Edited by Helaine E. Resnick, PhD, MPH
Incretin-Based Therapies: Are They Safe?
New work by Butler et al. shows increased β-cell and α-cell mass resulting from incretin therapy in type 2 diabetes mellitus (DM). A deficit in β-cell mass is one characteristic of DM. One class of drugs for treating DM, the glucagon-like peptide-1 (GLP-1) mimetic drugs known as incretins, has been shown to induce β-cell regeneration in rodents. However, it is unknown whether GLP-1 has the same property in adult humans, and there is a concern that GLP-1 might cause an unwanted expansion of the exocrine pancreas. In this issue of Diabetes (p. 2595), Butler et al. analyzed 34 human pancreata from brain-dead organ donors with and without DM. Eight of the donors with DM had undergone incretin therapy (seven treated with sitagliptin and one with exenatide). In the DM group that had not undergone incretin therapy (12 individuals), the investigators reported a 60% deficit in β-cell mass with no change in α-cell mass compared with individuals without diabetes (14 individuals). Individuals treated with incretins showed a significant expansion of the exocrine and endocrine pancreatic compartments. The investigators believe this may suggest an increased risk of neuroendocrine tumors as a result of GLP-1 therapy. An accompanying commentary on p. 2178 by Steven E. Kahn, MB, ChB, however, underscores the need for further research before conclusions are drawn regarding the safety of incretin therapy. Kahn points out several differences between the study group and the control group, and cautions that these differences, as well as other considerations, could have a substantial impact on interpretation of the data. While the study by Butler et al. raises a need for caution and further human research, according to Kahn it is too early to consider pulling incretin therapy from the toolbox of available DM treatments. — Laura Gehl, PhD
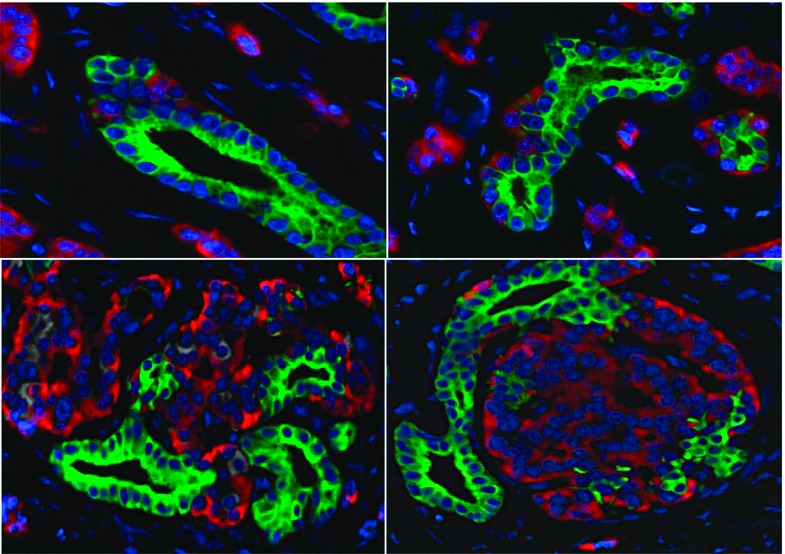
Glucagon immunohistochemistry in pancreas in DM after incretin therapy
Clock Genes in White Adipose Tissues Regulate the Release of Free Fatty Acids
In this issue of Diabetes (p. 2195), work from Shostak et al. shows that clock genes in white adipose tissue (WAT) regulate lipolysis. Data from rodent and human studies have demonstrated an important link between circadian clock disruption, obesity, and type 2 diabetes. WAT acts as a lipid storage area. During fasting, triglycerides from WAT are hydrolyzed to free fatty acids (FFAs) and glycerol, providing necessary energy for other organs. Mouse and human adipose tissues exhibit rhythmic clock gene expression. In a series of experiments using wild-type (WT) mice, Shostak et al. show variations in WAT lipolysis rates and blood FFA levels. In ClockΔ19 and Bmal1-/- mice, these variations decreased, pointing to a problem with lipid mobilization. The mRNA of rate-limiting lipolytic enzymes Atgl and Hsl showed circadian variation in WT mice. These mRNA levels were reduced and disturbed in ClockΔ19 mutants. Atgl and Hsl levels in Bmal1-/- mice were also lower, while fat pads from Bmal1-/- mice had lower lipolysis rates. Cultures prepared from white and brown adipose tissue fat explants from PER2::LUCIFERASE circadian reporter mice all showed luminescent oscillations for several days. Rhythmic oscillations in Atgl and Hsl mRNA expression were observed in WT fat pads. Analogous to the in vivo studies, Atgl and Hsl levels were reduced, and variations in transcription levels disappeared in explants from ClockΔ19 mice. When ClockΔ19 mutants were deprived of food for 12- and 24-h periods, the lipolytic response was dampened compared with WT mice. These data show that clock genes regulate the release of FFAs from adipose stores. Further research is needed to clarify the extent to which this phenomenon contributes to the obese phenotype observed in ClockΔ19 mutants. — Laura Gehl, PhD
Greater Than Expected Diurnal Variability in Postprandial Insulin Sensitivity in Type 1 Diabetic Patients
Among patients with type 1 diabetes, improved understanding of everyday perturbations in insulin sensitivity (SI) resulting from routine factors such as food intake would represent a major step forward toward the design of an artificial pancreas based on widely applicable control algorithms. With this idea in mind, new work by Hinshaw et al. in this issue of Diabetes (p. 2223) summarizes data from the first experimental use of the triple tracer stable isotope technique to track diurnal patterns in postprandial glucose kinetics in type 1 diabetic patients. Over 3 consecutive days, 19 C-peptide–negative subjects on insulin pump therapy were monitored as they consumed fixed meals and followed a prescribed physical activity protocol. A subset of the study meals contained triple-labeled glucose, which enabled the investigators to distinguish meals derived from endogenously produced glucose and also allowed them to measure glucose disappearance. Preprandial and postprandial plasma glucose and insulin concentrations were analyzed periodically over a 6-h window after each meal. Insulin concentration was higher and endogenous glucose production was less suppressed at breakfast than at lunch. There were no differences in meal glucose appearance or in glucose disappearance between meals. Although breakfast, lunch, and dinner were not significantly different in terms of modeled SI, an overall diurnal trend of increasing SI in type 1 diabetic individuals contrasted with a trend of decreasing diurnal SI in healthy subjects that was previously reported by the same group. The authors conclude that the high degree of variability in SI among diabetic subjects suggests a need for closed-loop systems to be more highly personalized than formerly suspected, a conclusion that has far-reaching implications for the development of an artificial pancreas. — Wendy Chou, PhD
IGF-I Receptor Signaling Under Long-Term Insulin Glargine Therapy Comparable to That of NPH Insulin Therapy
Recent literature has raised concerns that use of the insulin analog glargine (GLA) may be linked to higher cancer risk. Until now, however, few studies on insulin-related growth effects have looked beyond single-dose data at the consequences of longer-term GLA use. In this issue of Diabetes (p. 2539), Varewijck et al. evaluate factors affecting activation of the insulin receptor (IR) and IGF-I receptor (IGF-IR) in type 2 diabetic patients receiving high-dose GLA or NPH insulin treatment. The authors tested several explanations that mitogenic effects from insulin arise from 1) increased IGF-IR signaling, 2) activation of the two IR isoforms (IR-A and IR-B), or 3) from activation of IR-A, IR-B, and/or IGF-IR by the GLA metabolites M1 and M2. In a study of 104 diabetic patients randomized to receive either GLA or NPH insulin therapy, plasma concentrations of GLA, M1, and M2 were assessed at baseline and 36 weeks. Kinase receptor activation assays measured IR-A, IR-B, and IGF-IR activation (phosphorylation). These experiments showed that plasma concentrations of GLA and M2 remained static at undetectable levels, whereas M1 concentrations rose from an undetectable level to a median value of 1.5 ng/mL at 36 weeks, indicating effective removal of GLA. In vitro IR activation, which was induced using patient serum, did not differ between treatments at 36 weeks. Among individuals receiving NPH insulin, insulin dose correlated positively with serum-induced activation of IR-A but not IR-B. Similarly, among patients receiving GLA, M1 concentration correlated with serum-induced activation of IR-A, not IR-B. By contrast, insulin (GLA) dose was not correlated to serum-induced activation of either IR isoform. The new data challenge the premise that GLA increases IGF-IR signaling during insulin therapy and suggest that GLA metabolites, not GLA, mediate its mitogenic effects. — Wendy Chou, PhD


