Abstract
Gastrointestinal mechanisms involved in the suppression of appetite are compromised in obesity. Glucagon-like peptide-1 (GLP-1) is released in response to nutrients, suppresses food intake, and has been shown to play a role in regulation of energy balance. It is not known whether obese-prone (OP) rats exhibit dysfunctional GLP-1 signaling that could contribute to decreased nutrient-induced satiation and hyperphagia. Therefore, we examined the effects of exogenous intraperitoneal administration of the GLP-1R agonist, exendin-4 (Ex-4), on food intake in OP and obese-resistant (OR) rats during chow or high-energy/high-fat (HE/HF) feeding. All doses of Ex-4 effectively suppressed intake in OP and OR rats fed chow; however, during HE/HF-feeding, OP rats suppressed intake significantly less than OR rats at all Ex-4 doses tested. This was associated with downregulation of GLP-1R mRNA expression in the vagal nodose ganglia of OP rats. Furthermore, HE/HF-fed OP rats had significantly lower plasma GLP-1 levels, decreased protein levels of GLP-1 in the intestinal epithelium, and reduced number of L cells in the distal ileum. These results demonstrate that HE/HF-feeding, coupled with OP phenotype, results in reduced endogenous GLP-1 and GLP-1R activation, indicating that impaired GLP-1 signaling during obesity may exacerbate hyperphagia and weight gain.
The number of overweight and obese individuals continues to increase as new estimates predict that more than 50% of the United States’ population will be obese by 2030 (1). The interaction between genetics and the environment, such as dietary and lifestyle influences, plays a major role in the development of obesity, because up to 70% of human obesity is inherited in a polygenic fashion (2,3). Therefore, the use of select inbred obesity-prone (OP) and obesity-resistant (OR) polygenetic rodent models reflecting human obesity provides a useful means of unraveling these interactions. When exposed to a high-energy/high-fat (HE/HF) diet, OP rats become obese, which is accompanied by increased caloric intake (4), possibly from impaired postingestive intestinal feedback signaling (5,6). We recently showed that diet-induced obese rats fed an HE/HF diet are less sensitive to the suppressive effects of lipid gastric loads and that this was associated with decreased expression of several gut peptides (5). Similarly, obese humans exhibit decreased levels of gut peptides, such as cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), and peptide YY (7). Therefore, it is plausible that HE/HF-feeding in those susceptible to obesity leads to alterations in intestinal peptide signaling, resulting in increased energy intake and subsequent weight gain.
GLP-1 is a potent incretin and plays a physiological role in satiation, because administration of GLP-1 and GLP-1 receptor (GLP-1R) agonists (exendin-4 [Ex-4] and liraglutide) reduce food intake, whereas blockade of GLP-1R increases intake and attenuates nutrient-induced satiety (8). Several studies have implicated GLP-1 in the pathogenesis of obesity (7). For example, obese humans exhibit blunted postprandial GLP-1 release (9), whereas weight loss after bariatric surgery results in increased plasma GLP-1 (10). In the rat, HF-feeding causes decreased circulating GLP-1 and attenuated anorexigenic response to GLP-1 and Ex-4 (11). However, no study has addressed the role of GLP-1 in the OP animal model encompassing the gene and environment interaction that closely resembles human obesity, allowing us to distinguish between the effects of the phenotype from those of the diet. Therefore, in this study, we first examined the effect of Ex-4, a GLP-1R agonist, on food intake in OP and OR rats maintained on chow or an HE/HF diet. Second, to determine if changes in sensitivity to Ex-4 are the result of alterations in vagal afferent receptors, we evaluated GLP-1R mRNA expression in the nodose ganglia. Third, we assessed intestinal peptide protein expression and circulating levels of GLP-1 and quantified GLP-1–expressing enteroendocrine cells (EECs) in the distal ileum to determine if HE/HF-feeding leads to decreases in endogenous GLP-1 in OP rats.
RESEARCH DESIGN AND METHODS
Animals.
Twenty OP and OR male rats (n = 10 per phenotype, Charles River, Wilmington, MA) were housed individually in a temperature-controlled vivarium with 12:12-h light/dark cycle (lights on at 0700). Except where otherwise noted, rats had ad libitum access to standard rat chow (3.1 kcal/g; SDS Diets, Essex, U.K.). All experiments were carried out in accordance with the European Guidelines for the Care and Use of Laboratory Animals.
Feeding responses to Ex-4.
Eight-week-old OP and OR rats (287.1 ± 3 and 223.3 ± 3 g, respectively) were separated into two groups (n = 5 per phenotype and diet) and fed chow or the HE/HF diet (Research Diets, NJ, D12334B, 4.2 kcal/g) for 5 weeks before testing. After a 16-h fast (1700–0900), rats were given Ex-4 (American Peptides, Sunnyvale, CA) or saline vehicle. Food intake was measured at 1, 3, and 24 h after injection. Ex-4 (0.625, 1.25, 2.5, 5 μg/kg, i.p.) was administered in random order, at least twice, with each dose bracketed by vehicle injection, with a minimum of 72 h elapsing between injections.
Tissue collection.
Animals were killed 4 weeks after the Ex-4 tests, and proximal intestinal epithelial cells and bilateral nodose ganglia were collected and processed for quantitative real-time PCR and Western blotting (5). Also removed were 3-cm sections of the distal ileum for confocal immunofluorescence. Plasma was extracted from vena cava blood, and total and active GLP-1 were measured with ELISA (Millipore, Molsheim, France). Retroperitoneal, visceral, and epididymal fat pads were removed and weighed, and the adiposity index was calculated (total fat/body weight × 100).
Immunofluorescence.
The distal ileum was fixed, and 4-µm paraffin-cut sections were incubated with rabbit polyclonal antibody raised against GLP-1 (1:200, Abcam, Paris, France, ab22625), followed by donkey anti-rabbit IgG H&L (DyLight 488) secondary antibody (1:200, Abcam, ab96891). Images were acquired with an LSM510 confocal microscope (LSM Image Browser), and GLP-1–containing EECs were quantified by counting total villi and GLP-1–positive cells throughout the entire length of the section (more than 20 nonoverlapping microscopic areas) for each animal.
Statistical analyses.
All statistics were computed with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA), and SAS 9.13 (SAS Institute, Cary, NC) software. Biweekly average body weights and 24-h food intake were analyzed with repeated-measures ANOVA and post hoc Bonferroni adjustment. For all Ex-4 tests, raw food intake and as percentage suppression of food intake was analyzed by three-way (phenotype, treatment, diet) repeated-measures ANOVA with post hoc Bonferroni adjustment. Fat pads, plasma GLP-1, quantitative PCR, Western blots, and GLP-1 cell counts were analyzed by two-way ANOVA (diet × phenotype) with Bonferroni post hoc tests. Significance was considered at α < 0.05 for all tests.
RESULTS
Body weight, adiposity, and 24-h food intake.
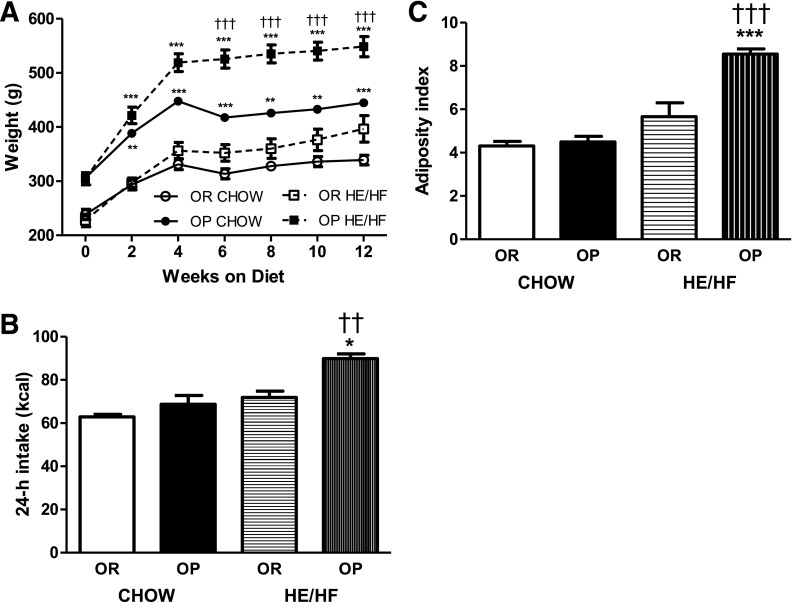
OP rats were significantly heavier than OR rats 2 weeks after HE/HF-feeding (P < 0.0001; Fig. 1A) and their chow-fed counterparts after 6 weeks of HE/HF-feeding (P < 0.0001). OP rats consumed more calories from the HE/HF diet than OR (P = 0.0057) or OP rats fed chow (P < 0.001; Fig. 1B). In addition, OP rats had a significantly larger adiposity index than OR rats fed the HE/HF diet (P < 0.0001; Fig. 1C), with no difference during chow-feeding.
FIG. 1.
Body weights (A), 24-h caloric intake (B), and adiposity index (C) in OP and OR animals fed chow or the HE/HF. A: OP chow and OP HE/HF animals weighed more than respective OR chow and OR HE/HF rats after 2 weeks on the diet, and OP rats fed the HE/HF diet weighed more than OP rats fed the chow diet after 6 weeks. B: HE/HF-fed OP rats consumed significantly more calories per day than HE/HF-fed OR and chow-fed OP rats. C: The adiposity index was significantly greater in HE/HF-fed OP rats than in the HE/HF-fed OR and chow-fed OP animals. Data are expressed as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.0001 denoting a significant difference from OR rats within diet condition. ††P < 0.01 and †††P < 0.0001 denoting a significant difference from the chow-fed diet condition within phenotype.
Sensitivity to Ex-4.
Ex-4 produced a significant reduction in 1- and 3-h food intake in rats maintained on chow, irrespective of phenotype (P < 0.05). However, although the lowest doses of Ex-4 (0.625 and 1.25 μg/kg) suppressed intake significantly in OR rats fed the HE/HF-diet, it failed to decrease 1- and 3-h food intake in OP rats maintained on the same diet (P > 0.05; Table 1). The two highest doses of Ex-4 (2.5 and 5.0 µg/kg) reduced food intake at 1, 3, and 24 h compared with saline, and this effect was significant for both diets and phenotypes. Furthermore, the percentage suppression of food intake at 1 and 3 h was significantly lower in OP rats than in OR rats fed HE/HF diet for all Ex-4 doses tested (P < 0.05). However, there were no significant differences at 24 h in the percentage suppression between OP and OR rats fed chow or the HE/HF diet.
TABLE 1.
Food intake in OP and OR rats after Ex-4 administration
GLP-1 protein and mRNA expression in intestinal epithelium.
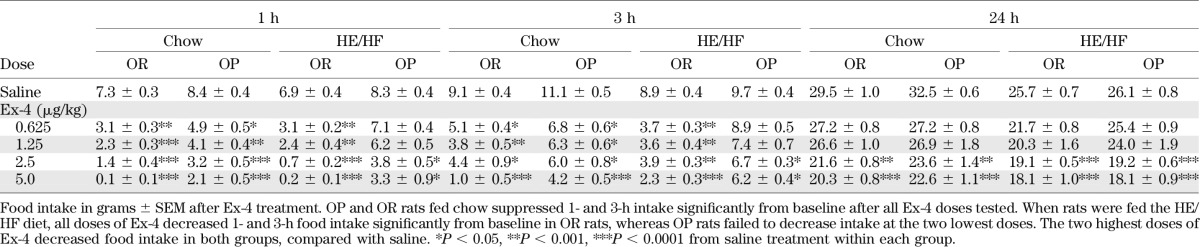
There was a significant decrease in GLP-1 protein, but not mRNA, expression in HE/HF-fed OP rats compared with HE/HF-fed OR rats and chow-fed OP rats (P < 0.05 for both; Fig. 2A).
FIG. 2.
Proximal intestinal epithelial cell expression of GLP-1 protein (A), mRNA transcript of GLP-1R in the nodose ganglia (B), and total (C) and active (D) GLP-1 plasma concentrations after brief (5-h) fast in OP and OR rats. A: OP rats had decreased GLP-1 protein expression during HE/HF-feeding compared with HE/HF-fed OR and chow-fed OP rats. B: HE/HF-fed OP rats had decreased gene expression of GLP-1R compared with chow-fed OP rats. HE/HF-fed OP rats had decreased circulating total (C) and active (D) GLP-1 compared with HE/HF-fed OR and chow-fed OP rats. Data are expressed as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.0001 denoting a significant difference from OR rats within diet condition. †P < 0.05 and ††P < 0.01 denoting significant difference from chow-fed diet condition within phenotype.
GLP-1R mRNA expression in the nodose ganglia.
HE/HF-feeding resulted in significant downregulation of nodose ganglia GLP-1R mRNA in OP, but not OR, rats (P < 0.05; Fig. 2B). There were no significant differences in GLP-1R mRNA expression between phenotypes when rats were maintained on chow.
Circulating GLP-1.
Total and active GLP-1 plasma levels were significantly decreased in HE/HF-fed OP rats compared with HE/HF-fed OR rats (total: P < 0.01; active: P < 0.001) and chow-fed OP rats (total: P < 0.05; active: P < 0.01; Fig. 2C and D). There was no significant difference in circulating GLP-1 between phenotypes when rats were maintained on chow, and there was no effect of the diet on GLP-1 in OR rats.
Immunofluorescence.
GLP-1 was present in cells lining the villi of the distal ileum (Fig. 3A). The number of GLP-1– containing L cells was similar between strains during chow-feeding; however, OP rats fed the HE/HF diet exhibited significantly fewer GLP-1–positive EECs than OR HE/HF-fed rats (P < 0.0001) and OP rats fed standard chow (P < 0.001; Fig. 3B).
FIG. 3.
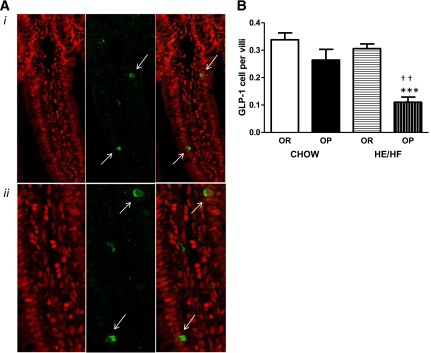
Representative images (A) and total count (B) of GLP-1–containing enteroendocrine cells (indicated by white arrows) in the distal ileum of OP and OR rats. A, i: Chow-fed OR rat villus with stained nuclei (left), stained with GLP-1 antibody (middle), and combined nuclei and GLP-1 staining (right) at original magnification ×40. A, ii: On the images above, we used a digital zoom function, resulting in three times the original magnification. B: HE/HF-feeding led to a decreased number of L cells per villi in the distal ileum of OP rats compared with chow-fed OP and HE/HF-fed OR rats. Data are expressed as means ± SEM. ***P < 0.0001 denoting significant difference from OR rats within diet condition. ††P < 0.01 denoting significant difference from chow-fed diet condition within phenotype.
DISCUSSION
Our results demonstrate that HF-feeding in animals prone to obesity results in impaired GLP-1 satiation signaling. Specifically, OP animals maintained on the HE/HF diet exhibit decreased sensitivity to the satiating effects of Ex-4, a GLP-1R agonist, compared with OR rats fed a similar diet or their counterparts fed chow. Furthermore, and similar to previous studies, we found that during HE/HF-feeding, OP rats gained significantly more weight, had increased body adiposity, and consumed more calories during 24 h than OR rats (4,5). Increased energy intake in HE/HF-fed OP rats is a function of increased meal size (4), indicating an inability of OP rats to effectively suppress appetite and calorie intake after a meal. Indeed, diet-induced obese rats exhibit decreased sensitivity to intraintestinal lipid-induced satiation compared with diet-resistant rats, an effect associated with diminished GLP-1 protein expression in the intestinal epithelium (5).
Fat is a potent GLP-1 secretagogue (12), and GLP-1 is thought to contribute to meal-induced satiation (8) by acting in a vagal-dependent manner (13). Therefore, we hypothesized that decreased responsiveness to lipids in OP rats may result from defective GLP-1 signaling during HE/HF-feeding; however, whether this phenomenon is an effect of HF-feeding, or of the ensued obesity or both, is not known. Here, we found that chow-fed OP and OR rats suppress food intake equally after all doses of Ex-4. In contrast, during HE/HF feeding, Ex-4 failed to inhibit intake or was less efficacious in suppressing intake in OP compared with OR rats, suggesting that obesity and HF-feeding interact to aggravate the deficits in GLP-1 signaling.
GLP-1 exerts its anorectic effect most likely in a paracrine-like fashion on vagal afferent terminals (13). Therefore, the decrease in short-term responsiveness to Ex-4 during HE/HF-feeding in OP rats is likely a result of reduced peripheral vagal afferent activation. Indeed, HF-fed animals exhibit reductions in vagal afferent sensitivity and receptor expression (14,15), and likewise, GLP-1R mRNA in the nodose ganglia was decreased in HE/HF-fed OP rats in the current study. Reduced vagal responsiveness to Ex-4 in OP rats may also be due to dysregulation between GLP-1 and leptin signaling, because vagal afferents contain leptin and GLP-1 receptors (5,16) and leptin enhances the response to GLP-1 (17). Indeed, leptin resistance occurs rapidly in the vagal afferents of obese rats fed an HF diet (18), and diminished vagal response to leptin may impair the ability of GLP-1 to activate vagal afferents, as has been demonstrated for CCK (19). Although the synergistic effect of leptin and GLP-1 is not vagally mediated (17), it is possible that leptin resistance in OP rats contributes to reduced responsiveness to Ex-4 via postvagal afferent activation on downstream central nervous system neurons (20).
Reduced sensitivity to Ex-4 by HE/HF-feeding during obesity was seen at 1 and 3 h after injection, whereas 24-h suppression of food intake remained similar between OP and OR rats fed both diets tested. Because Ex-4 has a much longer half-life than endogenous GLP-1 (21) and can cross the blood–brain barrier (22), the long-term reduction of food intake after Ex-4 is likely mediated by central GLP-1R populations, possibly in the nucleus tractus solitarius (NTS) or paraventricular hypothalamus (13). This long-term sensitivity to Ex-4 during obesity is consistent with previous reports (23,24).
The decrease in endogenous GLP-1 protein levels may further contribute to impaired satiation signaling in OP animals, ultimately exacerbating hyperphagia and weight gain. This agrees with our previous work, albeit in a different rat model, which showed that diet-induced obese rats exhibit decreased responsiveness to intestinal lipid, which was associated with reduced GLP-1 protein expression in intestinal mucosa (5). Furthermore, plasma levels of GLP-1 were decreased in OP rats only during HE/HF-feeding and were likely a consequence of decreased intestinal GLP-1 protein and L-cell numbers in the distal ileum. Reductions in active GLP-1 levels were likely secondary to reduced total GLP-1 levels observed and not due to enhanced DPP-4 activity, which is similar to the finding in obese humans (25).
Taken together, decreased endogenous GLP-1 signaling, through decreased nutrient-induced GLP-1 release and decreased vagal sensitivity to GLP-1, likely results in reduced sensitivity to luminal nutrients in diet-induced obese rats (5). The observed effects are only present during HE/HF-feeding and obesity, indicating an interaction of the diet and the genetic make-up of OP rats. In conclusion, our studies demonstrate an overall impairment in the ability of GLP-1 to reduce meal size that may contribute to overconsumption and excess weight gain after HF-feeding in the obese, thus perpetuating obesity.
ACKNOWLEDGMENTS
This study was supported by INRA through a scientific package awarded to M.C.
No potential conflicts of interest relevant to this article were reported.
F.A.D. and M.C. designed the study, researched data, and wrote the manuscript. Y.S. researched data and reviewed the article. M.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378:815–825 [DOI] [PubMed] [Google Scholar]
- 2.Bouchard C, Pérusse L. Genetics of obesity. Annu Rev Nutr 1993;13:337–354 [DOI] [PubMed] [Google Scholar]
- 3.Levin BE. Developmental gene x environment interactions affecting systems regulating energy homeostasis and obesity. Front Neuroendocrinol 2010;31:270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 2003;11:845–851 [DOI] [PubMed] [Google Scholar]
- 5.Duca FA, Swartz TD, Sakar Y, Covasa M. Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int J Obes (Lond) 1 May 2012. Available at http://www.nature.com/ijo/journal/vaop/ncurrent/full/ijo201245a.html Accessed 1 July 2012 [DOI] [PubMed] [Google Scholar]
- 6.Greenberg D, McCaffery J, Potack JZ, Bray GA, York DA. Differential satiating effects of fats in the small intestine of obesity-resistant and obesity-prone rats. Physiol Behav 1999;66:621–626 [DOI] [PubMed] [Google Scholar]
- 7.Duca FA, Covasa M. Current and emerging concepts on the role of peripheral signals in the control of food intake and development of obesity. Br J Nutr 2012;108:778–793 [DOI] [PubMed] [Google Scholar]
- 8.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 2009;150:1680–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Näslund E, Hellström PM. Glucagon-like peptide-1 in the pathogenesis of obesity. Drug News Perspect 1998;11:92–97 [DOI] [PubMed] [Google Scholar]
- 10.Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc 2010;110:571–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams DL, Hyvarinen N, Lilly N, et al. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav 2011;103:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little TJ, Feltrin KL, Horowitz M, et al. Dose-related effects of lauric acid on antropyloroduodenal motility, gastrointestinal hormone release, appetite, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol 2005;289:R1090–R1098 [DOI] [PubMed] [Google Scholar]
- 13.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 2010;100:503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol 2011;589:2857–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulino G, Barbier de la Serre C, Knotts TA, et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab 2009;296:E898–E903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vahl TP, Tauchi M, Durler TS, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 2007;148:4965–4973 [DOI] [PubMed] [Google Scholar]
- 17.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 2006;55:3387–3393 [DOI] [PubMed] [Google Scholar]
- 18.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab 2011;301:E187–E195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS ONE 2012;7:e32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner . Int J Obes (Lond) 2012;36:1522–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab 2009;94:1843–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 2003;27:313–318 [DOI] [PubMed] [Google Scholar]
- 23.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 2011;19:1342–1349 [DOI] [PubMed] [Google Scholar]
- 24.Reidelberger RD, Haver AC, Apenteng BA, Anders KL, Steenson SM. Effects of exendin-4 alone and with peptide YY(3-36) on food intake and body weight in diet-induced obese rats. Obesity (Silver Spring) 2011;19:121–127 [DOI] [PubMed] [Google Scholar]
- 25.Carr RD, Larsen MO, Jelic K, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 2010;95:872–878 [DOI] [PubMed] [Google Scholar]