Abstract
Proliferative diabetic retinopathy (PDR) is the most severe vision-threatening complication of diabetes. For investigation of genetic association between TCF7L2 and PDR in Caucasian type 2 diabetes mellitus (T2DM) and its functional consequences, 383 T2DM patients with PDR (T2DM-PDR) and 756 T2DM patients without diabetic retinopathy (T2DM–no DR) were genotyped with rs7903146 in TCF7L2. We found that risk allele (T) frequency of rs7903146 was significantly higher in T2DM-PDR patients (allelic P = 2.52E-04). In lymphoblastoid cells induced to undergo endoplasmic reticulum (ER) stress by treatment of tunicamycin, higher fold change of TCF7L2 and VEGFA mRNA levels were observed in rs7903146-TT cells than in rs7903146-CC cells (P = 0.02 for TCF7L2; P = 0.004 for VEGFA), suggesting that ER stress plays a role in PDR pathogenesis. Silencing TCF7L2 resulted in decreased mRNA levels of both TCF7L2 and VEGFA (P < 0.001). Retinas of oxygen-induced retinopathy mice (a model for PDR) had higher TCF7L2 and VEGFA mRNA levels than those of controls (P = 2.9E-04 for TCF7L2; P = 1.9E-07 for VEGFA). Together, data from our study show that TCF7L2-rs7903146 is associated with PDR in Caucasian T2DM and suggest that TCF7L2 promotes pathological retinal neovascularization via ER stress–dependent upregulation of VEGFA.
The prevalence of diabetes mellitus is steadily increasing worldwide. Currently, diabetes mellitus affects 25.8 million people in the U.S. Diabetic retinopathy is the most common cause of diabetes mellitus complications and the leading cause of new cases of blindness among adults aged 20–74 years. Proliferative diabetic retinopathy (PDR) is the most severe vision-threatening complication of diabetes mellitus (1,2). Ischemia-induced angiogenesis and expansion of extracellular matrix in association with neovascularization are the pathological hallmarks in PDR. Neovascularization can ultimately result in vitreous hemorrhage and tractional retinal detachment, producing severe and often irreversible vision loss (3,4). Previous studies demonstrate that severe diabetic retinopathy aggregates in families (5), suggesting that genetic susceptibilities contribute to disease development. However, only a few genes, such as VEGF and EPO, have been identified to be associated with PDR to date (2,6).
Transcription factor 7-like 2 (TCF7L2 [also known as TCF4]) is a key component in the Wnt-signaling pathway, which regulates fundamental processes such as vascular development and has been found to mediate pathological neovascularization in proliferative retinopathy (4,7). Common variant rs7903146 in TCF7L2 has been reported to be strongly associated with type 2 diabetes mellitus (T2DM), and this finding has been replicated in many studies (8,9).
It was reported in some small studies that TCF7L2 gene is linked with microvascular and macrovascular complications, such as diabetic retinopathy (10), but the mechanism remains unclear. Here, we investigated the role of TCF7L2 in PDR using a genetic association study and by analyzing the expression of TCF7L2 and VEGFA in human lymphoblastoid cells, ARPE-19 cells, and oxygen-induced retinopathy (OIR) mice.
RESEARCH DESIGN AND METHODS
This study was approved by the institutional review boards of University of California, San Diego, and West China Hospital. All subjects gave informed consent prior to participation. A two-stage approach was performed for this study. In stage one, 209 T2DM patients with PDR (T2DM-PDR) and 442 T2DM patients without diabetic retinopathy (T2DM–no DR) were used as a discovery cohort. In stage two, 174 T2DM-PDR and 314 T2DM–no DR patients served as the replication cohort. All patients were of European descent. The control subjects were defined as having no retinopathy and having T2DM for a minimum of 15 years. Characteristics of participants in the study are listed in Supplementary Table 1.
To investigate whether TCF7L2 is also associated with PDR in type 1 diabetes mellitus (T1DM), 372 T1DM-PDR and 417 T1DM–no DR Caucasian patients were studied. The control subjects were defined as having no retinopathy and having T1DM for a minimum of 15 years.
Clinical assessment.
Participants underwent detailed eye examinations using the Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol with seven-standard-field stereoscopic fundus photography. Retinopathy status was determined by evaluation of fundus photographs and graded according to clinical ETDRS criteria. Patients with any disk neovascularization, neovascularization elsewhere, vitreous hemorrhage, fibrovascular proliferation, or tractional retinal detachment were considered to have PDR. Retinopathy grading was performed without prior knowledge of genotypes.
Genotyping.
Genomic DNA was extracted from peripheral blood leukocytes with a Qiagen kit (Qiagen, Chatsworth, CA) according to the manufacturer’s instructions. rs7903146 (C/T) in TCF7L2 was genotyped using single-nucleotide primer extension assay (ABI Prism SNaPShot Multiplex kit; Applied Biosystems) on an ABI 3130xl genetic analyzer as previously described (11). The primers used for genotyping are listed in Supplementary Table 2.
Tunicamycin-treated lymphoblastoid cell lines.
The human lymphoblastoid cell lines were generated by the method “general protocol for the immortalization of human B-lymphocytes using EBV” (http://www.unclineberger.org/tissueculture/protocols/b-lymphocytesprotocol). Nine lymphoblastoid cell lines with the genotypes of TCF7L2 rs7903146-CC and another nine with the genotypes of TCF7L2 rs7903146-TT were used. The cells were cultured in RPMI base medium (61870127; Invitrogen) supplemented with 20% FBS and 1×Penicillin-Streptomycin (P0781; Sigma). On the day before treatment, the cells were diluted to ∼2 × 105/mL. On the day of treatment, tunicamycin (T7765; Sigma) was added to a final concentration of 10 µg/mL. RNA was extracted at 12 h posttreatment. The total mRNA levels of TCF7L2 and VEGFA were measured by quantitative RT-PCR (qRT-PCR). For monitoring for endoplasmic reticulum (ER) stress in human lymphoblastoid cell lines, RNA expression of XBP1 isoforms was used as a positive marker (12).
Lentivirus-mediated TCF7L2 short hairpin RNA knockdown in ARPE-19 cells.
Retinal pigment epithelium ARPE-19 cells were cultured in Dulbecco’s modified Eagle’s medium/F12 (1:1) base medium (11320; GIBCO) supplemented with 10% FBS and 1×Penicillin-Streptomycin (P0781; Sigma) and 1×MEM NEAA (11140; GIBCO). Short hairpin RNA (shRNA) sets in pLKO.1 clones against TCF7L2 (NM_030756.1) were purchased from Sigma-Aldrich. ARPE-19 cells were infected with control lentivirus or lentivirus-mediated TCF7L2 shRNA. RNA was extracted at 48 h posttreatment. The total mRNA levels of TCF7L2 and VEGFA were measured by qRT-PCR. DNMT1 (DNA methyltransferase 1) gene was used as a control gene to test whether there are off-target effects for the shRNA in our study.
OIR mouse model.
Retinal neovascularization was produced in C57BL/6 mice by placing postnatal day 7 (P7) mice and their mothers in an atmosphere of 75 ± 3% oxygen for 5 days (13). Oxygen concentration was automatically monitored and controlled by an oxygen controller (BioSpherix). At P12, the mice were returned to room air for 5 days. At P17, the mice were killed and the retinas were removed. Total RNA was isolated from the retinas of OIR mice and littermate controls. TCF7L2 and VEGFA mRNA levels were measured by real-time qRT-PCR.
Real-time quantitative PCR.
Total RNA extraction from mouse tissues or human lymphoblastoid cells, cDNA synthesis, and qRT-PCR experiments were performed as previously described (14). Assays were performed in triplicate. Relative mRNA levels were calculated by normalizing results using GAPDH and then compared with tissues from wild-type littermates or untreated human lymphoblastoid cells. The primers used for qRT-PCR are listed in Supplementary Table 2.
Western blot analysis.
Retinal protein extracts were lysed in radioimmunoprecipitation assay buffer with a mixture of protease inhibitors (Santa Cruz Biotechnology, Santa Cruz, CA). The total protein concentration was quantified by BCA protein assay (15). Twenty micrograms of total protein were used for blotting with anti–phosphorylated (p)–eukaryotic initiation factor (p-eIF2)α (Ser51) (Cell Signaling Technology, Boston, MA). The same membrane was stripped and reblotted with an anti–β-actin antibody (Santa Cruz Biotechnology) as internal control. The intensities of Western blot bands were quantified using the Multi Gauge, version 3.0 software (Fuji Film, Tokyo, Japan).
Statistical analysis.
Logistic regression analysis was used to compare the difference between the T2DM-PDR and T2DM–no DR groups. SNP genotyping results were screened for deviation from Hardy-Weinberg equilibrium using χ2 tests. The differences in quantitative PCR data were analyzed with independent two-sample t test. Covariable analysis was done by SPSS software. Statistical significance was defined as P < 0.05. Pearson correlation coefficient was used to calculate the correlation between TCF7L2 and VEGFA (16).
RESULTS
Association of TCF7L2-rs7903146 genotypes with PDR.
We performed genetic association study and genotyped a candidate gene TCF7L2 variant rs7903146 in T2DM-PDR case and T2DM–no DR control subjects. Information on demographics of case and control groups is presented in Supplementary Table 1. While there were no significant differences between the T2DM-PDR and T2DM–no DR groups with regard to sex, there was a statistically significant difference in age and BMI between the two groups (P < 0.05). The lower BMI was observed in the T2DM–no DR group.
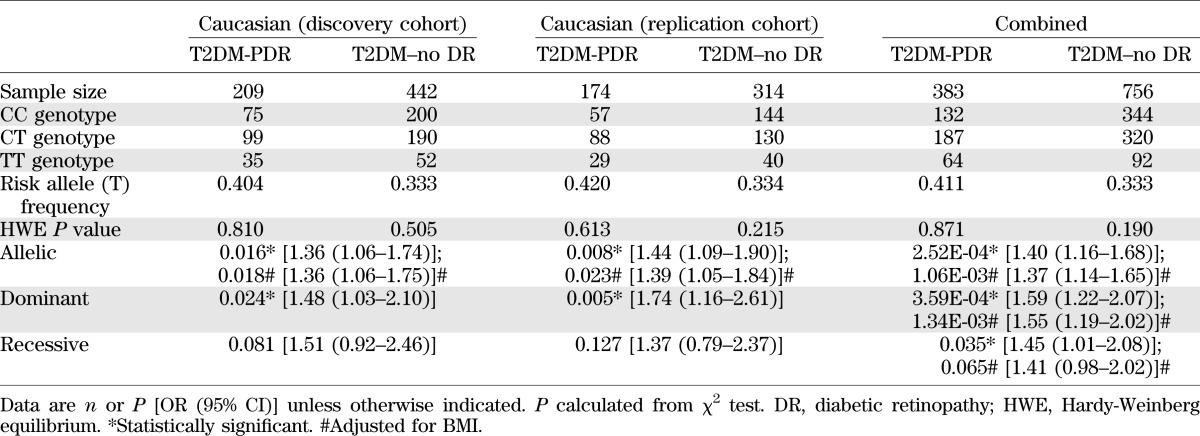
In the discovery cohort, the T risk allele frequency of rs7903146 in TCF7L2 was significantly higher in patients with PDR (T2DM-PDR, 40.4%) than in patients without diabetic retinopathy (T2DM–no DR, 33.3%) (allelic P = 0.016). We replicated this association in a second T2DM cohort. In this replication cohort, the T-allele frequency of rs7903146 was also significantly higher in T2DM-PDR (42.0%) than in T2DM–no DR (33.4%) (allelic P = 0.008). Together, a combined analysis showed that the risk allele (T) of rs7903146 was strongly associated with T2DM-PDR (allelic P = 2.52E-04, odds ratio [OR] 1.40 [95% CI 1.16–1.68]). After adjustment for BMI, the risk allele (T) of rs7903146 remained significantly associated with T2DM-PDR (adjusted allelic P = 1.06E-03; OR 1.37 [1.14–1.65]). (Table 1).
TABLE 1.
Genotype and association results of TCF7L2-rs7903146 in patients with T2DM-PDR and T2DM–no DR
To test whether this positive association can also be applied to a T1DM population, we genotyped 372 T1DM-PDR patients and 417 T1DM–no DR patients. There is no significant difference for the frequency of rs7903146 T allele between T1DM-PDR (28.0%) and T1DM–no DR (30.3%) (allelic P = 0.300) (Supplementary Table 3).
ER stress increases VEGFA and TCF7L2 expression.
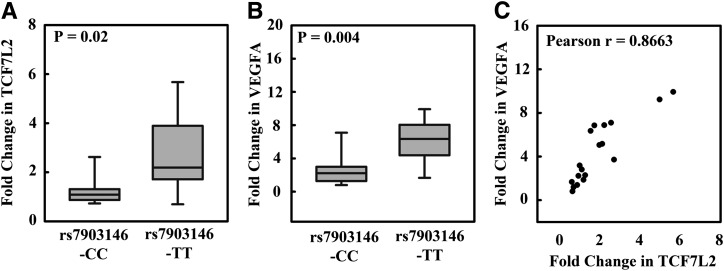
ER stress, which can be induced with tunicamycin, has been linked to vascular abnormalities in diabetes (17). We therefore tested whether treating human lymphoblastoid cells with tunicamycin could alter gene expression profiles of TCF7L2 and VEGFA. After treating human lymphoblastoid cells with tunicamycin, we observed a significantly higher fold change of mRNA levels of TCF7L2 in cells with a TCF7L2 rs7903146-TT risk genotype (P = 0.02) (Fig. 1A). Similarly, we observed increased fold change of mRNA level of VEGFA in cells with an rs7903146-TT risk genotype (P = 0.004) (Fig. 1B). There was also a positive correlation between the fold change of the expression of TCF7L2 and VEGFA in tunicamycin-treated cells (Pearson r = 0.8663) (Fig. 1C).
FIG. 1.
Quantitative gene expression of VEGFA and TCF7L2 in tunicamycin-treated lymphoblastoid cells. A: Compared with that in untreated cells, the TCF7L2 mRNA level in tunicamycin-treated cells was 1.31 ± 0.18-fold higher with an rs7903146-CC genotype and 2.68 ± 0.54-fold higher with an rs7903146-TT (P = 0.02). B: The VEGFA mRNA level in tunicamycin-treated cells was 2.66 ± 0.60-fold higher in rs7903146-CC cells than that in untreated cells and 6.09 ± 0.86-fold higher in rs7903146-TT cells than that in untreated cells (P = 0.004). C: The fold change of VEGFA mRNA level correlated well with that of TCF7L2 mRNA level, Pearson r = 0.8663.
We did not observe a significant difference in the total expression levels of TCF7L2 in the cell lines with an rs7903146-CC or rs7903146-TT genotype at the baseline or after tunicamycin treatment (at baseline, P = 0.853; after tunicamycin treatment, P = 0.390) (Supplementary Fig. 1A).
Similarly, there was no significant difference in the total expression level of VEGFA in cell lines with an rs7903146-CC or rs7903146-TT genotype at the baseline (P = 0.927). However, there was a significant higher expression of VEGFA in the cell lines with rs7903146-TT than in rs7903146-CC after tunicamycin treatment (P = 0.004) (Supplementary Fig. 1B). There was no correlation between total transcripts of TCF7L2 and VEGFA without calculation of fold change (Pearson r = −0.1) (Supplementary Fig. 1C).
The changes in gene expression mentioned above were not due to alterations in the present experimental conditions, as the level of a housekeeping gene (GAPDH) remained the same throughout the experiment (Supplementary Fig. 1D) and was used for normalization of TCF7L2 and VEGFA expression in fold change calculations. In the cells treated with tunicamycin, ER stress was induced as shown by alternative splicing of XBP1. Two bands (unspliced and spliced band) were detected for the tunicamycin-treated cells (Supplementary Fig. 2).
Effect of silencing TCF7L2 on VEGFA expression.
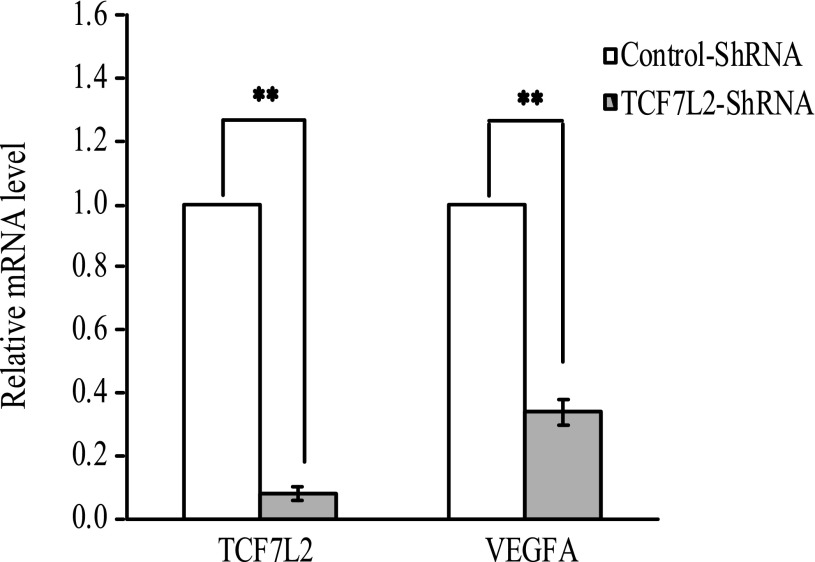
To validate the role of TCF7L2 in modulation of VEGFA expression, we performed an RNA silencing experiment by knocking down the expression of TCF7L2 in ARPE-19, and we observed concomitant 66% reduction of VEGFA mRNA level (P < 0.001) (Fig. 2).
FIG. 2.
Quantitative gene expression of TCF7L2 and VEGFA after silencing by TCF7L2-shRNA in ARPE-19 cells (n = 3). ARPE-19 cells infected with TCF7L2-shRNA lentivirus had a 92% decrease in TCF7L2 gene expression and 66% decrease in VEGFA compared with cells infected with control-shRNA lentivirus (P < 0.001). **P < 0.001.
For testing of whether there were off-target effects by shRNA, DNMT1 was used as an endogenous control in our study. The results showed that the expression of DNMT1 gene remained the same before or after shRNA treatment (Supplementary Fig. 3).
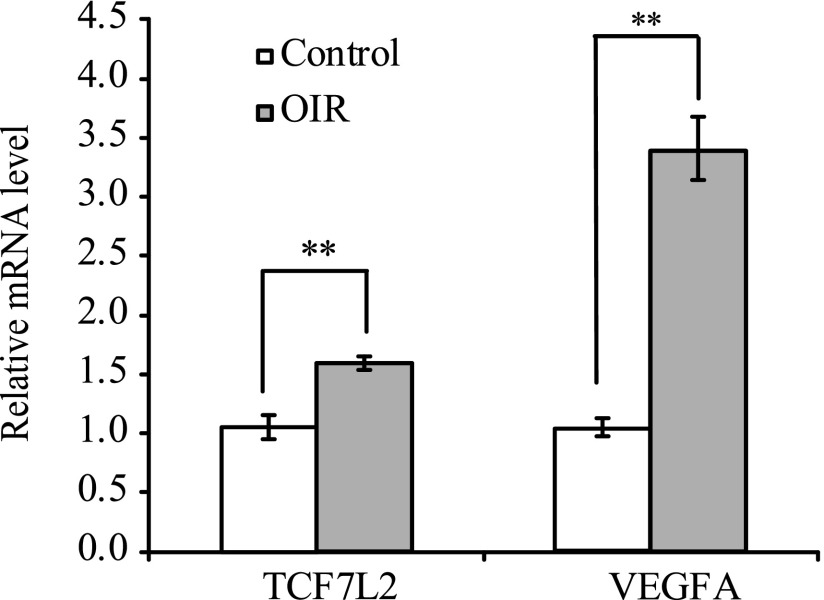
TCF7L2 and VEGFA mRNA levels were elevated in OIR mice.
OIR mice model has been used extensively to study retinal ischemia and PDR. We measured mRNA levels in retinas from the OIR model. TCF7L2 and VEGFA mRNA expression was significantly higher in retinas from OIR mice compared with controls (P = 2.90E-04 for TCF7L2; P = 1.90E-07 for VEGFA) (Fig. 3). The expression of p-eIF2α, a positive marker for ER stress, was significantly upregulated in OIR mice retinas (1.72 ± 0.22-fold) (Supplementary Fig. 4).
FIG. 3.
Quantitative gene expression of TCF7L2 and VEGFA in OIR mouse retinas (n = 9). Compared with those in controls, OIR mice retinas had higher TCF7L2 mRNA expression (P = 2.90E-04) and VEGFA mRNA expression (P = 1.90E-07). **P < 0.001.
DISCUSSION
TCF7L2 is the most significant susceptibility gene for T2DM thus far identified (9,18). However, the association between TCF7L2 and diabetic retinopathy has been conflicting (10,19). The discordant results in these studies may be due to a small sample size or the lack of distinction between the two stages of diabetic retinopathy: nonproliferative diabetic retinopathy (NPDR) and PDR. There are patients with NPDR who never progress to PDR despite poor glycemic control and long-standing diabetes, suggesting that there may be genetic factors predisposing patients to the development of PDR from NPDR. Our study therefore differentiates from other prior studies by taking the most extreme diabetic retinopathy phenotype (most severe phenotype). Here, we found that TCF7L2-rs7903146 is associated not only with T2DM (Supplementary Table 4) but also with PDR in Caucasian patients with T2DM. The allele (T) of rs7903146 is the same risk allele for T2DM-PDR as well as T2DM.
In patients with PDR, the expression of VEGFA is markedly upregulated (20). In our OIR study, the expression of both TCF7L2 and VEGFA was found to be significantly higher compared with controls. These findings provide evidence that TCF7L2, a key component of the Wnt-signaling pathway, plays an important role in pathological neovascularization.
ER stress pathway has been linked to vascular abnormalities in diabetes and diabetic retinopathy (17,21). VEGFA is one of the target genes of TCF7L2 (22). To study whether ER stress played a role in TCF7L2 and VEGFA expression, we tested the expression of TCF7L2 and VEGFA in the tunicamycin-treated lymphoblastoid cell lines stratified with genotypes of rs7903146. We showed that after treatment of human lymphoblastoid cells with tunicamycin, a more significant increase in TCF7L2 and VEGFA expression was observed in cells with an rs7903146 TT risk allele than in those with a nonrisk CC allele (P = 0.02 and P = 0.004, respectively). There are two TCF7L2-binding sites in the VEGFA promoter region, which may play a role in upregulating VEGFA transcription through TCF7L2 binding (23). Our results are correlated with those of a previous study that showed that the expression of VEGFA in human lymphoblastoid cell lines is induced after treatment with an ER stressor (12). ER stress is also an important feature of chronic metabolic disease that is linked to both metabolic and immune regulation (24). It has been reported that lymphoblastoid cells from T2DM subjects with the genotypes of TT/TC have a higher expression of TCF7L2 in basal condition (25). One of the plausible explanations is that the lymphoblastoid cells from T2DM subjects may already suffer from ER stress and that the T risk allele confers additional susceptibility to ER stress. The results indicate that ER stress may play an important role in triggering the increase of the expression of TCF7L2 and VEGFA.
The OIR mouse model has been widely used in studies related to PDR (13). In this model, neovascularization and proliferative retinopathy develop reliably (and quantifiably) over 17 days (26). Activation of ER stress and increased VEGFA can be observed in OIR mice models (15). We found that the ER stress–positive marker p-eIF2α increased in OIR mice retinas. OIR mice retinas had higher TCF7L2 and VEGFA mRNA levels than those of controls. These findings suggested that TCF7L2 may be a potential mediator of retinal neovascularization activated by ER stress during retinal neovascularization.
Some evidence indicates that other risk factors, such as BMI, are implicated in the pathogenesis of diabetic retinopathy (27,28). Our study demonstrated a relationship between higher BMI and an increased risk of T2DM-PDR.
There are some limitations in our study: 1) human lymphoblastoid cell lines are a reasonable choice of cells to study the effect of genotype, but they will only be a surrogate model for cells in retina; 2) tunicamycin is a strong chemical ER stressor and not entirely relevant in vivo; and 3) it will be valuable to analyze more covariables like T2DM onset, HbA1c levels (uncontrolled glycemia), fasting glucose, fatty acid, lipid profile, or blood pressure.
In summary, our study shows that TCF7L2 is associated with PDR in Caucasian T2DM subjects. Our results demonstrate that TCF7L2 expression is altered under ER stress and promote pathological retinal neovascularization via upregulation of VEGFA. Our findings also provide genetic evidence further implicating the Wnt/β-catenin–signaling pathway in the pathogenesis of PDR and suggest that TCF7L2 may serve as a potential therapeutic target for the treatment of PDR.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from 973 Program (2011CB510200 and 2013CB967504); National Institutes of Health (EY018660, EY021374, EY019270, and EY014428); King Abdulaziz City for Science and Technology and the University of California, San Diego, Center of Excellence in Nanomedicine; Burroughs Wellcome Fund Clinical Scientist Award in Translational Research; Research to Prevent Blindness; Clinical and Translational Research Institute 1TL1RR031979-01; Cancer Training Grant T32 CA009523; and the VA Merit Award.
No potential conflicts of interest relevant to this article were reported.
J.Lu., L.Z., and A.Y.C. designed the study, performed the study, and analyzed and interpreted data. L.Z., A.Y.C., and J.Le. wrote the manuscript. X.Z., J.Zhu, J.Zha., H.O., H.L., Y.S., J.Le., S.P., P.X.S., and S.S. performed the study. Y.Z. designed the study and analyzed and interpreted data. M.G.R. supervised the overall project and designed the study. K.Z. supervised the overall project, designed the study, analyzed and interpreted data, wrote the manuscript, and was the principal investigator. K.Z. is the guarantor of this work and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the participating diabetic patients and their families, G. Hughes (University of California, San Diego), and members of Zhang laboratory for assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1093/-/DC1.
REFERENCES
- 1.Abbate M, Cravedi P, Iliev I, Remuzzi G, Ruggenenti P. Prevention and treatment of diabetic retinopathy: evidence from clinical trials and perspectives. Curr Diabetes Rev 2011;7:190–200 [DOI] [PubMed] [Google Scholar]
- 2.Tong Z, Yang Z, Patel S, et al. Genetics of Diabetes and Diabetic Complication Study Group Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci USA 2008;105:6998–7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong DS, Aiello L, Gardner TW, et al. American Diabetes Association Diabetic retinopathy. Diabetes Care 2003;26(Suppl. 1):S99–S102 [DOI] [PubMed] [Google Scholar]
- 4.Zhang B, Zhou KK, Ma JX. Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the WNT/beta-catenin pathway. Diabetes 2010;59:1809–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arar NH, Freedman BI, Adler SG, et al. Family Investigation of Nephropathy and Diabetes Research Group Heritability of the severity of diabetic retinopathy: the FIND-Eye study. Invest Ophthalmol Vis Sci 2008;49:3839–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–1487 [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Stahl A, Krah NM, et al. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation 2011;124:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 2006;38:320–323 [DOI] [PubMed] [Google Scholar]
- 9.Tong Y, Lin Y, Zhang Y, et al. Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: a large Human Genome Epidemiology (HuGE) review and meta-analysis. BMC Med Genet 2009;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciccacci C, Di Fusco D, Cacciotti L, et al. TCF7L2 gene polymorphisms and type 2 diabetes: association with diabetic retinopathy and cardiovascular autonomic neuropathy. Acta Diabetol. 28 July 2012 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 2006;314:992–993 [DOI] [PubMed] [Google Scholar]
- 12.Dombroski BA, Nayak RR, Ewens KG, Ankener W, Cheung VG, Spielman RS. Gene expression and genetic variation in response to endoplasmic reticulum stress in human cells. Am J Hum Genet 2010;86:719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35:101–111 [PubMed] [Google Scholar]
- 14.Zhang L, Lim SL, Du H, et al. High temperature requirement factor A1 (HTRA1) gene regulates angiogenesis through transforming growth factor-β family member growth differentiation factor 6. J Biol Chem 2012;287:1520–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett 2009;583:1521–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neter J, Kutner MH, Wasserman W, Nachtsheim C. Applied Linear Statistical Models. 4th ed. Boston, McGraw Hill, 1996 [Google Scholar]
- 17.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 18.Saxena R, Elbers CC, Guo Y, et al. Look AHEAD Research Group. DIAGRAM consortium Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. Am J Hum Genet 2012;90:410–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Y, Klein R, Heiss G, et al. The transcription factor 7-like 2 (TCF7L2) polymorphism may be associated with focal arteriolar narrowing in Caucasians with hypertension or without diabetes: the ARIC Study. BMC Endocr Disord 2010;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pe’er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol 1996;80:241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oshitari T, Hata N, Yamamoto S. Endoplasmic reticulum stress and diabetic retinopathy. Vasc Health Risk Manag 2008;4:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masckauchán TN, Kitajewski J. Wnt/Frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology (Bethesda) 2006;21:181–188 [DOI] [PubMed] [Google Scholar]
- 23.Clifford RL, Deacon K, Knox AJ. Novel regulation of vascular endothelial growth factor-A (VEGF-A) by transforming growth factor (beta)1: requirement for Smads, (beta)-CATENIN, AND GSK3(beta). J Biol Chem 2008;283:35337–35353 [DOI] [PubMed] [Google Scholar]
- 24.Hummasti S, Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res 2010;107:579–591 [DOI] [PubMed] [Google Scholar]
- 25.Elbein SC, Chu WS, Das SK, et al. Transcription factor 7-like 2 polymorphisms and type 2 diabetes, glucose homeostasis traits and gene expression in US participants of European and African descent. Diabetologia 2007;50:1621–1630 [DOI] [PubMed] [Google Scholar]
- 26.Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 2009;4:1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim LS, Tai ES, Mitchell P, et al. C-reactive protein, body mass index, and diabetic retinopathy. Invest Ophthalmol Vis Sci 2010;51:4458–4463 [DOI] [PubMed] [Google Scholar]
- 28.Cheung N, Wong TY. Obesity and eye diseases. Surv Ophthalmol 2007;52:180–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.