Abstract
There is a critical clinical need to develop therapies for nonhealing diabetic foot ulcers. Topically applied mesenchymal stromal cells (MSCs) provide a novel treatment to augment diabetic wound healing. A central pathological factor in nonhealing diabetic ulcers is an impaired blood supply. It was hypothesized that topically applied allogeneic MSCs would improve wound healing by augmenting angiogenesis. Allogeneic nondiabetic bone-marrow derived MSCs were seeded in a collagen scaffold. The cells were applied to a full-thickness cutaneous wound in the alloxan-induced diabetic rabbit ear ulcer model in a dose escalation fashion. Percentage wound closure and angiogenesis at 1 week was assessed using wound tracings and stereology, respectively. The topical application of 1,000,000 MSCs on a collagen scaffold demonstrated increased percentage wound closure when compared with lower doses. The collagen and collagen seeded with MSCs treatments result in increased angiogenesis when compared with untreated wounds. An improvement in wound healing as assessed by percentage wound closure was observed only at the highest cell dose. This cell-based therapy provides a novel therapeutic strategy for increasing wound closure and augmenting angiogenesis, which is a central pathophysiological deficit in the nonhealing diabetic foot ulcer.
Diabetes is reaching epidemic proportions worldwide. Diabetic foot ulceration is the most frequent reason for hospitalization, and nonhealing ulceration may progress to amputation in spite of current standards of care. Nonhealing diabetic foot ulceration poses a major burden on individual patient health and healthcare budgets. Foot ulceration will affect 15–25% of people suffering from diabetes throughout their lives (1). Diabetes-related lower extremity amputation arises from pre-existing ulceration in 85% of cases (2). The high rate of progression from ulceration to amputation occurs despite standard care protocols. A central pathological factor in the treatment of nonhealing diabetic ulcers is impaired angiogenesis in the wound.
There is a critical clinical need to develop novel treatments to improve healing of diabetic foot ulcers. Mesenchymal stromal cells (MSCs) provide a novel therapeutic treatment and have been shown to be beneficial in diabetic wound healing (3). The mechanisms underlying the beneficial effect of wound healing include paracrine secretion of growth factors and chemokines requisite for wound healing and the differentiation into keratinocytes and endothelial cells required for wound healing and angiogenesis. MSCs can be delivered in an allogeneic fashion and possess immunosuppressant and immunomodulatory properties (4).
To date, there have been encouraging results in preclinical models of diabetic wound healing. Treatment with MSCs resulted in increased wound closure, new granulation tissue formation, and increased blood vessel formation and cellularity (5–8). In addition, 10 humans have received autologous MSCs, resulting in augmented wound healing. There is one report of an effect related to dose with autologous MSCs seeded in a fibrin spray (9). Nonetheless, there have been no studies using allogeneic human MSC transplantation in the setting of diabetic cutaneous ulceration. There is a paucity of data on effective dosing strategies in the literature. In this study, we report for the first time a dose-response evaluation of allogeneic transplantation of MSCs delivered through a collagen scaffold to an ulcer in a diabetic animal model.
Previous research has shown that infusions of several cell types into the body rapidly undergo cell death (10). After intravenous delivery, MSCs are found at low or very low frequencies in target organs (11). The use of biomaterials in conjunction with stem cell therapy in vivo may ensure sustained viability and functionality of cells (10). Collagen supports angiogenesis (12). A biomaterial such as collagen allows targeted delivery and positioning of high numbers of cells at the wound site. It was hypothesized that topical application of a collagen scaffold seeded with allogeneic nondiabetic bone marrow–derived MSCs would support angiogenesis and augment cutaneous wound closure in a diabetic animal model of cutaneous ulceration. The therapeutic effect of collagen seeded MSC therapy in a preclinical model using wound tracings and stereology was investigated. This technique is a scientifically robust validated strategy to assess in vivo tissue responses to tissue-engineered constructs.
RESEARCH DESIGN AND METHODS
MSC culture and characterization.
In vivo experiments were carried out under a license from the Department of Health, Ireland, and the National University of Ireland Galway Institutional Animal Use Committee. Allogeneic bone marrow–derived MSCs were isolated from healthy rabbit bone marrow as previously described (12). In brief, the animal was killed and bone marrow MSCs were isolated using collagenase digestion. Bone marrow aspirates were washed with Dulbecco’s PBS (Sigma-Aldrich, Arklow, Ireland), and precipitated mononuclear cells were suspended in MSC culture medium. Cells were grown in α-minimum essential medium (α-MEM) media (Gibco; Invitrogen, Carlsbad, CA) supplemented with 10% FBS (PAA Laboratories Ltd., Yeovil, U.K.) and 5% penicillin/streptomycin. Cells were maintained at 37°C and 95% humidity and 5% CO2 in the same medium. Nonadherent cells were washed off after 5 days and fresh medium was added. Colonies formed after 9 days and were trypsinized after 60–90% coverage with 0.25% trypsin/0.53 mmol/L ethylenediamine tetra-acetic acid (Sigma-Aldrich). Under appropriate culture conditions, differentiation assays were performed to confirm cell differentiation into chondrogenic, osteogenic, and adipogenic lineages (13). MSC (200,000) aliquots were frozen in liquid nitrogen at passage three and these cells were used for future experiments.
Fabrication of collagen scaffold and cell seeding.
Type 1 bovine collagen solution was isolated and purified as described previously (14). A collagen sponge was created by pipetting 500 µL of 3% (weight) type 1 bovine atelocollagen solution into 48-well tissue culture plates (Sarstedt Ltd., Wexford, Ireland). This was then lyophilized using a VirTis freeze-dryer (Suffolk, U.K). After washing in Hanks' balanced salt solution (Sigma-Aldrich), 70% ethanol, sterile water, and media, the collagen scaffold was transferred to one well of a 48-well cell culture plate (Sarstedt Ltd.). The frozen aliquots of MSCs were plated in a T75 tissue culture flask (Nunc; Thermo Fisher Scientific, Odense, Denmark). After 4 days, confluent MSCs were trypsinized and seeded by injecting cells in 1,000 µL of α-MEM–supplemented media using an insulin syringe (BD, Oxford, U.K.). Cells were placed in an incubator for 16 h at 37°C and 5% CO2. Prior to application to the wound, the cell scaffold was washed three times with serum-free media and twice with PBS.
Metabolic activity and fluorescent labeling of MSCs.
The metabolic activity of cells was assessed using alamarBlue (resazurin) (Invitrogen). Twenty-four hours after seeding, the cells were washed once in Hanks’ balanced salt solution (Sigma-Aldrich) and incubated for 3 h in 10% alamarBlue. This was performed at 24, 96, 144, and 366 h. This was performed for 50,000 and 1,000,000 rabbit MSCs seeded on a collagen scaffold. The absorbance of each sample was measured in a 96-well plate at wavelengths of 550 and 595 nm using a microplate reader. The percentage of reduced alamarBlue was determined as previously described (15). In one animal experiment, MSCs were labeled using PHK-26 (Sigma-Aldrich) and DAPI nucleic acid stain (Sigma-Aldrich) according to the manufacturer’s instructions, and the cells were imaged using a fluorescent microscope (Olympus).
Scanning electron microscope.
Scaffolds and scaffolds seeded with MSCs were rinsed in 0.1 mol/L phosphate buffer, pH 7.2, and fixed with 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer for 2 h at room temperature. The samples were dehydrated with ethanol and then placed in hexamethyldisilazane for 30 min. The MSC-seeded scaffolds were then gold coated and analyzed using a scanning electron microscope (Hitachi S-4700).
In vivo model.
Nine male New Zealand white rabbits (3–3.5 kg) were used in the study. The animals were outbred, 3–6 months of age, and purchased from Harlan Ltd. (Blackthorn, U.K.). The protocol was approved by the ethics committee of the National University of Ireland Galway and the study conducted under a license granted by the Department of Health and Children Dublin, Ireland. Rabbits were housed in individual cages, with a 12-h light/dark cycle and controlled temperature and humidity. Rabbits were fed a standard chow and water ad libitum.
Induction of hyperglycemia.
Rabbits were sedated with intramuscular injection of ketamine, xylazine, and acepromazine. Hair was shaved off the back of the ears. Alloxan (150 mg/kg) (Sigma-Aldrich) was made up in 30 mL of saline and administered via an ear vein using an intravenous cannula at a rate of 1.5 mL/min. After treatment, water containing glucose was provided for 24 h in addition to the provision of molasses to the animals’ front feet to prevent hypoglycemia. Serum blood glucose was checked daily using Accucheck advantage strips (Roche). Insulin therapy was administered if the animal lost weight and had “high” (>33 mmol/L) glucose readings using insulin glargine (Sanofi, Dublin, Ireland).
Surgical procedure.
After 5 weeks of hyperglycemia, rabbits were anesthetized using intramuscular injection of 0.1 mL/kg xylazine and 0.12 mL/kg ketamine. Sterile, disposable 6-mm punch biopsies were used to create three wounds on one ear and two wounds on the other ear. The wounds were created and dermis exposed to bare cartilage. Each wound was treated with one of five randomized treatment groups: 1) no treatment, 2) collagen scaffold alone, 3) collagen scaffold seeded with 50,000 MSCs, 4) collagen scaffold seeded with 100,000 MSCs, and 5) collagen scaffold seeded with 1,000,000 MSCs. The MSC-scaffold treatment was applied with the superior surface of the construct, which contained the majority of cells being applied to the base of the wound. The wounds were covered with a polyurethane dressing (OpSite; Smith & Nephew, London, U.K.), and the ear was stitched and covered with adhesive dressing (Operfix; Promedicare, Clonee, Ireland) until day 7 (n = 9). The animal received 5 mg/kg enrofloxacin antibiotic (Baytril; Bayer, Newbury, U.K.) and opiate analgesia postoperatively. At 7 days, rabbits were killed with intravenous sodium pentobarbital (2 mL).
Wound closure.
Wound closure was assessed as previously described (16). The wound was traced on the day of sacrifice. A fresh wound was made on the day of sacrifice and the percentage wound area reduction over 1 week was calculated using formula A (formulae are presented in the Supplementary Data).
Histology.
The wounds were cut across the midline and fixed in 10% formalin for 24 h. The tissue was processed using a tissue processor (ASP300; Meyer Instruments, Houston, TX) and embedded in paraffin. Sections (5 µm) were taken when the tissue was reached. Six sections were cut using a microtome every 150 µm into the wound for analysis. Three sections were placed on one slide. Sections were stained with hematoxylin and eosin and Masson’s trichrome using standard protocols.
Wound volume.
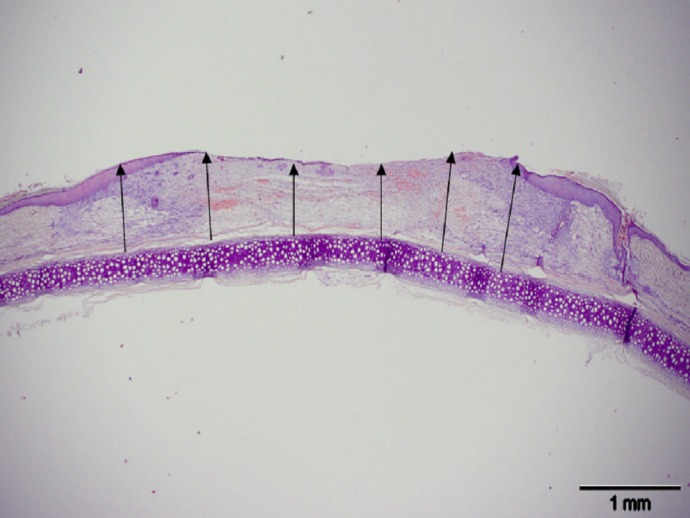
Wound volume was calculated by multiplying the average wound thickness by the area of the wound tracing 1 week after wounding (Fig. 1).
FIG. 1.
Example of cross-sectional image of wound stained with hematoxylin and eosin. Scale bar, 1 mm. Six measurements (black arrows) were taken from the cartilage to the wound surface and measured using Cell B software (Olympus), and the average thickness was calculated. The average thickness was used to calculate wound volume, which is used for the calculation of stereological end points. (A high-quality color representation of this figure is available in the online issue.)
Stereology.
Stereology is a means of assessing tissue responses to tissue constructs (17). It allows assessment of angiogenesis in vascular beds (18). In this present study, vertical sections of the tissue were examined using a systematic random sampling strategy to provide estimates of relevant stereological parameters (19). Methods used to measure the length of vessels in three dimensions were based using a vertical orientation design and a cycloid test system (20). A series of cycloid lines were placed on the histology sections using Image Pro Plus software (Media Cybernetics, Bethesda, MD), as previously described (16). In order to ensure that the areas of the wound had the same chance of being selected, selection of the fields was performed in a random manner. Five fields of view were obtained across the wound bed from one edge of the wound to the other edge (16). The fields were captured at ×40 magnification.
The parameters assessed were surface density of blood vessels, length density of blood vessels, and radial diffusion distance between capillaries. Surface density (SV) represents the amount of surface area (SA) contained in a reference volume (V). The surface area of a capillary represents the area available for gaseous transport to surrounding tissue. The higher the surface area of a capillary network, the higher the probability that the surface will intersect parallel lines placed on the image. Length density is a measurement of the length of blood vessel per unit volume of tissue (Lv), which is based on the principle that the longer and more convoluted a vessel, the greater the number of occasions its profile intersects a plane (16,17).
Length density and surface density of blood vessels were analyzed with and without multiplying by the wound volume. The surface density of blood vessels was calculated using formula B and the length of test line was 2,483 nm. The surface area of blood vessels was then calculated by multiplying the surface density by wound volume. To calculate the length density of blood vessels, a series of cycloid lines measuring 2,649 nm in length were rotated 90° and placed on the histological section. The length density of blood vessels was calculated using formula C. The total length of blood vessels in the wound was calculated by multiplying length density by wound volume. The radial diffusion distance was calculated using formula D. This allows for the measurement of the distance between blood vessels and is an indicator of the efficiency of a capillary network. The smaller the distance between blood vessels, the shorter the distance required for nutrients to diffuse into surrounding tissues. Blood vessel diameter was calculated using formula E (21).
The volume fraction of a feature within a particular reference space can be described as the proportion of space that the feature occupies in a unit volume (16). Inflammatory cells were counted and included lymphocytes and neutrophils. This was counted using a 192-point grid using Image-Pro Plus software (Media Cybernetics). Neutrophils were identified as small, dense, circular, multilobed cells and lymphocytes as small, round, dense cells with large nuclei. Formula F calculates inflammatory cell infiltrate in tissue.
Statistics.
Analysis between groups was assessed using analysis of variance and post hoc analysis with Fisher pairwise comparison. P < 0.05 was taken as significant. Minitab software was used. Bar graphs represent mean ± SD.
RESULTS
Induction of diabetes in the animal model.
The animals remained hyperglycemic post-alloxan infusion over the study time period (Supplementary Fig. 1). There was no mortality post-alloxan treatment. Two animals required insulin administration after alloxan treatment. Insulin therapy was administered if the animal lost weight and had high glucose readings using insulin glargine (Sanofi). High glucose readings were indicated on the glucometer as “high” and signified serum glucose of >33 mmol/L. The animal was administered 6 units of insulin subcutaneously if this occurred, and the blood glucose was monitored 12 h later.
MSC culture and characterization.
MSCs were successfully isolated from nondiabetic New Zealand white rabbit bone marrow. Cells were cultured to passage three and frozen in liquid nitrogen in 200,000-cell doses. When ready for use, cells were thawed and plated on tissue culture plastic. MSCs on tissue culture plastic demonstrated spindle-shaped morphology on becoming confluent. MSCs differentiated into chondrocytes, osteocytes, and adipocytes when exposed to appropriate conditions (Supplementary Fig. 2). Cell surface immunophenotyping was not carried out due to the lack of rabbit-specific antibodies available.
Metabolic activity of cells.
MSCs were seeded on collagen. After seeding the cells on collagen, metabolic activity was assessed at separate time points up until 2 weeks in vitro (Supplementary Fig. 3).
Histology and scanning electron microscopy.
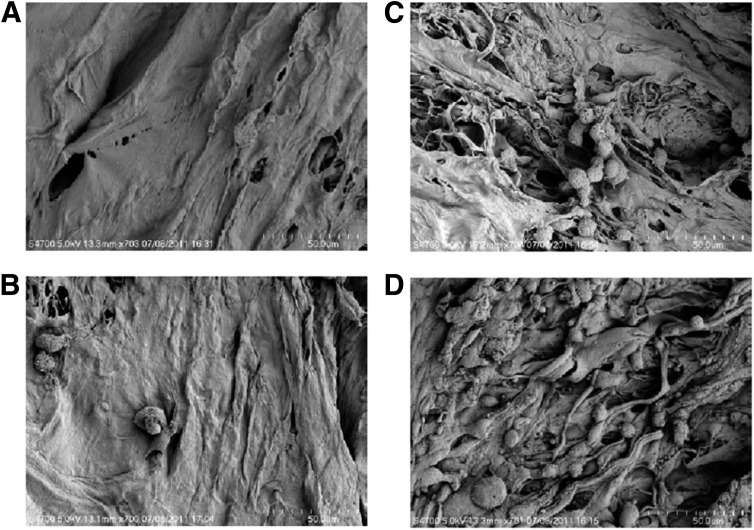
After immersion fixation, tissue processing, and sectioning of the MSC-collagen constructs, hematoxylin and eosin and Masson’s trichrome staining were performed. Cells were predominantly located on the superior border of the collagen scaffold (Supplementary Fig. 4). Scanning electron microscopy images (Fig. 2A–D) revealed densely populated MSCs within the collagen scaffolds seeded with 1,000,000 cells.
FIG. 2.
Scanning electron microscopy images of rabbit MSCs 24 h after seeding on a collagen scaffold. A: Unseeded scaffold. B: Scaffold seeded with 50,000 MSCs. C: Scaffold seeded with 100,000 MSCs. D: Scaffold seeded with 1,000,000 MSCs. The cells were adherent to the scaffold. MSCs were confluent on the scaffold at a dose of 1,000,000.
Detection of transplanted MSCs in the wound.
Supplementary Fig. 5 demonstrates PKH-26–labeled rabbit MSCs in the wound 1 week posttreatment. MSCs were present in the wound for at least 7 days, and the collagen scaffold was successful in mediating cell delivery to the wound.
Histology.
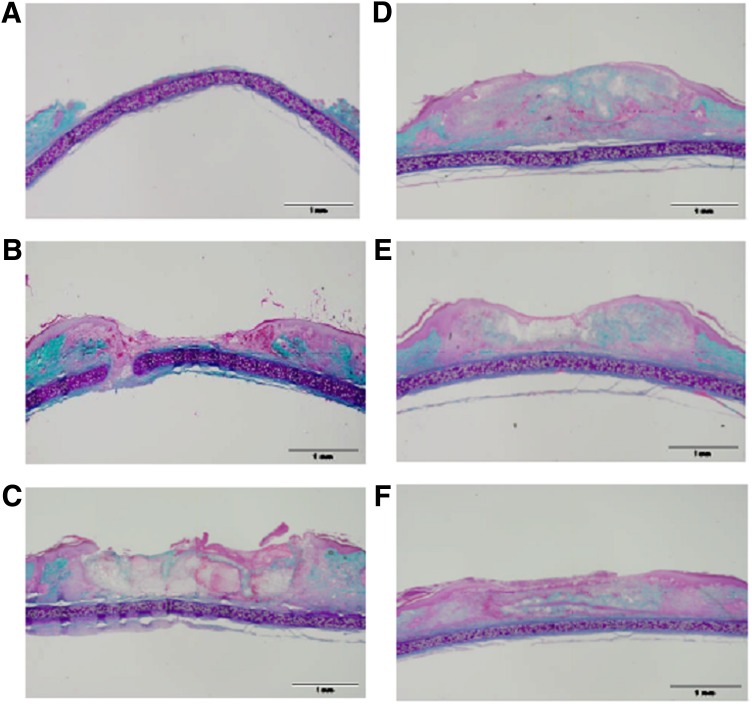
Figure 3 illustrates representative samples of Masson’s trichrome–stained histological sections of rabbit MSCs seeded in a collagen scaffold and delivered to an ulcer in a diabetic animal. MSCs delivered in a collagen scaffold demonstrate increased epithelial and granulation tissue formation in collagen seeded with MSC treatment group as compared with untreated wound and wounds treated with collagen alone. This benefit is observed in collagen seeded with MSCs groups at all of the treatment doses administered.
FIG. 3.
Masson’s trichrome stain of rabbit ear ulcer wounds. A: Fresh wound made on day of sacrifice. B: Untreated wound after 1 week. C: Wounds treated with collagen after 1 week. D: Wounds treated with collagen + 50,000 MSCs after 1 week. E: Wounds treated with collagen + 100,000 MSCs after 1 week. F: Wound treated with collagen + 1.000,000 MSCs after 1 week. Green stain represents collagen. Pink stain represents cytoplasm and epithelium. Purple stain represents cartilage. Original magnification ×2. Scale bar, 1 mm. There appears to be a dose-dependent increase in the epithelialization over the three doses. In the representative images of wounds, there is the subjective appearance of increased new granulation tissue in the wound and a more organized wound healing response in wounds treated with collagen-seeded MSCs in comparison with untreated wounds and wounds treated with collagen alone. (A high-quality color representation of this figure is available in the online issue.)
Percentage wound closure.
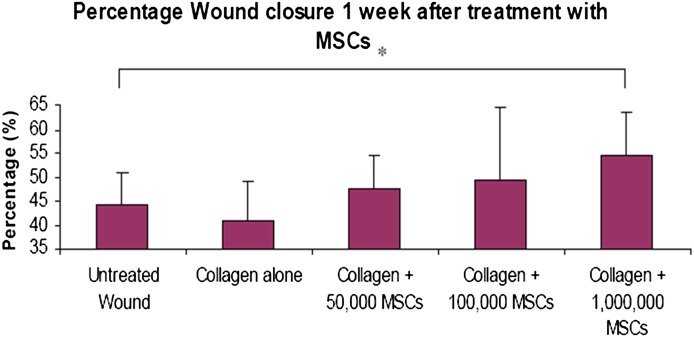
Transplantation of 1,000,000 rabbit MSCs seeded on a collagen scaffold resulted in a statistically significant increased percentage wound closure at 1 week as compared with untreated wounds (Fig. 4).
FIG. 4.
Percentage wound closure of cutaneous ulcers 1 week after treatment with MSCs seeded in a collagen scaffold. Analysis between groups using ANOVA and Fisher pairwise comparison. *P < 0.05. Error bars = SD. MSCs (1,000,000) seeded on a collagen scaffold result in a significantly increased percentage of wound closure as compared with control. There was no observed difference in the observed percentage wound closure between the other treatment groups, i.e., collagen + 50,000 MSCs, collagen + 100,000 MSCs, or collagen alone, when compared with untreated wounds. This result supports the hypothesis that the wound healing effect of MSC and collagen treatment occurs in a dose-dependent fashion. Increased cell doses increase the percentage wound closure and rate of wound healing. (A high-quality color representation of this figure is available in the online issue.)
Stereology.
Stereological analysis (Table 1) demonstrates significantly increased total length of blood vessels with enhanced neovasculature in wounds treated with 1,000,000 MSCs seeded on a collagen scaffold as compared with untreated wounds. The total length of blood vessels in wounds treated with collagen seeded with 50,000 or 100,000 MSCs or collagen alone is not significantly greater than untreated wounds.
TABLE 1.
Stereological analysis of wounds in diabetic animals
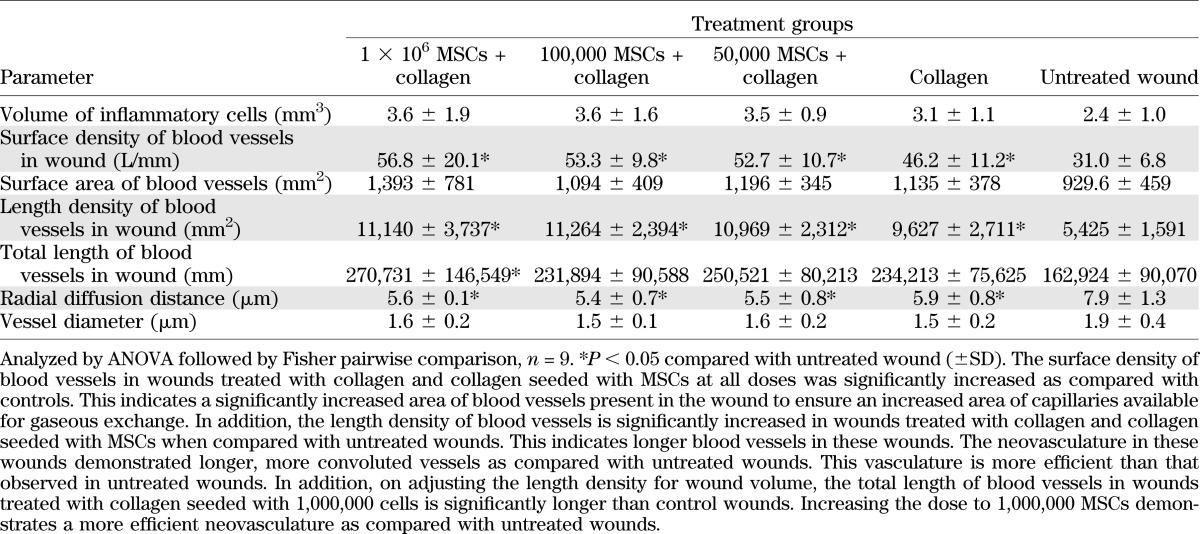
Blood vessels in collagen-treated wounds and collagen seeded with MSCs demonstrate significantly reduced radial diffusion distance when compared with untreated wounds. This occurs across all doses of MSCs. The distance for nutrients to travel from capillaries to tissue and cells is reduced and permits augmented tissue repair and regeneration. There was no statistically significant difference in blood vessel diameter between the groups 1 week after treatment. Figure 5 demonstrates representative images of blood vessels in the untreated wounds and wounds treated with MSC-seeded collagen scaffolds.
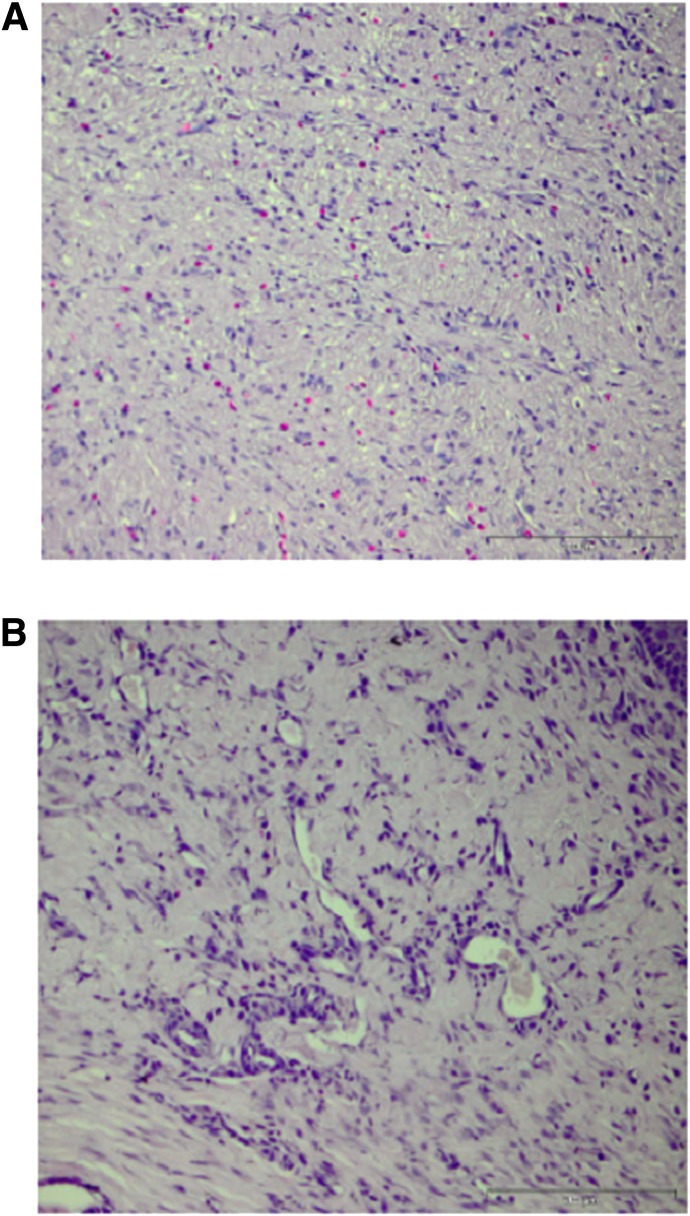
FIG. 5.
Representative images of neovasculature in control wounds (A) and wounds treated with 1,000,000 MSCs seeded in a collagen scaffold (B). Tissue samples are fixed in paraffin at 5-µm depth and stained with hematoxylin and eosin. Scale bar, 200 µm. An increased blood vessel density and reduced radial diffusion distance are evident in the wounds treated with MSCs and collagen as compared with untreated wounds, as evident in B and A, respectively. (A high-quality color representation of this figure is available in the online issue.)
Inflammation can be assessed in tissues using stereology. Inflammatory cell infiltrate is increased in healing tissue. In addition, inflammation may be increased in response to tissue-engineered biological construct implantation. The use of stereology that quantifies neutrophil and lymphocyte infiltration in tissue can assess inflammation in wounds 1 week after treatment (16). No significant difference was observed in inflammatory cell infiltrate between the groups. These data provide evidence that the MSC collagen treatment and collagen alone treatment do not result in increased inflammation, as compared with untreated wounds. The clinical relevance of this result is that the treatment does not result in an inflammatory reaction that would potentially result in a reduced healing rate.
The relationship between the various healing parameters was assessed in the treatment groups using Pearson correlations (Supplementary Table 2). The significance of the correlation between blood vessel morphology and inflammatory cells may be that the inflammatory cell infiltrate in wounds treated with 1,000,000 and 100,000 MSCs seeded in a collagen scaffold is associated with a more efficient neovasculature and arises from the paracrine effect of higher doses of transplanted MSCs.
DISCUSSION
Topical MSC therapy is a novel treatment for nonhealing human diabetic foot ulcers that are refractory to current standard care. This MSC-seeded biomaterial treatment may reduce amputation rates and alleviate the burden of nonhealing diabetic ulcers. A central pathological feature of diabetic foot ulceration is impaired angiogenesis. MSCs are known to promote angiogenesis in addition to improving cutaneous wound healing (7,22). This research is unique in several aspects: 1) investigating the effect of MSCs in a clinically relevant preclinical model, 2) the use of nondiabetic allogeneic MSCs, 3) the dose escalation strategy used, and 4) the use of a type 1 collagen biomaterial to mediate cell delivery to the cutaneous ulcer. The results of the research demonstrate that the topical delivery of MSCs to a diabetic wound using a biomaterial augments diabetic wound healing. The wound-healing benefit is observed by increased percentage wound closure with an associated more efficient neovasculature.
The animal model of diabetic cutaneous wound healing used in this study is a preclinical model, with healing occurring similar to human wound healing. Animal models used in the investigation of topical MSC therapy are predominantly murine, where skin heals by contraction. This is due to the presence of the panniculosus carnosus layer, which is present in rats and mice but absent in the rabbit ear and in humans. This functional anatomical layer contracts on wounding. Excisional wounds in the rabbit ear heal by re-epithelialization and new granulation tissue formation, as occurs in the human situation (16). Our group has previously described impaired wound healing in the diabetic model used in the current study (16). The duration of hyperglycemia in the current study was 5 weeks. The wound is a full-thickness cutaneous ulcer and facilitates assessment of wound closure and new granulation tissue formation, angiogenesis, and inflammation. Using stereological methodology, a comprehensive assessment of host tissue responses to cell-seeded biomaterial constructs can be achieved.
This study investigated the therapeutic efficacy of allogeneic nondiabetic bone marrow–derived MSCs delivered to a diabetic wound in an immunocompetent animal. The use of allogeneic MSC transplantation from a nondiabetic donor is an approach that may have advantages over autologous cell transplantation in which disease-induced cell dysfunction may limit therapeutic efficacy (23,24). To facilitate this approach, cryopreserved cells were used. Nondiabetic bone marrow–derived MSCs remained viable after freezing in liquid nitrogen and differentiated into three mesodermal cells, adipocytes, chondrocytes, and osteocytes. MSCs were successfully seeded in the scaffold. The MSC-scaffold treatment retains cellular viability in vitro. This provides evidence for the use of nondiabetic MSCs that are frozen in liquid nitrogen as an “off the shelf” product.
The animals used in the study were outbred; however, it is possible that genetic variability within the rabbit colony for major histocompatibility complex proteins could be limited and, as such, might resemble more closely a cell transplant between related individuals than between unrelated, fully major histocompatibility–mismatched individuals. The implication being that immunogenicity based on major histocompatibility complex disparity could be lower than that which would apply after fully mismatched cell delivery. However, we demonstrate in this paper that cells isolated from nondiabetic animals can be successfully transplanted to diabetic animals after cryopreservation.
As the body of data from preclinical research investigating the cutaneous wound healing benefit of MSCs is increasing, the positive results in preclinical studies will require the focus to switch to the translation of MSC-based therapy in human subjects (25). A majority of studies have investigated direct injection of cell suspensions around the wound but little is known of cell engraftment or retention at the wound site. It is known that cells injected directly into the body undergo cell death rapidly (10). Biomaterials may support cell viability and thus enhance therapeutic efficacy. Topical delivery of MSCs reduces the potential toxicity associated with systemic administration. One previous report demonstrates that MSC treatment when injected around the wound augments wound healing and increases percentage wound closure but fails to increase angiogenesis at the wound site (26). MSCs were injected intradermally around the cutaneous wounds in diabetic rats. Angiogenesis end points in histological sections were assessed using similar stereological methodology as used in our study. In contrast to the results of our current study, this study demonstrated that MSCs augmented wound healing, which was not associated with increased angiogenesis (26). Collagen is a natural biomaterial that promotes sustained cellular viability and functionality in addition to maintaining the cells at the wound site (9,10) and was used to deliver MSCs to the wound surface in our study. Collagen is known to support angiogenesis when used alone (12).
This study investigates an allogeneic strategy using a dose escalation regimen. In this dose escalation study of topical MSC therapy, transplantation of 1,000,000 cells on a collagen scaffold revealed increased percentage wound closure when compared with untreated wounds. This end point is highly relevant clinically. It provides a noninvasive measurement of wound healing, and increased percentage wound closure is associated with accelerated wound healing (27). This study reviewed extensive histological sections throughout the wound. Increased angiogenesis is reported in all treatment groups as compared with controls but enhanced wound closure was only observed in the high-dose cell group. Both surface density and length density were significantly increased in wounds treated with collagen alone and collagen seeded with MSCs when compared with untreated wounds after 1 week. In addition, the radial diffusion distance was significantly less in wounds treated with collagen alone and collagen seeded with MSCs when compared with untreated wounds. Furthermore, the total length of blood vessels in the wound was significantly greater in wounds treated with collagen seeded with 1,000,000 MSCs as compared with other groups. This increased blood vessel length suggests that at increasing doses of MSCs, to 1,000,000 in the case of this study, there is a more efficient blood vessel network not seen with lower doses of MSCs. It should be acknowledged, however, that the translation of this information to human trials is not completely clear. For example, should the dose chosen for phase 1 human studies be based on wound area or body weight in rabbits versus humans?
The stereological analysis and comparison between groups revealed no difference in inflammatory cell infiltrate between treatment groups and untreated wounds. This is an important observation to ensure that the tissue-engineered construct does not illicit an inflammatory response due to the allogeneic nature of the MSCs and the xenogeneic bovine collagen scaffold.
These data provide evidence of the wound healing benefit associated with collagen and collagen seeded with MSCs transplantation. Collagen seeded with 1,000,000 MSCs resulted in a significantly increased percentage wound closure and a superior vascular supply when compared with untreated wounds at 1 week. This is the first extensive analysis of MSCs delivered to a wound using a collagen scaffold in the context of diabetes. It confirms that the wound healing benefit of MSC transplantation on a collagen scaffold occurs with increased angiogenesis, as reported in previous studies, and for the first time assesses the optimal dose and the use of a collagen scaffold for cell delivery (22).
Supplementary Material
ACKNOWLEDGMENTS
Molecular Medicine Ireland provided funding for A.O. to complete this research as part of a clinical scientist training program. This work was also supported by Science Foundation Ireland, Strategic Research Cluster (Grant SFI: 09/SRC B1794), and the European Regional Development Fund.
T.O. is a founder, director, and equity holder in Orbsen Therapeutics Ltd. No other potential conflicts of interest relevant to this article were reported.
A.O. designed the study, performed the experiments, analyzed the data, and wrote the manuscript. M.K., M.C., E.M., G.S., and M.M. performed the experiments. E.E.V. and P.D. analyzed the data. A.P. and T.O. designed the study, analyzed the data, and wrote the manuscript. T.O. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was previously published in abstract form for the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1822/-/DC1.
REFERENCES
- 1.Frykberg RG, Zgonis T, Armstrong DG, et al. American College of Foot and Ankle Surgeons Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45(Suppl.):S1–S66 [DOI] [PubMed] [Google Scholar]
- 2.O’Loughlin A, McIntosh C, Dinneen SF, O’Brien T. Review paper: basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds 2010;9:90–102 [DOI] [PubMed] [Google Scholar]
- 3.Fu X, Li H. Mesenchymal stem cells and skin wound repair and regeneration: possibilities and questions. Cell Tissue Res 2009;335:317–321 [DOI] [PubMed] [Google Scholar]
- 4.Zhang QZ, Su WR, Shi SH, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 2010;28:1856–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Rocco G, Gentile A, Antonini A, et al. Enhanced healing of diabetic wounds by topical administration of adipose tissue-derived stromal cells overexpressing stromal-derived factor-1: biodistribution and engraftment analysis by bioluminescent imaging. Stem Cells Int 2010;2011:304562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH. Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg 2010;65:565–572 [DOI] [PubMed] [Google Scholar]
- 7.Nambu M, Kishimoto S, Nakamura S, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg 2009;62:317–321 [DOI] [PubMed] [Google Scholar]
- 8.Javazon EH, Keswani SG, Badillo AT, et al. Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen 2007;15:350–359 [DOI] [PubMed] [Google Scholar]
- 9.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–1312 [DOI] [PubMed] [Google Scholar]
- 10.Silva EA, Kim ES, Kong HJ, Mooney DJ. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci USA 2008;105:14347–14352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang SK, Shin IS, Ko MS, Jo JY, Ra JC. Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int 2012;2012:342968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweeney SM, DiLullo G, Slater SJ, et al. Angiogenesis in collagen I requires alpha2beta1 ligation of a GFP*GER sequence and possibly p38 MAPK activation and focal adhesion disassembly. J Biol Chem 2003;278:30516–30524 [DOI] [PubMed] [Google Scholar]
- 13.O’Shea CA, Hynes SO, Shaw G, et al. Bolus delivery of mesenchymal stem cells to injured vasculature in the rabbit carotid artery produces a dysfunctional endothelium. Tissue Eng Part A 2010;16:1657–1665 [DOI] [PubMed] [Google Scholar]
- 14.Ward J, Kelly J, Wang W, Zeugolis DI, Pandit A. Amine functionalization of collagen matrices with multifunctional polyethylene glycol systems. Biomacromolecules 2010;11:3093–3101 [DOI] [PubMed] [Google Scholar]
- 15.Calderon L, Collin E, Velasco-Bayon D, Murphy M, O’Halloran D, Pandit A. Type II collagen-hyaluronan hydrogel—a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater 2010;20:134–148 [DOI] [PubMed] [Google Scholar]
- 16.Breen A, Mc Redmond G, Dockery P, O’Brien T, Pandit A. Assessment of wound healing in the alloxan-induced diabetic rabbit ear model. J Invest Surg 2008;21:261–269 [DOI] [PubMed] [Google Scholar]
- 17.Dockery P, Fraher J. The quantification of vascular beds: a stereological approach. Exp Mol Pathol 2007;82:110–120 [DOI] [PubMed] [Google Scholar]
- 18.Garcia Y, Breen A, Burugapalli K, Dockery P, Pandit A. Stereological methods to assess tissue response for tissue-engineered scaffolds. Biomaterials 2007;28:175–186 [DOI] [PubMed] [Google Scholar]
- 19.Howard CV, Reed MG. Unbiased Stereology 2nd ed. Howard CV, Reed MG, Eds. Belfast, QTP Publications, 2010 [Google Scholar]
- 20.Gohkale AM. Unbiased estimation of curve length in 3D using vertical sections. J Microsc 1990;159:133–141 [Google Scholar]
- 21.Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol 1999;10:1100–1123 [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–2659 [DOI] [PubMed] [Google Scholar]
- 23.Khan M, Akhtar S, Mohsin S, N Khan S, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev 2011;20:67–75 [DOI] [PubMed] [Google Scholar]
- 24.Keats E, Khan Z. Unique responses of stem cell-derived vascular endothelial and mesenchymal cells to high levels of glucose. PLoS One. 6 June 2012 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 25.Chen JS, Wong VW, Gurtner GC. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front Immunol 2012;3:192 [DOI] [PMC free article] [PubMed]
- 26.Maharlooei MK, Bagheri M, Solhjou Z, et al. Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract 2011;93:228–234 [DOI] [PubMed] [Google Scholar]
- 27.Cardinal M, Eisenbud DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen 2008;16:19–22 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.