Abstract
Harnessing control of human β-cell proliferation has proven frustratingly difficult. Most G1/S control molecules, generally presumed to be nuclear proteins in the human β-cell, are in fact constrained to the cytoplasm. Here, we asked whether G1/S molecules might traffic into and out of the cytoplasmic compartment in association with activation of cell cycle progression. Cdk6 and cyclin D3 were used to drive human β-cell proliferation and promptly translocated into the nucleus in association with proliferation. In contrast, the cell cycle inhibitors p15, p18, and p19 did not alter their location, remaining cytoplasmic. Conversely, p16, p21, and p27 increased their nuclear frequency. In contrast once again, p57 decreased its nuclear frequency. Whereas proliferating β-cells contained nuclear cyclin D3 and cdk6, proliferation generally did not occur in β-cells that contained nuclear cell cycle inhibitors, except p21. Dynamic cytoplasmic-nuclear trafficking of cdk6 was confirmed using green fluorescent protein–tagged cdk6 and live cell imaging. Thus, we provide novel working models describing the control of cell cycle progression in the human β-cell. In addition to known obstacles to β-cell proliferation, cytoplasmic-to-nuclear trafficking of G1/S molecules may represent an obstacle as well as a therapeutic opportunity for human β-cell expansion.
In another article in this issue of Diabetes (1), we developed a novel human β-cell G1/S molecule atlas that reveals that essentially all of the G1/S molecules are present not only in the human islet but also in the human β-cell. Surprisingly, although the G1/S molecules are widely considered to be nuclear proteins, we encountered them principally in the cytoplasm, where they presumably would be unable to direct cell cycle progression. The only G1/S molecules encountered in the nucleus of the human β-cell were cell cycle inhibitors pRb, p57, and, variably, p21. In contrast, all of the cell cycle–activating cyclins and cdks were restricted to the cytoplasm. These studies were performed in quiescent human β-cells and shed no light on the functional activities of G1/S molecules during cell cycle progression.
In this report, we explored whether G1/S molecules might be able to be induced to shuttle from the cytoplasm to the nuclear compartment in association with activation of cell cycle progression. We found that several cell cycle inhibitors and activators do actively traffic from the cytoplasm to the nucleus in association with activation of proliferation. These results lead to a substantially altered model of G1/S trafficking and its control in the human β-cell.
RESEARCH DESIGN AND METHODS
Human cadaveric and rat islets.
One hundred sixty-four different cadaveric islet preparations were used for these studies. The demographics and sources of the islets are described in another report (1). Dispersal of the human islets was performed as described in detail previously (1–5). Rat islets were isolated from 2- to 3-month-old Sprague-Dawley rats, dispersed, and cultured as detailed previously (5,6). Rat studies were approved in advance by the University of Pittsburgh Institutional Animal Care and Use Committee.
Adenovirus production and transduction.
Adenovirus preparation has been described previously (1). The efficiency of adenoviral transduction, assessed using β-galactosidase and insulin costaining of human islets transduced with Ad.lacZ, was (mean ± SEM) 65.1 ± 3.0, 67.9 ± 2.5, and 75.7 ± 2.8% at 24, 48, and 72 h after transduction, respectively. In addition, to prepare a green fluorescent protein (GFP)-tagged cdk6 adenovirus, human cdk6 cDNA was subcloned into pcDNA3.1/CT-GFP plasmid (Invitrogen, Carlsbad, CA) using a GFP fusion TOPOTA expression kit (Invitrogen), which places the GFP at the C-terminus of cdk6. This was subcloned into the adenovirus shuttle vector, pACCMV, and adenovirus was prepared as described (1–7).
Immunocytochemistry.
Islets were dispersed to single cells, fixed, and labeled as described (1–7). For studies with proliferating conditions, dispersed islets were transduced with either Ad.LacZ or Ad.cdk6 plus Ad.cyclin D3 (100 multiplicity of infection) for 2 h, cultured for 24, 48, and 72 h (as described in the figures), and immunolabeled using antisera as described in the Supplementary Table 1 of our accompanying original article (1). Labeled cells were visualized using laser confocal microscopy. Each experiment shown is representative of 3–6 human islet preparations.
Immunoblotting.
Immunoblotting was performed as described (1–7). Antibodies used to detect the G1/S molecules are described in detail in Supplementary Table 1 of accompanying article (1). Each experiment shown is representative of 3–6 human islet preparations.
Live cell imaging.
Rat insulinoma cells (Ins1 832/13) were washed in PBS twice and trypsinized for 5 min. Complete medium (RPMI medium; Gibco, Grand Isle, NY) containing 5.5 mmol/L glucose, 1% penicillin and streptomycin, 10% FBS, 10 mmol/L HEPES, 2 mmol/L glutamine, 1 mmol/L sodium pyruvate, and 50 μmol/L β-mercaptoethanol was added and a suspension of 200,000 cells was plated on a glass-bottom microwell dish (MatTek, Ashland, MA). The cell suspension was transduced with 100 multiplicity of infection of Ad.cdk6-GFP for 2 h. Human islets (200 islet equivalents) were dispersed as described and plated on a glass-bottom microwell dish (MatTek) and were transduced for 2 h with 100 multiplicity of infection Ad.cdk6-GFP. The transduction was stopped by adding 1 mL complete medium to the Ins1 cells or the dispersed human islets. Transduced Ins1 cells or dispersed human islets were imaged 24 h after infection using a Nikon A1 Confocal Live Cell System and the NLS-Element software (Nikon, Melville, NY).
Statistics.
Statistical analysis for Figs. 4 and 5 was performed using one-way ANOVA with Bonferroni post hoc correction. Student paired two-tailed t test was used in Fig. 2A and C. All values are expressed as means ± SEM. P ≤ 0.05 was considered significant.
FIG. 4.
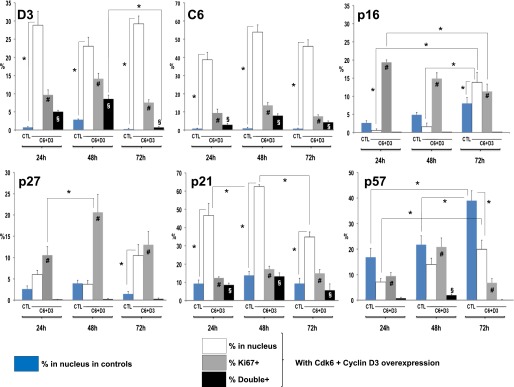
Quantification of changes in cyclin D3, cdk6, p16, p27, p21, and p57 with Ki67 in human β-cells in response to expression of cdk6 and cyclin D3. These data represent the quantification data in Fig. 3 from different experiments using islets from four to six different donors for each cell cycle molecule at 24, 48, and 72 h after cdk6/cyclin D3 transduction. The blue bars indicate the percent nuclear localization of the six molecules shown in insulin-positive (β) cells from control, nontransduced, dispersed human islet cells. The white bars represent the percent of insulin-positive (β) cells that contain the indicated G1/S molecule in question in the nucleus at 24, 48, and 72 h when cdk6/cyclin D3 are expressed. The gray bars represent the percent of Ki67+/insulin-positive cells. The black bars represent insulin-positive cells that are doubly positive for both Ki67 and nuclear presence of the G1/S molecule indicated. Bars indicate mean ± SEM. *P < 0.05 for differences shown by the solid lines. #P < 0.05 for Ki67+ β-cells (gray bars) vs. Ki67+ untransduced β-cells at 24, 48, and 72 h. §P < 0.05 vs. untransduced β-cells. Unless a symbol is shown, the changes are not significant. The range of Ki67 induction (gray bars) is large and spans ∼10–20% of cells, in accord with previous studies. This variability likely reflects differences among human islet preparations, adenoviral transduction efficiency, and other variables. CTL, control.
FIG. 5.
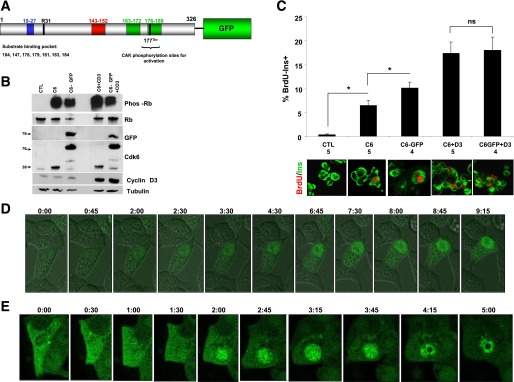
The cdk6 cytoplasmic-to-nuclear trafficking with live cell imaging. A: Schematic representation of the Ad.cdk6-GFP construct used in the live cell imaging experiments, with a GFP tag at the C-terminus. Numbers above indicate amino acid residues. The blue box designates the ATP-binding domain, the black box designates the INK4-binding domain, the red box designates the catalytic loop, and the two green boxes designate the activation loops. The cdk6 can be phosphorylated on Thr177 by cdk-activating kinase (CAK). Key amino acids in the substrate-binding pocket are indicated. B: Representative immunoblots of human islets transduced with Ad.lacZ (control [CTL]), Ad.cdk-6-GFP, nontagged Ad.cdk6, with or without Ad.cyclin D3, showing phosphorylated Rb (phos-Rb), total Rb (Rb), GFP, cdk6, and cyclin D3, and tubulin as loading control. Small numbers on the left indicate molecular weight markers. These studies indicate that the Ad.cdk6-GFP is biologically active because it can phosphorylate pRb. C: BrdU-positive and insulin-positive cells as a percentage of total insulin-positive cells. Representative pictures of BrdU (red) and insulin (green) in the conditions are shown at the bottom of the panel. Dispersed human islets were transduced with control Ad.lacZ (CTL) or with adenoviruses encoding cdk6 (C6), cdk6-GFP (C6-GFP), cdk6 and cyclin D3 (C6+D3), or cdk6-GFP and cyclin D3 (C6-GFP+D3). The numbers below each bar indicate the numbers of different human islet preparations studied. Bars indicate mean ± SEM. *P < 0.05; ns, nonsignificant. These studies confirm that the Ad.cdk6-GFP is biologically active. D: Photomicrographs derived from live cell imaging of Ins1 cells transduced with cdk6-GFP (the full movie available as Supplementary Movie 1). E: Photomicrographs derived from live cell imaging of dispersed human islet cells transduced with cdk6-GFP. The numbers above each picture in D and E indicate the time from the beginning of the movie (the full movie available asSupplementary Movie 2). Ins, insulin.
FIG. 2.
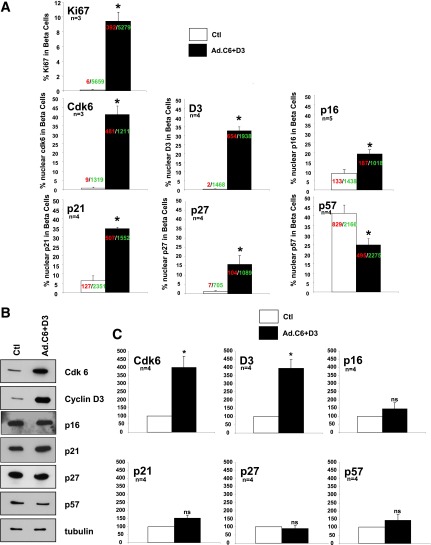
Quantification of the nuclear translocation of the G1/S molecules and their absolute expression in human β-cells in response to overexpression of cdk6 and cyclin D3. A: White bars represent control dispersed human β-cells and black bars represent those transduced with Ad.cdk6+cyclin D3. Each panel represents observations for the G1/S molecule indicated. The numbers shown within or above the bars indicate the number of insulin-positive cells showing nuclear G1/S molecule (red) and the total number of insulin-positive (green) cells counted. Bars indicate mean ± SEM. *P < 0.05 vs. control. Quantification of Ki67+ and insulin-positive cells as a function of total insulin-positive cells. B: Representative immunoblots of cdk6, cyclin D3, p16, p21, p27, and p57 from islet preps used for C. C: Densitometric quantification of four immunoblots for cdk6, cyclin D3, p16, p21, p27, and p57. *P < 0.05 vs. control; ns, nonsignificant vs. control. These panels make the point that when p16, p21, and p27 increase in nuclear intensity, there is no overall increase in the amounts of these three molecules, and the inverse is true for p57, suggesting that the changes in subcellular localization observed are not simply a reflection of a generalized increase in p16, p21, p27, and p57, but likely reflect changes in their nuclear trafficking or stability. Ctl, control.
RESULTS
Induction of proliferation leads to nuclear translocation of some, but not all, G1/S molecules.
In the absence of mitogenic growth factors or nutrients that are able to induce robust human β-cell proliferation, we have used adenoviral expression of D-cyclins, cyclin E, or cdk2, cdk4, and cdk6 to drive human β-cell proliferation (2–4,7). For the current studies, we selected the combination of Ad.cdk6 plus Ad.cyclin D3 because this combination yielded the highest rates of BrdU and Ki-67 incorporation (3). We asked whether proliferation associated with adenoviral expression of cyclins and cdks might influence the subcellular localization of G1/S components.
As previously reported (3,4), the expression of Ad.cdk6 and Ad.cyclin D3 led to β-cell proliferation and also the nuclear appearance of these two molecules (Fig. 1A). Specifically, after transduction with Ad.cdk6/cyclin D3, Ki67 labeling increased from 0.1% to ∼9.4% of β-cells; the percent of β-cells that contained nuclear cdk6 increased from 0.8 to 46.1% and the percent of β-cells that contained nuclear cyclin D3 increased from 0.3 to 29.2% (Fig. 2A).
FIG. 1.
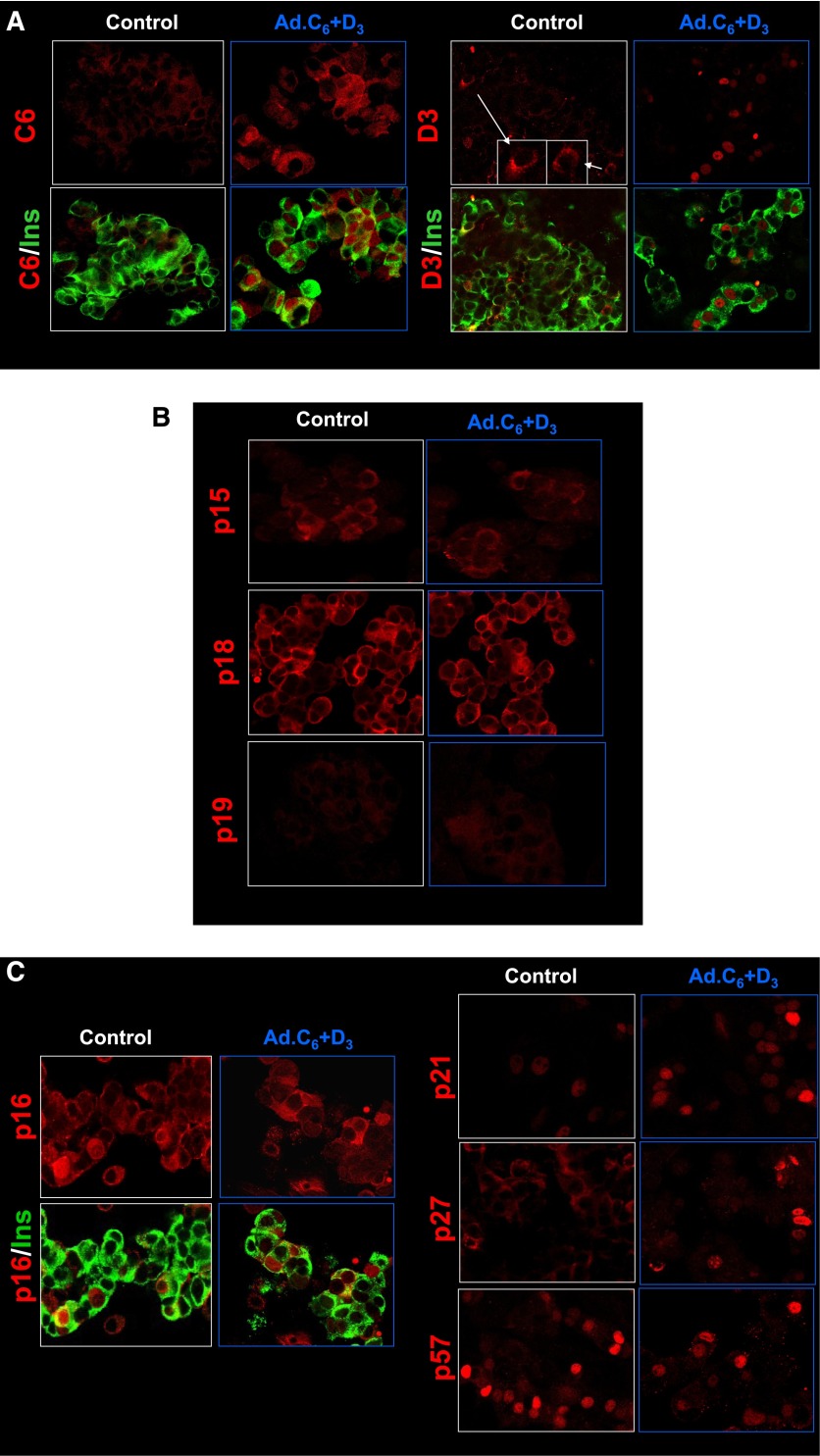
Nuclear translocation of the cdk6, cyclin D3, p16, p21, and p27, but not p15, p18, p19, or p57, in human β-cells in response to expression of cdk6 and cyclin D3. A: Dispersed human islets were transduced with control adenovirus (control, white frames) or with Ad.cdk6 and Ad.cyclin D3 (Ad.C6+D3, blue frames). Immunolabeling for cdk6 (four left panels) and cyclin D3 (four right panels) are shown in red, and insulin (Ins) is shown in green. Note that the nuclear frequency of both cdk6 and cyclin D3 increase with their adenoviral expression, confirming a previous report (4). B: In contrast to cdk6 and cyclin D3, there is no change in the cellular location of p15, p18, or p19. C: In contrast, p16, p21, p27, and p57 alter their subcellular distribution in response to cdk6/cyclin D3. These experiments are representative of three to five separate human cadaver islet preparations. In some panels, insulin staining is removed to allow visualization of the relevant G1/S molecule in the cytoplasm; however, in all cases, the cells shown are also insulin-positive as shown for cdk6 and p16.
Exploring whether other G1/S molecules altered subcellular location in response to induction of proliferation, three INK4 family members were found to be not altered: p15, p18, and p19 all remained cytoplasmic (Fig. 1B). However, p16, p21, and p27 all shifted into the nuclear compartment (Figs. 1C and 2A). In quantitative terms, p16, which was nuclear in only 8.4% of β-cells under basal conditions, became nuclear in 16.1%. Similarly, p21, which was nuclear in 7.9% of quiescent β-cells, became nuclear in 35.6%; p27, which had been nuclear in 1.9% of quiescent β-cells, became prominently nuclear in 11.2%. Surprisingly, p57, which had been so predominantly nuclear in quiescent cells (42.1%), became less prominent in the nucleus (23.8%) with induction of proliferation (Figs. 1C and 2A).
To determine whether the changes in nuclear abundance of p16, p21, and p27 and the decrease in p57 were a result of changes in the total abundance of these molecules, we examined their relative abundance with or without expression of cdk6 and cyclin D3. As shown in Fig. 2B and C, although these molecules shifted location with proliferation, there were no changes in their absolute abundance. Collectively, these results suggest that p16, p21, p27, and p57 markedly alter their subcellular localization, but not their overall abundance, in response to induction of proliferation, with p16, p21, and p27 displaying a pronounced increase in their nuclear frequency, whereas nuclear p57 declined in nuclear frequency.
Human β-cell proliferation broadly correlates with nuclear cdks and cyclins and inversely with nuclear cell cycle inhibitors.
We next studied G1/S molecule nuclear presence versus proliferation (Ki67) as well as the 72-h time courses of translocation of cdk6, cyclin D3, p16, p21, p27, and p57. Ki67 was selected rather than BrdU because it reflects cell cycle progression at the time of labeling, whereas BrdU incorporation reflects both active and past proliferation. Examples of these studies are shown in Fig. 3, and their quantifications are shown in Fig. 4. In Fig. 4, the blue bars represent nontransduced control β-cells at 24, 48, and 72 h after dispersal and plating. The subsequent three bars reveal events in β-cells transduced with Ad.cdk6 plus Ad.cyclin D3 at these same time points (white indicates the percent of β-cell nuclei that contain the G1/S molecule in question; gray indicates the percent of β-cells containing Ki67 in their nuclei; and black indicates the percent of β-cells that are doubly positive for Ki67 plus nuclear presence of the G1/S molecule in question).
FIG. 3.
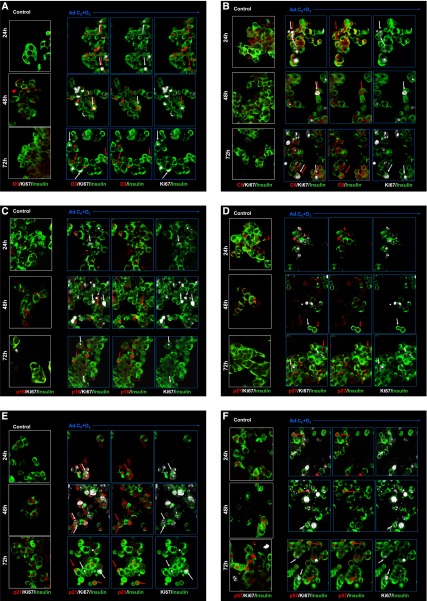
Time courses of subcellular localization of G1/S molecules and Ki67 in human β-cells in response to expression of cdk6 and cyclin D3. Dispersed human islets were transduced with control adenovirus (control, white frames) or with Ad.cdk6 and Ad.cyclin D3 (Ad.C6+D3, blue frames). Cells were fixed 24, 48, or 72 h after transduction and immunolabeled for the G1/S molecule indicated, cyclin D3 (A), cdk6 (B), p16 (C), p27 (D), p21 (E), or p57 (F). White arrows indicate examples of proliferative β-cells as assessed by Ki67. Red arrows illustrate examples of the nuclear G1/S molecule in question. Double red and white arrows indicate colocalization of a nuclear G1/S molecule with Ki67 in insulin-positive cells.
The cyclin D3 (Figs. 3A and 4) in control (blue bar) β-cells appears in the nucleus of only 0.7% of β-cells at 24 h, increases to 2.8% at 48 h, and declines to 0.3% at 72 h. With Ad.cdk6 plus cyclin D3 transduction, 25–30% of β-cells display nuclear cyclin D3 at all three time points (white bars), and the percent of Ki67+ β-cells initially increases from 9.7 to 14.1% and then decreases to 7.5% (gray bars). Interestingly, cyclin D3 colocalizes in the nuclei with Ki67 in 5.0, 8.6, and 0.7% of β-cells at 24, 48, and 72 h (black bars), suggesting that it may enter the nucleus early, partner with cdk6, activate proliferation, and then may be exported, degraded, or both by 72 h; cdk6 reveals a similar biphasic pattern, suggesting that it enters the nucleus transiently and then declines through one of these mechanisms (Figs. 3B and 4).
For p16 (Figs. 3C and 4), in control nontransduced β-cells (blue bars), its nuclear abundance increases with time, increasing from 2.7 to 8.0% between 24 and 72 h. With activation of proliferation, nuclear p16 increases to levels above baseline and above controls by 72 h. Significantly, p16 is never in the nucleus of a β-cell that is also Ki67+ (black bars) at any of the three time points, suggesting that nuclear p16 is incompatible with proliferation.
p27 (Figs. 3D and 4) reveals a pattern that is generally similar to p16, with the principal observations being that it is only occasionally (1.5–4.0%) nuclear in control β-cells, it markedly increases with induction of proliferation at 72 h (10.5%), and it never is in the nucleus of β-cells that are proliferating (black bars), suggesting that its nuclear presence also may be incompatible with proliferation.
p21 (Figs. 3E and 4) reveals a different pattern. It does not change substantially in control β-cells (blue bars) over time, but its nuclear abundance (white bars) increases dramatically in transduced β-cells at every time point, ranging from 9.4 to 13.9% in controls and to 34.8 to 62.5%. Additionally, p21 is the only cell cycle inhibitor that coincides with Ki67 in the nucleus of β-cells, and it may do this in a time-dependent manner, increasing from 8.6 to 13.3% of β-cells and then declining to 5.7% at 24, 48, and 72 h, respectively. These observations are compatible with the assembly/chaperon and nuclear translocation functions of p21 in other cell types.
p57 (Figs. 3F and 4) reveals a different pattern. It is the only INK4 or KIP/CIP molecule that is present in the nuclei of large numbers of control β-cells and increases with time in culture (blue bars). Also, although its nuclear abundance increases with induction of proliferation at every time point (white bars), it is always lower in transduced β-cells than in control β-cells. Like p16 and p27, it never is present in the nuclei of proliferating β-cells (black bars).
Confirmation of G1/S molecule cytoplasmic-to-nuclear trafficking with live cell imaging.
The preceding results suggest a model in which G1/S molecules may be able to traffic into (cdk6, cyclin D3, p16, p21, p27) or out of (cyclin D3, p57) the nucleus of β-cells in a manner that is associated with proliferation. To directly confirm whether G1/S molecule trafficking can occur, an adenovirus in which human cdk6 was coupled to green fluorescent protein (GFP) (Fig. 5A) was prepared so that trafficking could be directly observed in real time, if it occurred. Ad.cdk6-GFP was selected because cdk6 can colocalize with Ki67 in human β-cells (Figs. 3B and 4), and because Ad.cdk6 is capable of driving human β-cell proliferation in vitro and in vivo and enhances human β-cell engraftment in vivo (1–3). To confirm that the Ad.cdk6-GFP construct was fully biologically active, it was compared with an adenovirus expressing wild-type untagged human cdk6 (Ad.cdk6), examining their relative levels of expression and their abilities, with or without Ad.cyclin D3, to induce pRb phosphorylation and human β-cell proliferation. These studies confirmed the efficiency of expression, bioactivity, and potency, as well as induction of pRb phosphorylation (Fig. 5B) and β-cell proliferation (Fig. 7C), of the Ad.cdk6-GFP as compared with wild-type cdk6.
FIG. 7.
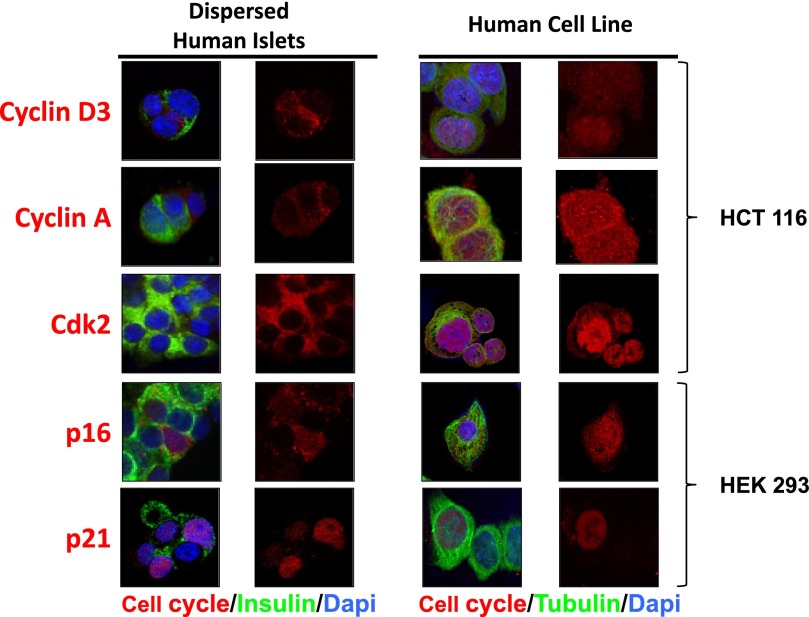
Comparison of G1/S molecule subcellular localization in human β-cells and transformed human cell lines. Left two columns show dispersed human islets immunolabeled for cyclin D3, cyclin A, cdk2, p16, and p21. Right two columns show immunolabeling for the same five G1/S molecules in HCT116 human colon cancer cells or HEK293 human embryonic kidney cells. Culture and fixation conditions were identical (4% paraformaldehyde for 15 min), as were antisera (as shown in Supplementary Table 1 of accompanying article) (1). Each image is representative of three different human islet preparations or cell line experiments. Note that cyclin D3, cyclin A, cdk2, and p16 appear cytoplasmic in quiescent human β-cells, but each is observed in the nucleus of HEK293 or HCT116 cells. p21, which may be nuclear in some human β-cells, also can be nuclear in HEK293 cells. These studies are compatible with the concepts that these G1/S molecules can be readily observed in the nucleus, when present, using the reagents and conditions used, and that their localization generally correlates with quiescent vs. proliferative state.
The rat insulinoma line, Ins1, and dispersed human islet cells were transduced with the Ad.cdk6-GFP construct and live cell imaging was performed beginning 24 h after transduction. As can be seen in the live cell time-lapse images in Fig. 5D and E (also seen in video format, see Supplementary Movies 1 and 2), cdk6-GFP can be observed clearly in the cytoplasmic compartment initially, and then it shifts into the nuclear compartment with time. That this is not simply the natural progression of cytoplasmic translation of what will ultimately be a protein that is then targeted to the nuclear compartment is evident from the observations in Figs. 1–4 (1,3,4) that cdk6 is present in the cytoplasm but does not appear in the nuclear compartment in quiescent cells. Thus, these studies document in dynamic terms that cdk6 is a principally cytoplasmic protein in quiescent nonproliferating human β-cells but it is able to shuttle to the nucleus in association with proliferation.
The quiescent-to-proliferative G1/S molecule shift applies to rat β-cells.
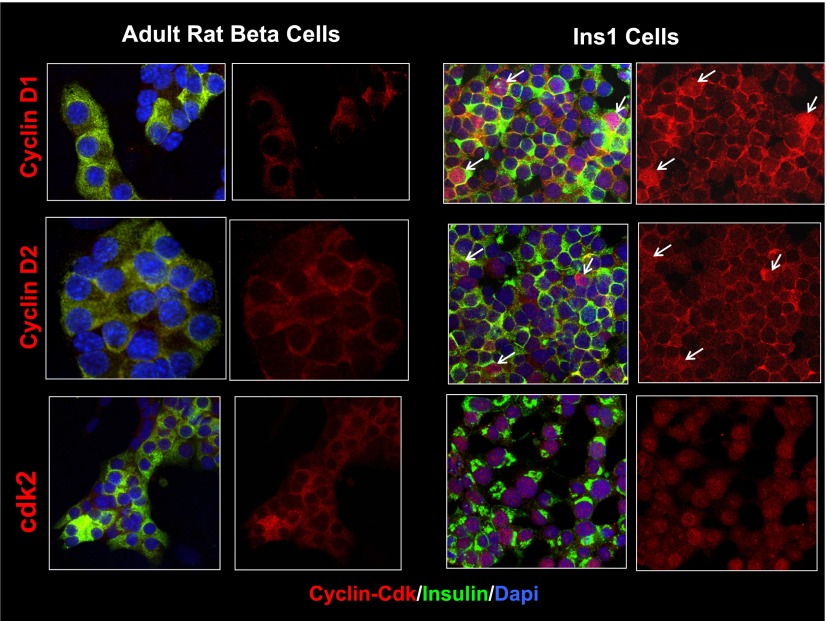
To determine whether the cytoplasmic G1/S molecule paradigm is restricted to human β-cells or if it might be a feature of rodent β-cells as well, we examined cyclin D1, cyclin D2, and cdk2 in rat islets (Fig. 6) and compared their localization to that observed in the rat insulinoma cell line, Ins1 832/13. We selected cyclin D1 and cdk2 because they are present in the cytoplasm of human islets, and we chose cyclin D2 because it is the most important D-cyclin in rat islets and therefore comparable with cyclin D3 in human islets. As can be seen in the figure, these three molecules are most intensely apparent in the cytoplasmic compartment in rat β-cells. However, using the same antisera, the same fixation methods, and the same species, these three molecules are readily apparent in the nuclei of Ins1 cells.
FIG. 6.
Comparison of distribution of cyclin D1, cyclin D2, and cdk2 in rat β-cells and rat Ins1 cells. Left two columns show the subcellular location of cyclin D1, cyclin D2, and cdk2 in primary cultures of dispersed rat pancreatic β-cells. Right two columns show the subcellular location of the same three molecules in proliferating Ins1 cells. White arrows show example of Ins1 cells in which cyclins D1 and D2 appear in the nucleus. In each pair of columns, the left column displays the merged images of insulin (green), the cyclin/cdk of interest (red), and the nuclear marker DAPI (blue), whereas the right column shows the cyclin/cdk separated from insulin and DAPI images. Each experiment is representative of three different rat islet preparations or Ins1 experiments. Rat islets were isolated and dispersed as detailed previously (5,6) and both rat islets and Ins1 cells were cultured for 72 h in RPMI 1640 with 10% FBS before fixing with 4% paraformaldehyde, as for the human islet samples. The primary antisera were the same used for human islets (in the Supplementary Table of accompanying article) (1). Note that cyclin D1, cyclin D2, and cdk2 are cytoplasmic and nonnuclear in quiescent rat β-cells as they are in human β-cells, but each can be readily detected in the nucleus of proliferating Ins1 cells. These studies indicate that cyclin D1, cyclin D2, and cdk2 can be easily observed in the nucleus, when present, using the reagents and conditions used, and that their localization is correlated with quiescent vs. proliferative state.
Comparison of cytoplasmic human β-cell G1/S molecules in transformed human cell lines.
To determine whether the molecules that appeared cytoplasmic in nonproliferating human β-cells could be observed in the nucleus of transformed proliferating human cell lines using the same antisera and same fixation methods in a human homologous system, we re-examined cyclin D3, cyclin A, cdk2, p16, and p21 in human β-cells, and we compared them with the human colonic cancer cell line, HCT116, and with HEK293 human embryonal kidney cells. As can be seen in Fig. 7, in human β-cells, each of the molecules showed the same principally cytoplasmic pattern described. In contrast, in HCT116 and HEK293 cells, cyclin D3, cyclin A, cdk2, and p16 could be readily observed in the nuclei of many cells; p21, which is nuclear or cytoplasmic in human β-cells, also was observed in both nuclei and cytoplasm in HEK293 cells.
DISCUSSION
Most previous models of human β-cell replication assumed that all G1/S molecules are present in the nuclear compartment, as depicted in Fig. 8A, awaiting activation (1–21). Instead, in quiescent adult human β-cells, most are found in the cytoplasm, as shown in the model in Fig. 8B (1). The only three abundant nuclear molecules in quiescent human β-cells are the following cell cycle inhibitors: pRb; p57, and, in some cases, p21 (1). All of which would be anticipated to discourage cell cycle progression. Further, the quiescent human β-cell model depicted in Fig. 8B is not static, but one that is active, motile, fluid, and characterized by shuttling of G1/S molecules to and from the cytoplasm into the nuclear compartment in association with β-cell proliferation, as shown in the models in Fig. 8C and D. These models demonstrate that several G1/S molecules can be induced to traffic into the nucleus in association with induction of proliferation. Further, the observations suggest that this trafficking may be selective, with some G1/S molecules engaging in nuclear trafficking (e.g., cdks, cyclins, p16, p21, p27, p57), whereas others may not (p15, p18, p19), and also suggest a directionality to this trafficking, with some molecules entering the nucleus (cyclins, cdks, p16, p21, p27) and some others exiting, degrading, or otherwise disappearing (cdk6, cyclin D3, p57).
FIG. 8.
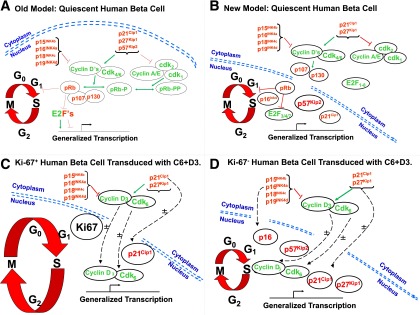
Previous and revised models of the G1/S cell cycle transition regulation in human β-cells. The red arrows indicate inhibition, the green arrows indicate activation, and the dotted black lines indicate trafficking. A: A previous model of G1/S control in human β-cells (2–13) in which most cell cycle molecules were assumed to be present in the nucleus. B: A revised model for G1/S molecule distribution in quiescent human β-cells: most G1/S molecules are cytoplasmic, and the only other frequently nuclear molecules are the cell cycle inhibitors, pRb, p57, accompanied in occasional β-cells by p16, or, in some preparations, p21. C: G1/S molecules in Ki67+ human β-cells that recently have proliferated or are proliferating. The larger G1/S circle, as compared with the others, indicates that these cells have entered the cell cycle. After transduction, cdk6 and cyclin D3 transit to the nuclear compartment in association with p21. D: G1/S molecules in human β-cells that remain Ki67− (fail to proliferate) despite transduction with cdk6 and cyclin D3. The cdk6 and cyclin D3 still enter the nucleus in many β-cells but are accompanied variably by p16, p21, p27, or p57, all or some of which may attenuate the induction of proliferation. The ± symbols in C and D indicate that these movements may or may not occur in a given β-cell after cdk6–cyclin D3 transduction.
The evidence for G1/S molecule cytoplasmic-nuclear trafficking seems clear. Specifically, cdk6 and cyclin D3 clearly become more abundant in the nucleus, as we have shown previously for cyclins D1 and D2, when overexpressed (1–4). In parallel, the cell cycle inhibitors p16, p21, and p27 also become more abundant in the nuclear compartment, without an overall increase in the amounts of these molecules. Conversely, the nuclear abundance of p57 declines, without an overall change in the amount of p57. In contrast yet again, p15, p18, and p19 reveal the following third pattern: no shift in intracellular location in response to proliferation. The most unequivocal evidence for trafficking was observed for cdk6-GFP, which is absent from the nuclear compartment under basal conditions but can be observed trafficking into the nucleus using live-cell GFP imaging approaches in Ins1 and human islet cells.
Superficially, the mechanism of proliferation seems obvious; cdk6 and cyclin D3 enter the nucleus, phosphorylate resident pRb, and cell cycle progresses. On reflection, however, the models raise fundamental questions about the control of trafficking of cell cycle molecules. In a widely held model (11,12,22–27), cdk6, cdk4, and the D-cyclins lack a nuclear localization signal (NLS) and require assembly in the cytosol by p21 and p27 (and perhaps p57) after their translation. Paradoxically, p21 and p27 serve not only as chaperones but also as members of cyclin–cdk inhibitor complexes. These complexes are guided into the nuclear compartment by the NLS that is present in both p21 and p27. This NLS is in proximity to phosphorylation sites, suggesting that kinases may phosphorylate p21 or p27 and activate the nuclear translocation of these molecules and their cdk–cyclin cargo. In this model, the abundance of p21 and p27 “titrates” proliferation, such that complete lack of p21 and p27 causes cell cycle arrest because cyclins/cdks cannot enter the nucleus and, conversely, marked upregulation of p21 and p27 causes cell cycle arrest because of their ability to block cyclin A/cyclin E/cdk1/cdk2 activity. Proliferation only occurs when p21 and p27 are present in the correctly titrated concentration. Of course, most of these data are derived from rapidly proliferating genetically modified mouse embryonic fibroblasts and cancer cell lines, in which all of these cyclins and cdks as well as CIPs/KIPs are nuclear (22–27). These events may be specific to cell type.
With this background, the data summarized in Figs. 3 and 4 may indicate that among the cell cycle inhibitors studied, p21 is the one most commonly present in the nucleus of Ki67+ β-cells and therefore may serve the chaperone/nuclear translocator function in the human β-cell. Conversely, since p27 and p57, like p16, essentially never appear in the nucleus of Ki67+ β-cells, they may serve a conventional cell cycle inhibitor function. A similar trafficking-mediated proliferation-inhibitory function also has been suggested for p21 (28). Further, the observations that p16, p21, p27, and p57 all increase in nuclear frequency or abundance with the induction of proliferation (although for p57, always lower than in control cells) may reflect chaperone/nuclear translocator functions, e.g., for p21, or an overall reactive increase in inhibitory cell cycle “tone” in cells driven to proliferate. This model raises the question of what is restraining the cdks and cyclins, the CIP/KIP family, and the INK4 family in the cytoplasmic compartment of quiescent adult human β-cells and preventing them from entering the nucleus? Perhaps they are assembled in the cytoplasm by the CIP/KIP molecules, but for some reason (e.g., possibly lack of peri-NLS phosphorylation) these complexes are constrained to the cytoplasm and cannot transport the cdk/cyclins into the nucleus. Perhaps the INK4s or other as-yet-unidentified cytoplasmic molecules anchor the cdk/cyclins in the cytoplasm. Unraveling these issues will require silencing the CIPs/KIPs and INK4s to see which, if any, influences trafficking of cdks/cyclins or proliferation. It also will require immunoprecipitation and proteomic studies to identify additional molecules that are bound to the cdks/cyclins. These studies will be particularly challenging given the complexity of human islet cellular composition (2,29,30), the relative paucity of β-cells within intact islets, and the lack of availability of large numbers of pure populations of human β-cells for study.
As good working models should, these models raise many additional questions. For example, we have not studied the trafficking of all potential G1/S molecules. Do E2Fs or late G1/S molecules (cyclins A/E, cdks1/2) or p107/p130 also traffic to the nucleus when proliferation is activated? Does pRb exit the nucleus? If so, then what orchestrates all of this? Or, if the E2Fs remain in the cytoplasm, then how can the cell cycle become active? Or, as suggested by some (31,32), are E2Fs dispensable for proliferation? As another example, Figs. 3 and 4 suggest that cdk–cyclin D complexes activate proliferation in certain cells that lack cell cycle inhibitors in the nuclear compartment, but what decides whether a given β-cell does or does not contain nuclear cell cycle inhibitors? And, if cdk6 and cyclin D3 are present in the nucleus of 30–40% of transduced cells (Figs. 2–4), then why are all of these cells not proliferating? Of course, we could not study the subcellular localization and partnering of every G1/S molecule in a single β-cell, so that knowledge of the precise molecular complexes and the stoichiometry of their components within the cytoplasm or nucleus of a given β-cell remains incomplete. Finally, these studies were performed using adult human β-cells, which are recalcitrant to proliferation. Because replication is believed to occur at greater rates in fetal and neonatal human β-cells (17,20,33), it will be informative to develop a model such as that shown in Fig. 8 using these types of islets.
The observation that G1/S molecules that are cytoplasmic in primary rat β-cells can be nuclear in proliferating rat Ins1 insulinoma cells (Fig. 6) is consonant with the concept that the cytoplasmic-to-nuclear translocation occurs in association with activation of proliferation. Coupled with data regarding intact human pancreas in another article (1), it is further suggest that the subcellular localization to the cytoplasm in human β-cells is not an artifact of fixation, antiserum choice, brain death, or islet isolation, because all of these variables can be controlled for in the rodent models. The cytoplasmic G1/S quiescence model may apply more broadly beyond the human or rodent β-cell, extending to quiescent differentiated mammalian cells in general. Thus, in addition to the data of our other article documenting cytoplasmic localization of G1/S molecules in differentiated quiescent non–β-cells, the concept is further supported by Fig. 7, contrasting quiescent human β-cells to proliferating human HEK293 and HCT116 cancer cells immunolabeled under identical conditions.
Nonetheless, this work has important limitations. First, we have used cdk6 and cyclin D3 to force proliferation, an artificial approach. Although it would have been more “physiological” to have used growth factors or nutrients to induce proliferation, no growth factors or nutrients that can induce robust proliferation in adult human β-cells have been identified, so the cdk/cyclin approach represents the only currently feasible approach. In addition, although it seems reasonable to assume that G1/S molecules traffic into and out of the nucleus, most of the data provided are descriptive and not functional or mechanistic, with the exception of the live cell imaging for cdk6. Along these lines, whereas several G1/S molecules appear in the nucleus and are less frequent at later time points, we have not documented that this nuclear diminution is a result of nuclear exit, intranuclear degradation, or another process, all of which are known to occur in other systems (26,27,34–38). Also, it also should be emphasized that the majority of studies were performed in isolated, dispersed, cultured, cadaveric β-cells, all conditions that might alter the normal distribution, function, and immunodetection of cell cycle molecules. This was unavoidable because the usual harsh fixation methods used for intact pancreas pathology specimens, e.g., long-term 10% formalin, prevented immunolabeling for most of the G1/S molecules studied. However, we were able to confirm the subcellular location in intact human pancreas of pRb p18, p57, p107, and p16, i.e., in five of five cases in which immunolabeling of intact pancreas was possible (1). Further, the data are complimented by similar data in nonproliferating rodent β-cells (Fig. 7) (36,37), so these observations seem firm. Also, whereas we use the broad term “G1/S” throughout, and although many of these same molecules participate in the G0/G1 transition, whether there are additional molecules that may “gate” the G0/G1 interface in β-cells is unknown. Importantly, we also have used terms such as “proliferation” and “replication” advisedly, using a surrogate for β-cell proliferation, Ki67, rather than authentic expansion of β-cell numbers. Unfortunately, this is unavoidable because no laboratory has developed techniques that provide unequivocal evidence of productive human β-cell expansion in vitro or in vivo (14). In a related manner, we variously use the terms “quiescent,” “senescent,” and “irreversible” interchangeably in the context of nonproliferating human β-cells. Although some would argue that these are different states, distinguishing among them in cadaveric human β-cells is difficult or impossible. Another important consideration is that although we have demonstrated an association between nuclear-cytoplasmic trafficking and cell cycle activation, we have not proven in formal terms that this is required for cell cycle entry in the human β-cell. It also is important to emphasize that whereas it is likely that G1/S molecule trafficking is one important regulatory process involved in β-cell cycle entry, it is only one of many important mechanisms, with other examples being phosphorylation and dephosphorylation of key substrates, transcriptional and posttranscriptional induction, or stabilization/destabilization of cyclins/cdks, or comparable reductions in cell cycle inhibitors, to name a few. Finally, although we believe we have controlled as well as reasonably possible for immunocytochemical/histochemical variation and error, antibody specificity can be troublesome. Thus, it must be emphasized that the models in Fig. 8 are just that, working models that can be used for confirmation, modification, correction, extension, and hypothesis-generation; they likely will evolve further with time.
In summary, these studies provide three new models of human β-cell G1/S cell cycle control, one in quiescence (Fig. 8B), one for proliferating β-cells induced by cdk6/cyclin D3 (Fig. 8C), and one for β-cells that fail to proliferate despite cdk6/cyclin D3 expression (Fig. 8D). The models should be useful for hypothesis-generation and provide new questions and potential therapeutic approaches to human β-cell expansion, such as high-throughput drug screens examining nuclear translocation or kinome profiling to identify kinases that phosphorylate key cell cycle inhibitors and induce their translocation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the Beta Cell Biology Consortium through National Institutes of Health grants U-01 DK 089538, R-01 DK55023, R56 DK065149, and T32-07052, by Juvenile Diabetes Research Fund grants 1-2008-39 and 34-2008-630, by American Diabetes Association grant 7-12-BS-046, and by a University of Pittsburgh Junior Faculty Award (to N.M.F.-T.).
No potential conflicts of interest relevant to this article were reported.
N.M.F.-T. researched data, contributed to the discussion, and wrote the manuscript. J.W.K., F.G.S., R.T., R.W., M.T., G.C., A.E.C., K.K.T., and H.S. researched data. D.K.S. contributed to the discussion. A.F.S. contributed to the discussion and wrote the manuscript. N.M.F.-T. and A.F.S. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Adolfo Garcia-Ocaña, PhD, Rupangi C. Vasavada, PhD, and Laura C. Alonso, MD, at the University of Pittsburgh School of Medicine for many helpful discussions during the preparation of these studies. The authors also thank the Kroh and Wagner Family Foundations for their support of this work. Human islets were generously supplied by the National Institute of Diabetes and Digestive and Kidney Diseases–supported and Juvenile Diabetes Research Fund–supported Integrated Islet Distribution Program, and by Tatsuya Kin, MD, at the University of Alberta.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0778/-/DC1.
D.K.S. and A.F.S. are currently affiliated with the Diabetes Obesity and Metabolism Institute, Mount Sinai School of Medicine, New York, New York.
See accompanying original article, p. 2450.
REFERENCES
- 1.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, et al. Human pancreatic β-cell G1/S molecule cell cycle atlas. Diabetes 2013;62:2450–2459 [DOI] [PMC free article] [PubMed]
- 2.Cozar-Castellano I, Takane KK, Bottino R, Balamurugan AN, Stewart AF. Induction of beta-cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes 2004;53:149–159 [DOI] [PubMed] [Google Scholar]
- 3.Fiaschi-Taesch NM, Bigatel TA, Sicari BM, et al. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes 2009;58:882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiaschi-Taesch NM, Salim F, Kleinberger J, et al. Induction of human beta-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes 2010;59:1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karslioglu E, Kleinberger JW, Salim FG, et al. cMyc is a principal upstream driver of beta-cell proliferation in rat insulinoma cell lines and is an effective mediator of human beta-cell replication. Mol Endocrinol 2011;25:1760–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cozar-Castellano I, Harb G, Selk K, et al. Lessons from the first comprehensive molecular characterization of cell cycle control in rodent insulinoma cell lines. Diabetes 2008;57:3056–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guthalu Kondegowda N, Joshi-Gokhale S, Harb G, et al. Parathyroid hormone-related protein enhances human β-cell proliferation and function with associated induction of cyclin-dependent kinase 2 and cyclin E expression. Diabetes 2010;59:3131–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavine JA, Raess PW, Davis DB, et al. Overexpression of pre-pro-cholecystokinin stimulates beta-cell proliferation in mouse and human islets with retention of islet function [retracted in Mol Endocrinol 2010;24;472]. Mol Endocrinol 2008;22:2716–2728 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Davis DB, Lavine JA, Suhonen JI, et al. FoxM1 is up-regulated by obesity and stimulates beta-cell proliferation. Mol Endocrinol 2010;24:1822–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatrai S, Elghazi L, Balcazar N, et al. Akt induces β-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 2006;55:318–325 [DOI] [PubMed] [Google Scholar]
- 11.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, et al. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 2006;27:356–370 [DOI] [PubMed] [Google Scholar]
- 12.Heit JJ, Karnik SK, Kim SK. Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol 2006;22:311–338 [DOI] [PubMed] [Google Scholar]
- 13.Cozar-Castellano I, Weinstock M, Haught M, Velázquez-Garcia S, Sipula D, Stewart AF. Evaluation of beta-cell replication in mice transgenic for hepatocyte growth factor and placental lactogen: comprehensive characterization of the G1/S regulatory proteins reveals unique involvement of p21cip. Diabetes 2006;55:70–77 [PubMed] [Google Scholar]
- 14.Rieck S, Li Z, Sandhu AK, Liu C, Naji Al, Takane KK, Fiaschi-Taesch NM, Stewart AF, Kushner JA, Kaestner KH. Overexpression of hepatocyte nuclear factor 4α initiates cell cycle entry, but is not sufficient to promote β-cell expansion in human islets. Mol Endocrinol 2012;26:1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 2012;61:2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong L, Georgia S, Tschen SI, Nakayama K, Nakayama K, Bhushan A. Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J Clin Invest 2007;117:2869–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, Gu X, Liu Y, et al. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 2011;478:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beattie GM, Itkin-Ansari P, Cirulli V, et al. Sustained proliferation of PDX-1+ cells derived from human islets. Diabetes 1999;48:1013–1019 [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 2006;443:453–457 [DOI] [PubMed] [Google Scholar]
- 20.Köhler CU, Olewinski M, Tannapfel A, Schmidt WE, Fritsch H, Meier JJ. Cell cycle control of β-cell replication in the prenatal and postnatal human pancreas. Am J Physiol Endocrinol Metab 2011;300:E221–E230 [DOI] [PubMed] [Google Scholar]
- 21.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PubMed] [Google Scholar]
- 22.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev 2004;18:2699–2711 [DOI] [PubMed] [Google Scholar]
- 23.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999;13:1501–1512 [DOI] [PubMed] [Google Scholar]
- 24.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci 2005;30:630–641 [DOI] [PubMed] [Google Scholar]
- 25.Cobrinik D. Pocket proteins and cell cycle control. Oncogene 2005;24:2796–2809 [DOI] [PubMed] [Google Scholar]
- 26.Malumbres M. Physiological relevance of cell cycle kinases. Physiol Rev 2011;91:973–1007 [DOI] [PubMed] [Google Scholar]
- 27.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 2009;28:2925–2939 [DOI] [PubMed] [Google Scholar]
- 28.Ranta F, Leveringhaus J, Theilig D, et al. Protein kinase C delta (PKC∂) affects proliferation of insulin-secreting cells by promoting nuclear extrusion of the cell cycle inhibitor p21cip/waf1. PLoS ONE 2011;6:e28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–1097 [DOI] [PubMed] [Google Scholar]
- 31.Chong JL, Wenzel PL, Sáenz-Robles MT, et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 2009;462:930–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen D, Pacal M, Wenzel P, Knoepfler PS, Leone G, Bremner R. Division and apoptosis of E2f-deficient retinal progenitors. Nature 2009;462:925–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanova IA, Vespa A, Dagnino L. A novel mechanism of E2F1 regulation via nucleocytoplasmic shuttling: determinants of nuclear import and export. Cell Cycle 2007;6:2186–2195 [DOI] [PubMed] [Google Scholar]
- 35.Veiga-Fernandes H, Rocha B. High expression of active CDK6 in the cytoplasm of CD8 memory cells favors rapid division. Nat Immunol 2004;5:31–37 [DOI] [PubMed] [Google Scholar]
- 36.He LM, Sartori DJ, Teta M, et al. Cyclin D2 protein stability is regulated in pancreatic beta-cells. Mol Endocrinol 2009;23:1865–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Dross R, Yao S, Asad S, et al. Constitutively active K-cyclin/cdk6 kinase in Kaposi sarcoma-associated herpesvirus-infected cells. J Natl Cancer Inst 2005;97:656–666 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.