, T. Sagai, K. Moriwaki, T. Shiroshi, R. Maron, and T. Lund. 1999. Homologous recombination of the MHC class 1K regions defines new MHC-linked diabetogenic susceptibility gene(s) in nonobese diabetic mice. J. Immunol. 163:1721-1724. [PubMed] [Google Scholar]
- 13.Jespersgaard, C., G. Hajishengallis, Y. Huang, M. W. Russell, D. J. Smith, and S. M. Michalek. 1999. Protective immunity against Streptococcus mutans infection in mice after intranasal immunization with the glucan-binding region of S. mutans glucosyltransferase. Infect. Immun. 67:6543-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang, H. I., H. M. Fei, I. Saito, S. Sawada, S. I. Chen, D. Yi, E. Chan, C. Peebles, T. L. Bugawan, E. A. Erlich, and R. I. Fox. 1993. Comparison of HLA class II genes in caucasoid, Chinese, and Japanese patients with primary Sjögren's syndrome. J. Immunol. 150:3615-3623. [PubMed] [Google Scholar]
- 15.Keyes, P. H., and H. V. Jordan. 1964. Periodontal lesions in the Syrian hamster. III. Findings related to an infectious and transmissible component. Arch. Oral Biol. 9:377-400. [DOI] [PubMed] [Google Scholar]
- 16.Kolenbrander, P. E., and J. London. 1993. Adhere today, here tomorrow: oral bacterial adherence. J. Bacteriol. 175:3247-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolenbrander, P. E. 2000. Oral microbial communities: biofilms, interactions and genetic systems. Annu. Rev. Microbiol. 54:413-437. [DOI] [PubMed] [Google Scholar]
- 18.Kopec, L. K., A. M. Vacca Smith, D. Wunder, L. Ng-Evans, and W. H. Bowen. 2002. Influence of antibody on the structure of glucans. Caries Res. 36:108-115. [DOI] [PubMed] [Google Scholar]
- 19.Liljemark, W. F., and R. J. Gibbons. 1972. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect. Immun. 6:852-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindahl, G., E. Hedfors, L. Klareskog, and U. Forsum. 1985. Epithelial HLA-DR expression and T-cell subsets in salivary gland in Sjögren's syndrome. Clin. Exp. Immunol. 61:475-482. [PMC free article] [PubMed] [Google Scholar]
- 21.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund, T., L. O'Reilly, P. Hutchings, O. Kanagawa, E. Simpson, R. Geavely, P. Chandler, J. Dyson, J. K. Ricard, A. Edwards, D. Kioussis, and A. Cooke. 1990. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-Aβ-chain or normal I-Eα-chain. Nature 345:727-729. [DOI] [PubMed] [Google Scholar]
- 23.Mann, D. L., and H. M. Moutsopoulos. 1983. HLA-DR alloantigens in different subsets of patients with Sjögren's syndrome and in family members. Ann. Rheum. Dis. 42:533-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh, P. D. 1999. Microbiologic aspects of dental plaque and dental caries. Dent. Clin. N. Am. 43:599-614. [PubMed] [Google Scholar]
- 25.Miyazaki, T., M. Uno, M. Uehara, H. Kikutani, T. Kishimoto, M. Kimoto, H. Nishimoto, J. Miyazaki, and K. Yamamura. 1990. Direct evidence for the contribution of the unique I-Anod to the development of insulitis in non-obese diabetic mice. Nature 345:722-724. [DOI] [PubMed] [Google Scholar]
- 26.Moore, P. A., D. I. Bounous, R. L. Kaswan, and M. G. Humphreys-Befer. 1996. Histologic examination of the NOD-mouse lacrimal glands, a potential model for idiopathic autoimmune dacryoadenitis in Sjögren's syndrome. Lab. Anim. Sci. 46:125-138. [PubMed] [Google Scholar]
- 27.Moutsopoulos, H., D. Mann, A. H. Johnson, and T. M. Choused. 1979. Genetic differences between primary and secondary sicca syndrome. N. Engl. J. Med. 301:761-763. [DOI] [PubMed] [Google Scholar]
- 28.Newman, F., J. A. Beeley, and T. W. MacFarlane. 1996. Adherence of oral microorganisms to human parotid salivary proteins. Electrophoresis 17:266-270. [DOI] [PubMed] [Google Scholar]
- 29.Nyvad, B., and M. Kilian. 1987. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand. J. Dent. Res. 95:368-380. [DOI] [PubMed] [Google Scholar]
- 30.Ooshima, T., N. Sumi, A. Izumitani, and S. Sobue. 1988. Effect of inoculum size and frequency on the establishment of Streptococcus mutans in the oral cavities of experimental animals. J. Dent. Res. 67:964-968. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, C. P., S. Yamachika, D. I. Bounous, J. Brayer, R. Jonsson, R. Holmdahl, B. Peck, and M. G. Humphreys-Beher. 1998. A novel NOD-derived murine model of primary Sjögren's syndrome. Arthritis Rheum. 41:150-156. [DOI] [PubMed] [Google Scholar]
- 32.Rudiger, S. G., A. Carlen, J. H. Meurman, K. Kari, and J. Olsson. 2002. Dental biofilms at healthy and inflamed gingival margins. J. Clin. Periodontol. 29:524-530. [DOI] [PubMed] [Google Scholar]
- 33.Rudney, J. D., C. J. Larson, W. F. Liljemark, and K. L. Hickey. 1995. Saliva protein binding to layers of oral streptococci in vitro and in vivo. J. Dent. Res. 74:1280-1288. [DOI] [PubMed] [Google Scholar]
- 34.Rudney, J. D., and R. K. Staikov. 2002. Simultaneous measurement of the viability, aggregation, and live and dead adherence of Streptococcus crista, Streptococcus mutans and Actinobacillus actinomycetemcomitans in human saliva in relation to indices of caries, dental plaque and periodontal disease. Arch. Oral Biol. 47: 347-359. [DOI] [PubMed] [Google Scholar]
- 35.Senpuku, H., K. Matin, S. M. Abdus, I. Kurauchi, S. Sakurai, M. Kawashima, T. Murata, H. Miyazaki, and N. Hanada. 2001. Inhibitory effects of MoAbs against a surface protein antigen in real-time adherence in vitro and recolonization in vivo of Streptococcus mutans. Scand. J. Immunol. 54:109-116. [DOI] [PubMed] [Google Scholar]
- 36.Slattery, R. M., L. Kjer-Nielson, J. Allison, B. Charlton, T. E. Mandel, and J. F. Miller. 1990. Prevention of diabetes in non-obese diabetic I-Ak transgenic mice. Nature 345:724-726. [DOI] [PubMed] [Google Scholar]
- 37.Sreebny, L. M., and W. X. Zhu. 1996. The use of whole saliva in the differential diagnosis of Sjögren's syndrome. Adv. Dent. Res. 10:17-24. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, N., Y. Kurihara, and Y. Kurihara. 1998. Dental caries susceptibility in mice is closely linked to the H-2 region on chromosome 17. Caries Res. 32:262-265. [DOI] [PubMed] [Google Scholar]
- 39.Tao, L., and J. M. Tanzer. 2002. Novel sucrose-dependent adhesion co-factors in Streptococcus mutans. J. Dent. Res. 81:505-510. [DOI] [PubMed] [Google Scholar]
- 40.Van Houte, J., R. J. Gibbons, and S. B. Banghart. 1970. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch. Oral Biol. 15:1025-1034. [DOI] [PubMed] [Google Scholar]
- 41.Wicker, L. S., B. J. Miller, L. Z. Coker, S. E. McNally, S. Scott, Y. Mullen, and M. C. Appel. 1987. Genetic control of diabetes and insulitis in the nonobese diabetic (NOD) mouse. J. Exp. Med. 165:1639-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wicker, L. S., M. C. Appel, F. Dotta, A. Pressey, B. J. Miller, N. H. DeLarato, P. A. Fischer, R. C. Boltz, Jr., and L. B. Peterson. 1992. Autoimmune syndromes in major histocompatibility complex (MHC) congenic strains of nonobese diabetic (NOD) mice. The NOD MHC is dominant for insulitis and cyclophosphamide-induced diabetes. J. Exp. Med. 176:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, P., C. Jespersgaard, L. Lambert-Mallory, J. Katz, Y. Huang, G. Hajishengallis, and S. M. Michalek. 2002. Enhanced immunogenicity of a genetic chimeric protein consisting of two virulence antigens of Streptococcus mutans and protection against infection. Infect. Immun. 70:6779-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]