Abstract
We have previously demonstrated that Lactobacillus paracasei NCC2461 may help to prevent cow's milk allergy in mice by inducing oral tolerance to β-lactoglobulin (BLG). To investigate the mechanisms involved in this beneficial effect, we examined the possibility that L. paracasei induces tolerance by hydrolyzing BLG-derived peptides and liberating peptides that stimulate interleukin-10 (IL-10) production. L. paracasei peptidases have been shown to hydrolyze tryptic-chymotryptic peptides from BLG, releasing numerous small peptides with immunomodulating properties. We have now shown that acidic tryptic-chymotryptic peptides stimulate splenocyte proliferation and gamma interferon (IFN-γ) production in vitro. Hydrolysis of these peptides with L. paracasei peptidases repressed the lymphocyte stimulation, up-regulated IL-10 production, and down-regulated IFN-γ and IL-4 secretion. L. paracasei NCC2461 may therefore induce oral tolerance to BLG in vivo by degrading acidic peptides and releasing immunomodulatory peptides stimulating regulatory T cells, which function as major immunosuppressive agents by secreting IL-10.
The mucosal immune system is stimulated daily by the continuous passage of food and microbial antigens. Breakdown of proteins in the gut and activation of T-cell suppression lead to a systemic hyporesponsiveness to ingested protein antigens called oral tolerance. Three basic mechanisms are involved in antigen-driven tolerance: clonal deletion, clonal anergy, and active suppression (13). Active suppression is defined as a state of T-lymphocyte unresponsiveness induced by direct action of regulatory T cells secreting inhibitory factors such as transforming growth factor β and interleukin-10 (IL-10) (18, 38, 40). Many factors seem to affect the induction of tolerance, including the age of the host, the nature and dosage of the antigen, and the frequency of feeding (1, 14, 23). The intestinal microbiota has also been found to play a major role in the induction (21, 32, 34, 39) and maintenance (22, 32) of tolerance, but the mechanism has yet to be elucidated.
Bifidobacteria and lactobacilli are common anaerobes in the human intestinal microbiota (20), and some of them have been reported to display probiotic properties (26). Probiotics are live microorganisms that when ingested may have positive effects on human health (7). Probiotic bacteria, increasingly used as food supplements, especially in infant formulas, have been found to be transiently present in the intestine when administered daily at high doses (6). The beneficial effects of probiotics on the immune system are believed to be numerous (3, 26), but few studies have focused on their role in induction of oral tolerance. Bifidobacterium infantis has been shown to restore oral tolerance to ovalbumin in monoassociated mice (21, 34), and we have recently reported that oral tolerance to β-lactoglobulin (BLG) was strongly induced and maintained in mice monocolonized with Lactobacillus paracasei NCC2461 (32). The mechanisms by which L. paracasei induces and maintains the oral tolerance response are not yet understood.
It is now recognized that orally administered proteins are subjected to degradation in the gastrointestinal tract by digestive enzymes (pepsin, trypsin, and chymotrypsin) and intestinal bacteria (19). Peptides obtained by tryptic hydrolysis of bovine casein (12, 28) and BLG (27) induce specific oral tolerance in mice. Probiotics colonizing the gut have been shown to contribute to this degradation (31), but it has not yet been proven whether peptides from probiotic degradation induce oral tolerance to the whole proteins. Interestingly, the suppression of lymphocyte proliferation by bovine caseins hydrolyzed by Lactobacillus rhamnosus GG enzymes in vitro has been reported (29, 35), suggesting a potential effect of this strain in the oral tolerance response.
The objective of the present study was to investigate in vitro a mechanism by which L. paracasei NCC2461 may induce and maintain oral tolerance to BLG, namely, the hydrolysis of BLG-derived tryptic-chymotryptic peptides. Suppression of the in vitro lymphocyte proliferation-stimulating action of these peptides as result of hydrolysis by L. paracasei enzymes was evaluated with splenocytes from naïve and BLG-primed mice, and cytokine production was measured.
MATERIALS AND METHODS
Preparation of the tryptic-chymotryptic hydrolysate of BLG.
Bovine BLG (lot JE 002-8-922; Davisco, Le Sueur, Minn.) dissolved at 10% (wt/vol) in water containing 0.01 M CaCl2 was hydrolyzed by a 2:1 (wt/wt) mixture of trypsin VI-chymotrypsin XI (Inovatech, Abbotsford, Canada) at an enzyme/substrate ratio of 1:200 (wt/wt) at 40°C for 1 h. BLG was hydrolyzed by trypsin and chymotrypsin to simulate gastrointestinal digestion. The pH was maintained constant at 7.5 by addition of 1 M NaOH-KOH (2:1) during the hydrolysis. The reaction was stopped by ultrafiltration on a 10-kDa-molecular-mass-cutoff membrane (model S1Y30; Amicon Corp., Danvers, Mass.) to separate the peptides from the enzymes and nonhydrolyzed proteins. Filtration was carried out at 45°C at a transmembrane pressure of 25 lb/in2 and followed by diafiltration with an equal volume of water. Peptides in permeate were then freeze-dried and stored at room temperature until fractionation.
Preparation of basic and acidic peptide fractions.
Acidic and basic peptides were separated from the tryptic-chymotryptic hydrolysate of BLG by ampholyte-free isoelectric focusing by the protocol described by Groleau et al. (9). Briefly, hydrolysate (0.5 g) was rehydrated in 40 ml of deionized water and fractionated for 2 h at 4°C by liquid-phase isoelectric focusing in a preparative Rotofor cell (Bio-Rad Laboratories, Hercules, Calif.) at constant power (15 W). Twenty peptide fractions were collected, and the first 10 were pooled to constitute the acidic fraction (pI from 2 to 5), while the next 10 were pooled to give the basic fraction (pI from 5 to 12). Acidic and basic fractions were freeze-dried and stored at room temperature until use.
Bacterial strain and cell extract preparation.
L. paracasei NCC2461 (Nestlé Culture Collection, Lausanne, Switzerland) was isolated from feces of a healthy infant and subcultured twice for 18 h at 37°C under anaerobic conditions in de Man-Rogosa-Sharpe broth supplemented with 0.5 g of l-cysteine/liter. Cell extract was obtained by the method described by Fernandez-Espla et al. (5) with some modifications. Briefly, 3 liters of de Man-Rogosa-Sharpe medium was inoculated with L. paracasei culture (1%, vol/vol) and incubated at 37°C for 15 h without agitation or pH control. Cells were collected by centrifugation at 5,000 × g for 10 min at 4°C and washed three times with 0.01 M potassium phosphate buffer (pH 7). Pellets were then manually ground with alumina beads for at least 30 min at 4°C, and cell walls were discarded by centrifugation (16,000 × g; 1 h; 4°C). The supernatant cell extract was used the same day for hydrolysis. The protein content of the cell extract was measured by the Lowry method (17) with bovine serum albumin as a standard.
Hydrolysis of BLG and peptide fractions with L. paracasei extract.
BLG and peptide fractions dissolved at 10% (wt/vol) in water containing 0.01 M CaCl2 were hydrolyzed by L. paracasei extract at 40°C for 1 h without pH control. Prior to addition of the L. paracasei extract, the pH was adjusted to 7.5 with 1 M NaOH (acid fraction) or 1 M HCl (basic fraction). The hydrolysis mixture contained 20 μg of L. paracasei protein per 2,000 μg of substrate. The reaction was terminated by filtration through a 10-kDa-molecular-mass-cutoff Osmotics SEPA membrane (Minnetonka, Minn.) for 3 h at 4°C under constant pressure (120 lb/in2). Permeates were freeze-dried and kept at room temperature until used for in vitro tests. BLG hydrolyzed by L. paracasei extract was freeze-dried without filtration and stored under the same conditions. Peptide fractions not hydrolyzed with L. paracasei extract were also filtered under the same conditions and used as controls.
Characterization of BLG and peptide fractions by size-exclusion chromatography.
BLG and its acidic and basic peptide fractions, before and after hydrolysis with L. paracasei extract, were diluted with acetonitrile-trifluoroacetic acid (30%-0.1%) aqueous buffer to give a final protein concentration of 1% (wt/vol) and filtered on a 0.2-μm-pore-size membrane. The same buffer was used as running buffer. The samples (20 μl) were passed through a TSK-GEL Guard SWXL precolumn (6.0 mm [inside diameter] by 40 mm; Tosoh Biosep LLC) before being separated on a TSK-GEL G2000 SWXL column (7.8 mm [inside diameter] by 300 mm; Tosoh Biosep LLC), with a high-pressure liquid chromatography system (Waters, Milford, Mass.) equipped with two pumps (model 600) running at 0.6 ml/min and a UV-visible-light detector (model 486) set at 214 nm. Molecular weights were determined by comparison to the following standards: BLG, α-lactalbumin, bovine serum albumin, and RNase A (all from Sigma) and egg albumin and chymotrypsinogen A (both from Pharmacia Biotech). Peptides from BLG (positions 142 to 149 and 41 to 60), β-casein (positions 193 to 202), α-lactalbumin (positions 50 to 53), and casein-αs1 (positions 28 to 34) (all from Service de Synthese de Peptide de l'EST du Québec, Sainte-Foy, Canada) were also used as standards. The total surface area of the chromatograms was integrated and separated into five ranges (>10,000, 5,000 to 10,000, 1,000 to 5,000, 500 to 1,000, and <500 Da), expressed as percentages of the total area of the chromatogram.
Animals.
Two groups of BALB/c female mice aged from 4 to 6 weeks were used for lymphocyte proliferation tests. “Naïve” mice fed with a protein-free whey diet (<7 μg of BLG/g of protein) for at least three generations were purchased from Taconic Farms Inc. (Germantown, N.Y.). “BLG-primed” mice from Charles River (Saint-Constant, Quebec, Canada) received a daily diet containing 4 mg of BLG/g of protein. BLG in the diet was measured by the competitive-inhibition enzyme-linked immunosorbent assay (ELISA) described by Pecquet et al. (27).
Proliferation assay.
Individual spleens were removed from five naïve and five BLG-primed mice and were pressed separately through cellular sieves. Extracted cells were suspended in 7 ml of RPMI 1640 medium (Gibco) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U of penicillin-100 μg of streptomycin, 10 mM HEPES buffer, and 2 × 10−5 M β-mercaptoethanol. After centrifugation (135 × g, room temperature, 7 min), erythrocytes were removed from pellets by osmotic shock with 2 ml of 0.87% NH4Cl for 2 min at 37°C. Spleen cells were then washed three times at 4°C with RPMI 1640 medium, and mononuclear cells were counted using a Malassez chamber and 0.4% trypan blue.
BLG and its acidic and basic peptide fractions, before and after hydrolysis with L. paracasei extract, were diluted in supplemented RPMI to 200 and 4,000 μg/ml and microfiltered (0.22-μm pore size). However, BLG hydrolyzed by L. paracasei extract was not filtered after hydrolysis, indicating that 2 and 40 μg, respectively, of L. paracasei extract remained in the two diluted solutions. In contrast, acidic and basic peptide fractions were filtered after L. paracasei hydrolysis to separate peptides from the enzymes. Nevertheless, small amounts of L. paracasei-associated enzymes might pass through the ultrafiltration membrane and hence contaminate peptide fractions. Therefore, L. paracasei extract alone was diluted in supplemented RPMI to 40 and 2 μg of protein/ml, microfiltered, and used as a control for cell proliferation.
Flat-bottomed microtiter plates were loaded with 100 μl of each dilution in duplicate. One hundred microliters of cell suspension (5 × 105 cells/ml) was added to wells, and microplates were incubated at 37°C in a humidified atmosphere of 5% CO2 for 48 h. Wells containing cells suspended in RPMI 1640 medium only were incubated as controls. Lymphocyte proliferation was evaluated by bromodeoxyuridine incorporation for 18 h, and incorporated bromodeoxyuridine was measured by ELISA (Proliferation ELISA kit; Roche Diagnostics, Laval, Canada) according to the manufacturer's instructions. The stimulation index was expressed as the ratio of the optical density in the presence of antigen to that in the absence of antigen (means of duplicates).
IFN-γ, IL-10, and IL-4 quantification.
To measure cytokine production by splenocytes, cells were cultured with 10 μg of phytohemagglutinin/ml, at which concentration the stimulation index was not affected. The levels of gamma interferon (IFN-γ) and IL-10 in splenocyte culture supernatants were determined by ELISA with commercial kits (Quantikine Murine; R&D Systems, Minneapolis, Minn.). IL-4 was quantified by the sandwich ELISA technique with the monoclonal anti-mouse IL-4 rat immunoglobulin G2b clone 1D11 (1 μg/ml) and biotinylated monoclonal anti-mouse IL-4 rat immunoglobulin G1 clone 24G2 (0.25 μg/ml) as coating and detection antibodies, respectively (Endogen, Woburn, Mass.). Concentrations of IL-4 were extrapolated from standard curves calculated by using dilutions of recombinant mouse IL-4 (Endogen). Optical densities were measured at 450 nm after 15 min of incubation with tetramethylbenzidine and H2O2. Fresh cell-free complete RPMI-10% fetal calf serum medium was used as a negative control. Detection limits of IFN-γ, IL-10, and IL-4 were 2, 4, and 4 pg/ml, respectively.
Statistical analysis.
The statistical significance was assessed by using the Student t test. A P value of <0.05 was considered significant.
RESULTS
Hydrolysis of BLG and its peptide fractions by L. paracasei extract.
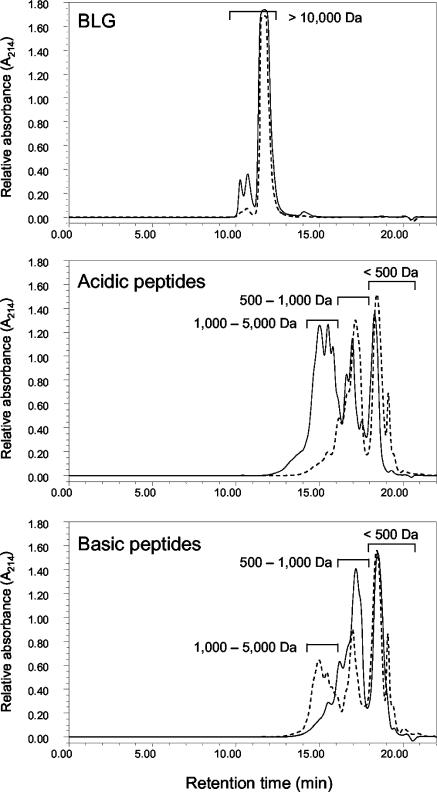
To assess whether L. paracasei extract hydrolyzes BLG and its peptide fractions, molecular weight profiles before and after hydrolysis were compared. As shown in Fig. 1, BLG was not hydrolyzed while both acidic and basic peptide fractions were. Large acidic peptides (1,000 to 5,000 Da) were almost completely degraded, releasing small peptides with molecular masses below 1,000 Da. Small peptides represented more than 75% of the L. paracasei-hydrolyzed fraction, of which 42% was below 500 Da. Compared to the acidic fraction, the basic fraction was degraded to a lesser extent. Peptides in the 500- to 1,000-Da range decreased, yielding peptides smaller than 500 Da, which represented 43% of the fraction after hydrolysis compared to 37% before hydrolysis. Surprisingly, peptides with molecular masses higher than 1,000 Da increased in the hydrolyzed fraction.
FIG. 1.
Effect of hydrolysis by L. paracasei extract on the mass distribution of BLG and its acidic and basic peptide fractions. Solid and dashed lines represent values before and after hydrolysis, respectively.
Effect of BLG and its peptide fractions on lymphocyte proliferation.
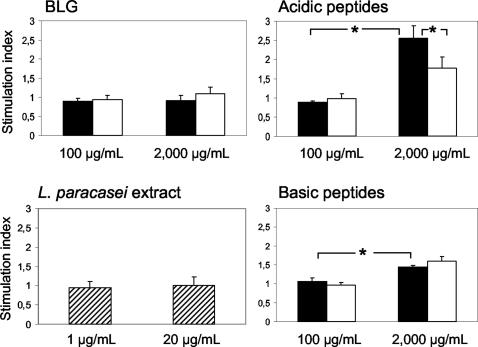
BLG had no significant effect on lymphocyte proliferation either before or after hydrolysis with L. paracasei extract, as shown by stimulation indices close to 1 at either concentration used (Fig. 2). In contrast, the acidic and basic peptide fractions stimulated lymphocyte proliferation at 2,000 μg/ml, but the strongest stimulation was observed with the acidic fraction. Stimulation indices of 1.5 and 2.5 were obtained in response to basic and acidic fractions, respectively. The stimulating effect of the acidic peptide fraction was significantly reduced after hydrolysis with L. paracasei extract, whereas that of the basic fraction remained unchanged. In addition, the attenuated stimulating effect of the acidic fraction was not due to residual L. paracasei extract in the fraction while L. paracasei extract alone had no effect on cell proliferation (stimulation index close to 1).
FIG. 2.
Proliferation of splenocytes from BLG-naïve mice in response to BLG and its acidic and basic peptide fractions, tested at 100 and 2,000 μg/ml, before (solid bars) and after (open bars) hydrolysis with L. paracasei extract. Splenocyte proliferation in response to L. paracasei extract, tested at 1 and 20 μg/ml, was determined as a control. Proliferation in duplicate wells 48 h after the initiation of the culture was determined by ELISA. The stimulation index was expressed as the ratio of the optical density in the presence of BLG, peptides, or L. paracasei extract (means of duplicates) to that in its absence. Values are means ± standard deviations of five mice. *, P < 0.05.
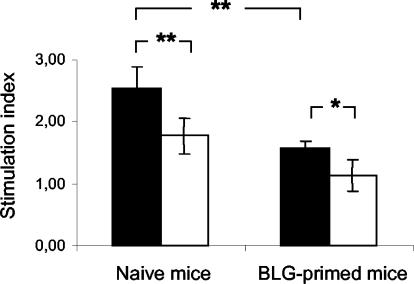
The effect of the acidic peptide fraction on cell proliferation has been investigated with splenocytes from mice never or already exposed to BLG (Fig. 3). The acidic peptide fraction stimulated splenocyte proliferation from both naïve and BLG-primed mice (stimulation indices higher than 1.5), but the strongest stimulation was observed with naïve mice. Moreover, the acidic fraction hydrolyzed by L. paracasei extract exhibited a significant attenuated stimulating effect in both naïve (P < 0.01) and BLG-primed (P < 0.05) mice.
FIG. 3.
Proliferation of splenocytes collected from naïve and BLG-primed mice in response to the acidic peptide fraction (2,000 μg/ml), before (solid bars) and after (open bars) hydrolysis with L. paracasei extract. Proliferation in duplicate wells 48 h after the initiation of the culture was determined by ELISA. The stimulation index was expressed as the ratio of optical density in the presence of acidic peptides to that in their absence (means of duplicates). Values are means ± standard deviations of five naïve and five BLG-primed mice. *, P < 0.05; **, P < 0.01.
Effect of BLG and its peptide fractions on cytokine production.
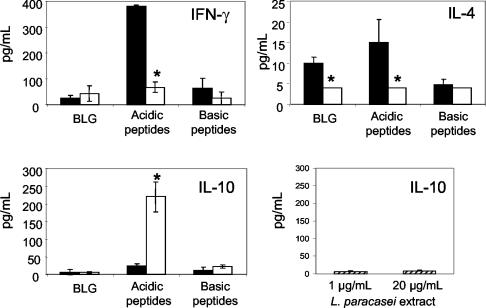
Low levels of IFN-γ and IL-10 were produced by splenocytes in response to BLG and the basic peptide fraction, whether hydrolyzed with L. paracasei extract or not (Fig. 4). The acidic fraction produced a high level of IFN-γ and a low level of IL-10, while its L. paracasei-hydrolyzed form did the opposite. Production of IL-4 by the acidic fraction was also conspicuously decreased by L. paracasei hydrolysis. The effect of the L. paracasei-hydrolyzed acidic peptide fraction appears to be due to peptides and not to ultrafilterable residual extract, since no effect on IL-10 production was observed with L. paracasei extract alone.
FIG. 4.
Production of IFN-γ, IL-10, and IL-4 by splenocytes collected from naïve mice in response to BLG and its acidic and basic peptide fractions (2,000 μg/ml), before (solid bars) and after (open bars) hydrolysis with L. paracasei extract. IL-10 production in response to L. paracasei extract, tested at 1 and 20 μg/ml, was determined as a control. Cytokine production in duplicate wells 48 h after the initiation of the culture was determined by ELISA, and means of duplicate determinations were calculated for each mouse. Values are means ± standard deviations of five mice. *, P < 0.05.
DISCUSSION
Probiotic bacteria are increasingly used in the production of a wide range of dairy products such as cheese, yogurts, and infant formulas and are found to transiently colonize the intestinal tract (6). Several metabolic properties of probiotics serve special health functions such as modulation of the immune system via the degradation of caseins (29, 35, 36). In this study, we investigated the proteolytic activity of L. paracasei NCC2461 and its potential role in the degradation of the principal allergen in bovine milk, BLG.
The proteolytic system of lactic acid bacteria includes essentially two functions: proteases that break down whole protein to peptides and peptidases that degrade peptides. Proteases occur outside the bacterial cell, whereas most peptidases are found in the cytoplasm (15, 16). However, high peptidase activity has been detected at the extracellular level for several bacterial strains, including probiotics (33). The quite similar specificities of intra- and extracellular peptidases (33) have led us to use cytoplasmic peptidases of L. paracasei NCC2461 to degrade native BLG and its peptides even if we hypothesized that L. paracasei hydrolyzes them in vivo with extracellular peptidases. L. paracasei-associated enzymes hydrolyzed mainly acidic peptides, while basic peptides were only slightly degraded and BLG was not at all degraded. Since BLG hydrolysate was shown to be composed mainly of acidic peptides (unpublished data), their hydrolysis by L. paracasei enzymes was of particular interest. The extract activity appeared to be primarily endopeptidasic and di- or tripeptidasic, since an appreciable increase in peptides with molecular masses in the 500- to 1,000-Da range and below 500 Da was observed. Aminopeptidase and dipeptidase activities have been reported for the L. casei group, including L. paracasei, by Shihata and Sha (33). The basic fraction was partially hydrolyzed by L. paracasei peptidases, but peptides with molecular masses higher than 2,000 Da appeared. Interactions between basic peptides and extract-derived components could explain this observation.
Several immunoregulatory peptides from bovine milk proteins have been reported (8). Little is known about the effect of purified BLG and its peptide derivatives on the immune system (41). The hydrolysis of acidic peptides by L. paracasei extract appears to have an attenuated stimulating effect, this being essential for induction of oral tolerance. Well-characterized mechanisms for the induction of oral tolerance include clonal deletion, clonal anergy, and active suppression via the induction of regulatory T cells (25). Type 1-regulatory T (Tr-1) cells have a low proliferation capacity and suppress naïve and memory T helper type 1 (Th1) and Th2 responses due to their ability to produce high levels of immunosuppressive cytokines such as IL-10 (2, 10, 11). IL-10 has been found to down-regulate expression of CD80 and CD86, which function as important costimulatory molecules for T-cell activation (4). Interestingly, IL-10 was found to be up-regulated by L. paracasei-degraded acidic peptides, indicating the potential of these peptides to induce oral tolerance to BLG by a mechanism of active suppression. IL-10 was initially identified as a product of antigen-stimulated murine Th2 cells (24), but in our study, the secretion of IL-10 resulted undoubtedly from the generation of Tr-1 cells, while no induction of IL-4 (Th2-related cytokine) was observed. The potential benefit of L. paracasei-degraded acidic peptides to stimulate oral tolerance response was manifested in BLG-naïve mice especially but also in BLG-primed mice in terms of suppression of lymphocyte proliferation, indicating the capability of hydrolyzed peptides to induce, maintain, and reinforce hyporesponsiveness of T cells. This implies that L. paracasei may aid the induction and maintenance of tolerance to milk proteins in infants and hence prevent later manifestations of allergy symptoms. Induction and maintenance of oral tolerance to BLG have recently been reported in gnotobiotic mice colonized with L. paracasei (32), but further studies of the released bioactive peptides are needed for us to better understand the mechanism of action.
L. paracasei NCC2461 thus seems to stimulate regulatory T cells through its proteolytic activity and liberation of bioactive peptides. Whole cells of the same strain have been found to stimulate regulatory T cells in vitro (37), while we have observed an immunosuppressive effect of its cytoplasmic content at concentrations higher than 80 μg/ml (data not shown). These findings suggest that bacteria such as L. paracasei NCC2461 may induce an immunosuppressive activity either directly via cell-to-cell interactions or indirectly via degradation of antigens or autolytic liberation of cytoplasmic contents. Similar findings have been reported for the cytoplasmic content of Lactobacillus GG (30).
The present study supports earlier findings of immunosuppressive effects of L. paracasei NCC2461 and provides key elements to understanding its mechanism of action in the oral tolerance response. Nevertheless, further investigation is needed to identify the immunosuppressive peptides and understand the molecular mechanisms involved.
Acknowledgments
This work was supported by a grant from Nestlé (Lausanne, Switzerland) and the Fond Québecois de la Recherche sur la Nature et les Technologies.
We thank Julie Brassard and Mélanie Alain for their deft assistance in the animal house; Vincent Leclerc, Lynda Labarre, Catherine Schwarz, and Christine Martin-Pashoud for their generous technical assistance; and Stephen Davids for critical reading of the manuscript.
REFERENCES
- 1.Chen, Y. H., and H. L. Weiner. 1996. Dose-dependant activation and deletion of antigen specific T cells following oral tolerance. Ann. N. Y. Acad. Sci. 778:111-121. [DOI] [PubMed] [Google Scholar]
- 2.Cottrez, F., S. D. Hurst, R. I. Coffman, and H. Groux. 2000. T regulatory cells 1 inhibit a Th2-specific response in vivo. J. Immunol. 165:4848-4853. [DOI] [PubMed] [Google Scholar]
- 3.Cross, M. L., L. M. Stevenson, and H. S. Gill. 2001. Anti-allergy property of fermented foods: an important immunoregulatory mechanism of lactic acid bacteria? Int. Immunopharmacol. 1:891-901. [DOI] [PubMed] [Google Scholar]
- 4.Ding, L., and E. M. Shevach. 1992. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J. Immunol. 148:3133-3139. [PubMed] [Google Scholar]
- 5.Fernandez-Espla, M. D., P. Garault, V. Monnet, and F. Rul. 2000. Streptococcus thermophilus cell wall-anchored proteinase: release, purification and biochemical and genetic characterization. Appl. Environ. Microbiol. 66:4772-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukushima, Y., Y. Kawata, H. Hara, A. Terada, and T. Mitsuoka. 1998. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int. J. Food Microbiol. 42:39-44. [DOI] [PubMed] [Google Scholar]
- 7.Fuller, R. 1991. Probiotics in human medicine. Gut 32:439-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill, H. S., F. Doull, K. J. Rutherfurd, and M. L. Cross. 2000. Immunoregulatory peptides in bovine milk. Br. J. Nutr. 84(Suppl. 1):S111-S117. [DOI] [PubMed] [Google Scholar]
- 9.Groleau, P.-E., R. Jimenez-Flores, S. F. Gauthier, and Y. Pouliot. 2002. Fractionation of β-lactoglobulin tryptic peptides by ampholyte-free isoelectric focusing. J. Agric. Food Chem. 50:578-583. [DOI] [PubMed] [Google Scholar]
- 10.Groux, H., M. Bigler, J. de Vries, and M. G. Roncarolo. 1996. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 184:19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groux, H., M. Bigler, A. O'Garra, M. Rouleau, S. Antonenko, J. E. de Vries, and M. G. Roncarolo. 1997. A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis. Nature 389:737-742. [DOI] [PubMed] [Google Scholar]
- 12.Hachimura, S., Y. Takahashi, Y. Fujikawa, C. Tsumori, A. Enomoto, U. Yoshino, and S. Kaminogawa. 1993. Suppression of the systemic immune response to casein by oral administration of tryptic digest of casein. Biosci. Biotechnol. Biochem. 57:1674-1677. [Google Scholar]
- 13.Kaminogawa, S. 1996. Food allergy, oral tolerance and immunomodulation. Their molecular and cellular mechanisms. Biosci. Biotechnol. Biochem. 60:1749-1756. [DOI] [PubMed] [Google Scholar]
- 14.Kato, C., K. Sato, Y. Eishi, and K. Nakamura. 1999. The influence of initial exposure timing to β-lactoglobulin on oral tolerance induction. J. Allergy Clin. Immunol. 104:870-878. [DOI] [PubMed] [Google Scholar]
- 15.Kunji, E. R. S., I. Mierau, A. Hagting, B. Poolman, and W. N. Konings. 1996. The proteolytic systems of lactic acid bacteria. Antonie Leeuwenhoek 70:187-221. [DOI] [PubMed] [Google Scholar]
- 16.Law, J., and A. Haandrikman. 1997. Proteolytic enzymes of lactic acid bacteria. Int. Dairy J. 7:1-11. [Google Scholar]
- 17.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 18.Lundin, B. S., M. R. Karlsson, L. A. Svensson, L. A. Hanson, U. I. H. Dahlgren, and E. Telemo. 1999. Active suppression in orally tolerized rats coincides with in situ transforming growth factor-beta (TGF-β) expression in the draining lymph nodes. Clin. Exp. Immunol. 116:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macfarlane, G. T., J. H. Cummings, and C. Allison. 1986. Protein degradation by human intestinal bacteria. J. Gen. Microbiol. 132:1647-1656. [DOI] [PubMed] [Google Scholar]
- 20.Mackie, R. I., A. Sghir, and H. R. Gaskins. 1999. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 69(Suppl.):1035S-1045S. [DOI] [PubMed] [Google Scholar]
- 21.Maeda, Y., S. Noda, K. Tanaka, S. Sawamura, Y. Aiba, H. Ishikawa, H. Hasegawa, N. Kawabe, M. Miyasaka, and Y. Koga. 2001. The failure of oral tolerance induction is functionally coupled to the absence of T cells in Peyer's patches under germfree conditions. Immunobiology 204:442-457. [DOI] [PubMed] [Google Scholar]
- 22.Moreau, M.-C., and G. Corthier. 1988. Effect of the gastrointestinal microflora on induction and maintenance of oral tolerance to ovalbumin in C3H/HeJ mice. Infect. Immun. 56:2766-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreau, M.-C., and V. Gaboriau-Routhiau. 1996. The absence of gut flora, the doses of antigen ingested and aging affect the long-term peripheral tolerance induced by ovalbumin feeding in mice. Res. Immunol. 147:49-59. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 25.Nagler-Anderson, C. 2000. Tolerance and immunity in the intestinal immune system. Crit. Rev. Immunol. 20:103-120. [PubMed] [Google Scholar]
- 26.Ouwehand, A. C., P. V. Kirjavainen, C. Shortt, and S. Salminen. 1999. Probiotics: mechanisms and established effects. Int. Dairy J. 9:43-52. [Google Scholar]
- 27.Pecquet, S., L. Bovetto, F. Maynard, and R. Fritsché. 2000. Peptides obtained by tryptic hydrolysis of bovine beta-lactoglobulin induce specific oral tolerance in mice. J. Allergy Clin. Immunol. 105:514-521. [DOI] [PubMed] [Google Scholar]
- 28.Peng, H. J., M. W. Turner, and S. Strobel. 1990. The generation of a tolerogen after the ingestion of ovalbumin is time-dependant and unrelated to serum levels of immunoreactive antigen. Clin. Exp. Immunol. 81:510-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pessi, T., E. Isolauri, Y. Sütas, H. Kankaanranta, E. Moilanen, and M. Hurme. 2001. Suppression of T-cell activation by Lactobacillus rhamnosus GG-degraded bovine casein. Int. Immunopharmacol. 1:211-218. [DOI] [PubMed] [Google Scholar]
- 30.Pessi, T., Y. Sütas, M. Saxelin, H. Kallioinen, and E. Isolauri. 1999. Antiproliferative effects of homogenates derived from five strains of candidate probiotic bacteria. Appl. Environ. Microbiol. 65:4725-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pessi, T., Y. Sütas, A. Marttinen, and E. Isolauri. 1998. Probiotics reinforce mucosal degradation of antigens in rats: implications for therapeutic use of probiotics. J. Nutr. 128:2313-2318. [DOI] [PubMed] [Google Scholar]
- 32.Prioult, G., I. Fliss, and S. Pecquet. 2003. Effect of probiotic bacteria on induction and maintenance of oral tolerance to β-lactoglobulin in gnotobiotic mice. Clin. Diagn. Lab. Immunol. 10:787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shihata, A., and N. P. Sha. 2000. Proteolytic profiles of yogurt and probiotic bacteria. Int. Dairy J. 10:401-408. [Google Scholar]
- 34.Sudo, N., S.-A. Sawamura, K. Tanaka, Y. Aiba, C. Kubo, and Y. Koga. 1997. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J. Immunol. 159:1739-1745. [PubMed] [Google Scholar]
- 35.Sütas, Y., E. Soppi, H. Korhonen, E.-L. Syväoja, M. Saxelin, T. Rokka, and E. Isolauri. 1996. Suppression of lymphocyte proliferation in vitro by bovine caseins hydrolyzed with Lactobacillus casei GG-derived enzymes. J. Allergy Clin. Immunol. 98:216-224. [DOI] [PubMed] [Google Scholar]
- 36.Sütas, Y., M. Hurme, and E. Isolauri. 1996. Down-regulation of anti-CD3 antibody-induced IL-4 production by bovine caseins hydrolysed with Lactobacillus GG-derived enzymes. Scand. J. Immunol. 43:687-689. [DOI] [PubMed] [Google Scholar]
- 37.von der Weid, T., C. Bulliard, and E. J. Schiffrin. 2001. Induction by a lactic acid bacterium of a population of CD4+ T cells with low proliferative capacity that produce transforming growth factor β and interleukin-10. Clin. Diagn. Lab. Immunol. 8:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakkack, A., N. Fournier, V. Brun, J.-P. Breittmayer, F. Cottrez, and H. Groux. 2003. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 18:605-617. [DOI] [PubMed] [Google Scholar]
- 39.Wannemuehler, M. J., H. Kiyono, J. L. Babb, S. M. Michalek, and J. R. McGhee. 1982. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J. Immunol. 129:959-965. [PubMed] [Google Scholar]
- 40.Weiner, H. L. 2001. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 3:947-954. [DOI] [PubMed] [Google Scholar]
- 41.Wong, K. F., N. Middleton, M. Montgomery, M. Dey, and R. I. Carr. 1998. Immunostimulation of murine spleen cells by materials associated with bovine milk protein fractions. J. Dairy Sci. 81:1825-1832. [DOI] [PubMed] [Google Scholar]