Abstract
The recent availability of numerous well-characterized Mycobacterium tuberculosis recombinant proteins has revived interest in the serological diagnosis of tuberculosis. Several promising results have been reported, particularly when more than one antigen is used in the test. However, thus far these antigens have not been used in routine diagnostic tests because they lack sufficient sensitivity. In addition, with the exception of one antigen, most recombinant M. tuberculosis proteins do not identify the majority of tuberculosis patients coinfected with human immunodeficiency virus (HIV). Here, we report a newer M. tuberculosis protein that is a promising candidate for increasing the sensitivity of the serological tests, in particular for patients coinfected with HIV. The protein was found in the urine of mice during the early stages of infection with M. tuberculosis (10 to 14 days), thus suggesting that the antigen is abundantly released during the in vivo growth of the mycobacterium. Reverse genetics was used to produce the recombinant protein, which we named U1 (for urine protein 1). Using a conventional enzyme-linked immunosorbent assay (ELISA), antibody to U1 could be detected in 60% of patients with pulmonary tuberculosis with no signs of coinfection with HIV (n = 83). Conversely, anti-U1 antibody was detected in 87% of the sera from tuberculosis patients coinfected with HIV (n = 47). Out of 12 HIV-infected nontuberculosis patients' sera, 9 did not react with U1 and three sera gave borderline ELISA signals (signal/cutoff of ≤1.75). These results suggest that the high efficiency of U1 in identifying tuberculosis patients coinfected with HIV may be related to abundant release of this protein during the initial phase of the HIV coinfection. The immediate availability of the antigen at a time point in which the patient's immune system is still competent would lead to a secondary immune response to U1 that persists for months in the patient's serum.
Tuberculosis remains one of the most serious public health threats for humanity. One-third of the world's population is infected with tubercle bacilli, and it is estimated that in the next decade 80 million people will develop disease, of which 19 million will die (11, 22, 26, 31). These alarming projections are in general attributed to the fact that tuberculosis is a highly contagious airborne disease (17), its treatment is long and usually requires three antibacterial drugs (19, 30, 32), the epidemic of AIDS has been responsible for the activation of latent tuberculosis and has been associated with the emergence of innumerous and serious cases of drug-resistant tuberculosis disease (27, 34), and finally, because there is no effective vaccine (13).
Among the tests used for the diagnosis of tuberculosis, the tuberculin skin test (Mantoux) has been used and accepted for more than 85 years (1, 24). Nevertheless, this test lacks specificity for Mycobacterium tuberculosis (2, 21) and requires special medical or paramedical personnel to perform it and read the results. However, the replacement of the native multiantigenic preparation used in the Mantoux test (purified protein derivative of tuberculin [PPD]) by purified recombinant proteins has recently been reported to successfully improve the specificity of this test (5, 9).
Conversely, despite the availability of several well-characterized specific M. tuberculosis recombinant antigens, the serological diagnosis of tuberculosis remains problematic. For example, although the 38-kDa antigen used in an enzyme-linked immunosorbent assay (ELISA) is useful in detecting up to 85% of the smear-positive cases of tuberculosis, its sensitivity drops considerably for smear-negative cases of the disease (33). Other antigens, such as 85B, 16-kDa heat shock protein, A60, ESAT-6, and CFP-10 (4, 8, 10, 15, 29), have been tested and shown to have lower sensitivity and/or specificity than the 38-kDa antigen in sera from both smear-positive and -negative tuberculosis patients. Moreover, all these antigens have very low sensitivity for detecting tuberculosis patients coinfected with human immunodeficiency virus (HIV) (3). More recently, though, a novel antigen named Mtb81 was described as a promising marker for tuberculosis patients coinfected with HIV (18). However, this antigen has low sensitivity for the diagnosis of patients with tuberculosis only.
Notwithstanding the different sensitivities and specificities of these recombinant antigens, they were all discovered and/or validated using immunological readouts (serological or cellular) from patients with chronic tuberculosis. However, at this stage of the disease, it is likely that a vast proportion of the antigenic repertoire of M. tuberculosis recognized by the immune system comprises both antigens associated with the microorganisms' growth and a complex mixture of antigens released from dead mycobacteria. Antigens that are produced by the bacteria during the early phases of the disease are perhaps more representative markers of recent acquisition of primary tuberculosis, disease reactivation, or exogenous reinfection. Potentially such antigens can represent specific tools both for the diagnosis of active infection in immunocompetent individuals and for early detection of reactivation of tuberculosis in patients coinfected HIV.
The present studies were undertaken to search for such antigens. Mice were infected with M. tuberculosis, and their urines were harvested and screened for mycobacterial antigens as early as 10 to 14 days postinfection, a time point at which the microorganisms are actively multiplying in the mouse lungs, spleens, and livers (28). One of the discovered M. tuberculosis antigens was more extensively evaluated and is described in detail herein. The amino acid sequence of the purified antigen was determined and used to instruct the cloning and expression of the corresponding gene. The purified recombinant protein, which we named U1 (for urine protein 1), was used in an ELISA format to test its reactivity against a panel of sera from patients with various forms of tuberculosis. The results point to an M. tuberculosis protein that is recognized by the sera from 60% of patients with tuberculosis in the absence of HIV infection and by 87% of the sera from patients coinfected with tubercle bacilli and HIV.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, Mass.). The mice were maintained under pathogen-free conditions and used at 8 weeks of age.
Bacteria, mouse infections, and electrophoresis analysis.
Virulent M. tuberculosis strain H37Rv (ATCC 35718; American Type Culture Collection, Manassas, Va.) suspended in phosphate-buffered saline (PBS)-Tween 80 (0.05%) was delivered intravenously (107 CFU/mouse). After infection, urine was collected daily from day 10 to day 14 from infected mice and for four consecutive days from a group of noninfected mice. Urine samples were centrifuged immediately after collection, sterile filtered in 0.2-μm-pore-size filters, and stored at −80°C until use. For comparative analyses, 5 μl of urine from both uninfected and infected mice were submitted to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (4 to 12% gradient gel) followed by silver staining.
Mass spectrometry.
Peptide mixtures were initially concentrated using a Capillary LC column filled with C18 resin (inside diameter, 100 μm; length, 12 cm). Peptides were eluted using a gradient of 0.5%/min increase from 5 to 65% buffer B (80% acetronitrile-0.2% acetic acid in water). Concentrated peptides were introduced into an LCQ-Ion Trap mass spectrometer (Finnigan, San Jose, Calif.) by electrospray ionization interface (Cytopeia, Seattle, Wash.) and analyzed by data-dependent mass spectrometry (MS) and tandem mass spectrometry (MS/MS) scanning. The collision-induced dissociation spectra (MS/MS) generated during the experiment were searched against mouse and M. tuberculosis protein databases using Sequest software to identify possible sequence matches (16, 35).
M. tuberculosis antigens.
Culture filtrate antigenic preparation (CF) was obtained from 2-week-old cultures of M. tuberculosis strain H37Rv grown in defined medium as described previously (9). CF was centrifuged at 2 000 × g for 20 min, and the supernatant was sterilized by passing through 0.2-μm-pore-size filters. CF was concentrated with an Amicon (Beverly, Mass.) 3 Centriprep concentrator 100× of the original volume, and protein content was determined with a bicinchoninic acid protein assay (Pierce, Rockford, Ill.). The recombinant antigen Mtb81 was obtained as previously described (18).
High-level expression and affinity purification of recombinant protein.
U1 oligonucleotide PCR primers were designed to amplify the full-length U1 open reading frame from genomic DNA of the virulent Erdman strain. U1 was amplified using primers 5′ (5′-CAATTACATATGCATCACCATCACCATCACATGGCACAGATAACCCTG-3′) and 3′ (5′-CAATTAAAGCTTCTAGGCGCCCAGCGCGGC-3′). The 5′ oligonucleotide contains an NdeI restriction site preceding the ATG initiation codon (underlined), followed by a sequence encoding six histidines (bold) and sequence derived from the gene (italic). The 3′ oligonucleotide contains protein-coding sequence followed by a stop codon (underlined) and HindIII restriction site. The resultant PCR product was digested with NdeI and HindIII and cloned into a pET17b vector (Novagen, Madison, Wis.) similarly digested with NdeI and HindIII for directional cloning. Ligation products were initially transformed into Escherichia coli XL1-Blue competent cells (Stratagene, La Jolla, Calif.) and were subsequently transformed into E. coli BL21(DE3)/pLysS (Novagen) for expression. Recombinant (His tag) U1 was purified from the insoluble inclusion body of 1 liter of isopropyl-β-d-thiogalactopyranoside-induced batch cultures by affinity chromatography using the one-step QIAexpress Ni-nitrilotriacetic acid (NTA) agarose matrix (Qiagen, Chatsworth, Calif.) in the presence of 8 M urea as previously described (6). The yield of recombinant protein varied from 8 to 10 mg per liter of induced bacterial culture. Purity of the recombinant protein was assessed by SDS-PAGE, followed by Coomassie blue staining, and N-terminal sequencing using Erdman chemistry with a Procise 494 protein sequencer (Perkin-Elmer/Applied Biosystems, Foster City, Calif.).
Human sera.
A total of 270 serum samples were employed in this study. The sera were subdivided into six distinct groups. (i) Eighty-three patients with pulmonary tuberculosis in the absence of coinfection with HIV. The diagnosis of patients with pulmonary tuberculosis was based on clinical signs and symptoms, such as fever, cough, and productive sputum, on the grounds of suggestive chest X ray, and on the identification of acid-fast bacilli in the patient's sputum smears. (ii) Forty-seven patients with pulmonary tuberculosis and coinfection with HIV, evidenced by both ELISA (Organon Teknika Corp., Durham, N.C.) and Western blotting (Genelabs Diagnostics Pte. Ltd., Singapore Science Park, Singapore). (iii) Twelve HIV patients with no signs of tuberculosis. (iv) Fifty-four patients with an extrapulmonary form of tuberculosis (excluding pleural tuberculosis). The diagnosis of this group of patients was based on clinical signs and symptoms, such as fever, presence of suggestive enlarged lymph node, and identification of acid-fast bacilli in biopsies of the affected tissues. (v) Thirty-seven patients with pleural tuberculosis only, as suggested by the clinical history and confirmed by chest X ray and pleural aspiration. (vi) Thirty-seven sera from healthy PPD-negative individuals (controls). All serum samples, including the controls, were obtained in Brazil and are from individuals representative of varied ethnic backgrounds (Caucasians, blacks, Asians, and mulattos). The donors were above 18 years of age and gave informed consent. The blood donation was noncoercive, following protocols approved by the Review Boards and Ethics Committees of the Medical School of TriÂngulo Mineiro, Uberaba, Minas Gerais, Brazil, and of the Hospital Universitário Edgard Santos, Federal University of Bahia, Salvador, Bahia, Brazil).
Immunoblot analysis of U1 antigen.
Purified recombinant U1 protein and M. tuberculosis CF were separated by electrophoresis on 4- to 20%-gradient SDS-PAGE gels and transferred to nitrocellulose using a semidry transfer apparatus (Bio-Rad Laboratories, Hercules, Calif.). Blots were blocked overnight with PBS-1% Tween 20 and probed with polyclonal sera from the same rabbit before and after immunization with the purified recombinant protein. Before incubation of blots, rabbit sera were preadsorbed with E. coli whole-cell extract for 1 h at 37°C. The blots were also probed with pooled sera from tuberculosis-infected individuals to detect the presence of U1 in human antituberculosis antisera.
Generation of rabbit anti-U1 antiserum.
The purified recombinant protein (100 μg) was mixed with 100 mg of muramyl dipeptide, brought up to 1 ml with PBS, and emulsified with 1 ml of incomplete Freund adjuvant. The emulsion was injected at multiple subcutaneous sites into a female New Zealand White rabbit (R&R Rabbitry, Stanwood, Wash.). The rabbit was given two subcutaneous boosters (100 μg of antigen in incomplete Freund adjuvant) 3 weeks apart. One week after the final boost, the rabbit was bled and serum was collected.
ELISA.
ELISA was performed using standard protocols. Briefly, maxisorp surface ELISA plates (Nalge Nunc International) were coated with 50 μl of 200-ng antigen in carbonate bicarbonate buffer/well and were incubated at 4°C overnight. The plates were aspirated and were blocked with PBS-1% bovine serum albumin at 250 μl/well, incubated at room temperature for 2 h. The blocking reagent was aspirated, and the plates were washed 5 times with PBS-0.1% Tween 20 plus 0.01% benzalkonium chloride. One hundred microliters of human anti-tuberculosis antisera was added per well at the indicated dilutions in PBS-0.1% bovine serum albumin-0.1% Tween 20 plus 0.01% benzalkonium chloride. Samples were incubated at room temperature for 30 min. The plates were washed as mentioned, and 50 μl of protein A-horseradish peroxidase conjugate was added per well at a 1:20,000 dilution and incubated for 30 min at room temperature. The conjugate was aspirated, and the plates were washed as described above. One hundred microliters of tetramethyl benzidine substrate (Kirkegaard & Perry Laboratories)/well was incubated for 15 min at room temperature, and the reaction was stopped with 100 μl of 1 N H2SO4/well. The plates were then read at 450 nm using an ELISA reader (ELX 808; Bio-TEK Instruments, Inc.). The cutoff for the assays was the mean of the negative population plus three standard deviations of the mean.
RESULTS
Detection of M. tuberculosis proteins in urine of infected mice.
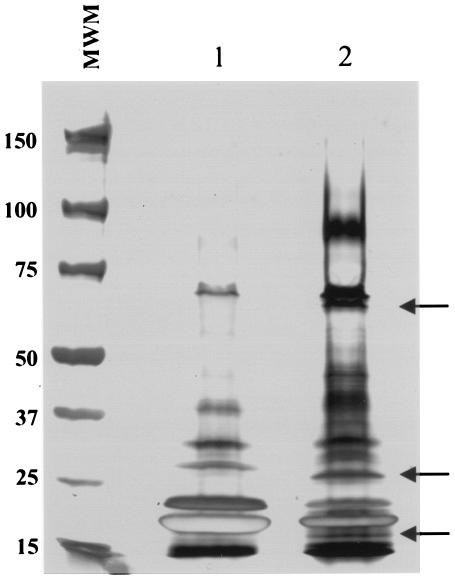
The detection of microbial antigens in biological fluids of infected hosts can be an important indicator of active disease caused by various organisms. With tuberculosis this is of particular interest, because most immunological diagnostic tests cannot distinguish between the several manifestations of infection in humans and animals. To identify and characterize possible M. tuberculosis protein antigens in biological fluids of infected hosts, C57BL/6 mice were initially infected intravenously with the virulent M. tuberculosis strain H37Rv. Urine was collected daily from day 10 to day 14 after infection and analyzed comparatively by SDS-PAGE and silver staining with urine of noninfected control C57BL/6 mice. Figure 1 illustrates the migration pattern of the proteins present in the two urine preparations. Several unique bands were present in the urine of infected mice only. These bands were excised from the gels and were in-gel tryptic digested and used for protein identification by microLC-ESI-mass spectrometry. The collision-induced dissociation spectra (MS/MS) generated during the experiment produced peptide sequences that were used to search matches in both M. tuberculosis and mouse protein databases using Sequest software. Several bands generated sequences with high matches with mouse proteins. However, the sequence of one band of 16.5 kDa showed homology with that of a hypothetical thiol peroxidase protein of M. tuberculosis, present in the genome database released by The Institute for Genomic Research (http://www.tigr.org; primary locus, Rv1932; GenBank accession no. CAB06529.1, coordinates 2183370 to 2183864). This protein was symbolically named urine protein 1 or simply U1.
FIG. 1.
Polyacrylamide gel electrophoresis of mouse urine. Approximately 15 μl of urine from either normal C57BL/6 mice (lane 1) or M. tuberculosis-infected C57BL/6 mice (lane 2) were subjected to gel electrophoresis (4 to 12% gradient gel) followed by silver staining. Numbers on the left indicate the molecular weights of the markers (MWM) expressed in thousands. Arrows point to protein bands that are apparently unique to the urine of infected mice.
Cloning of the gene and protein expression of an M. tuberculosis antigen (U1) excreted in the urine of infected mice.
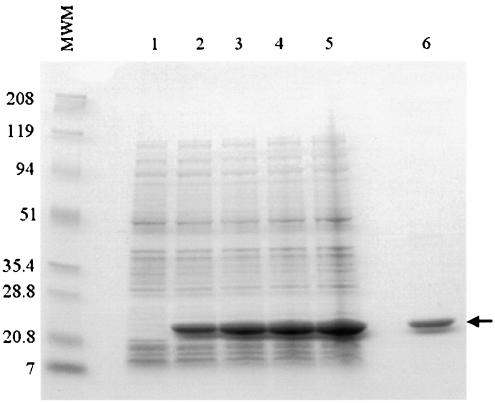
Oligonucleotides were synthesized and used to PCR amplify the coding open reading frame portion of U1 beginning 5′ to the possible initiation ATG codon and included sequence coding for an NdeI restriction enzyme site for subcloning followed by a stretch of sequence coding for six histidine residues in tandem. The NdeI site would reconstitute an ATG start codon upon ligation to the NdeI-digested vector, enabling expression of the six histidines immediately upstream of the U1 open reading frame. The 3′ primer sequence included coding for a stop codon and a HindIII restriction enzyme site. Standard PCR was carried out using M. tuberculosis H37Rv genomic DNA as a template, and the resulting DNA fragment was ligated with T4 DNA ligase into an NdeI-HindIII-digested pET17b plasmid vector and was transformed into E. coli XL-1 Blue competent cells. Sequencing confirmed that there were no mutations generated by the PCR amplification. For protein expression and purification, the histidine-tagged open reading frame of the full-length U1 gene cloned into pET17b was transformed into the BL21(DE3)/pLysS expression vector. The construct was designed to contain six N-terminal histidine residues for ease of purification by affinity chromatography over the Ni-NTA matrix. The recombinant protein was expressed and purified from inclusion bodies, with yields ranging from 8 to 10 mg/liter of induced culture. Figure 2 shows a Coomassie blue-stained SDS-PAGE gel of the E. coli cultures before and after induction and the respective purified recombinant antigen. Histidine-tagged U1 migrated to a little over 21 kDa, which is slightly higher than the predicted size of approximately 16.8 kDa.
FIG. 2.
Overexpression and purification of recombinant U1 in E. coli. Recombinant U1 was expressed in E. coli with six-His-tag amino-terminal residues, and the protein was purified by affinity chromatography using an Ni-NTA agarose matrix. E. coli lysates (10 μl) from noninduced cultures (lane 1) and isopropyl-β-d-thiogalactopyranoside-induced cultures for 1, 2, 3, and 4 h (lanes 2 to 5) and 2 μg of purified recombinant U1 (lane 6) were subjected to SDS-PAGE (15% polyacrylamide) and stained with Coomassie blue. Numbers on the left indicate the molecular weights of the markers (MWM) expressed in thousands.
Expression of native U1 in M. tuberculosis organisms.
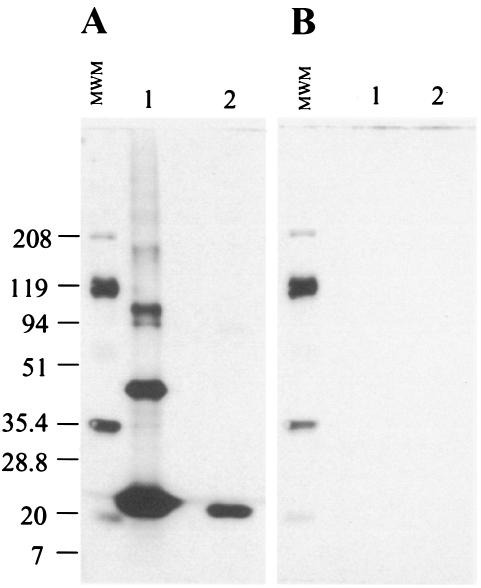
To investigate the expression of U1 in M. tuberculosis organisms, the purified recombinant protein was used to raise a rabbit anti-U1 antiserum. The serum was initially adsorbed with E. coli and then tested by Western blotting against the purified recombinant molecule and crude M. tuberculosis culture antigenic preparation. The results are expressed in Fig. 3a and show that rabbit anti-U1 recognizes the purified recombinant protein as well as a single molecule of ∼22 kDa in a crude extract of M. tuberculosis. No bands were seen in blots probed with the preimmune rabbit serum (Fig. 3B). While the anti-U1 antiserum recognized a single reactive band in the crude M. tuberculosis antigenic preparation, a major band of 22 kDa and several minor bands of higher molecular weight were present in the purified recombinant protein preparation. These minor bands could either be nonspecific aggregation of the recombinant molecule or, alternatively, minor contamination with E. coli proteins not previously seen in the Coomassie blue-stained gels.
FIG. 3.
Immunological recognition of the recombinant U1. U1 was submitted to Western blot analysis, probed with either a rabbit anti-U1 antiserum or the preimmune serum from this rabbit. Recombinant antigen (lanes 1) and a crude culture filtrate of M. tuberculosis (lanes 2) were subjected to electrophoresis under reducing conditions in a 4 to 20% gradient gel and transferred to a nitrocellulose membrane. Proteins were identified using rabbit anti-U1 antiserum (A) or preimmune rabbit serum (B). Numbers on the left are the molecular weights of the markers (MWM) expressed in thousands.
Recognition of U1 by sera from patients with pulmonary tuberculosis.
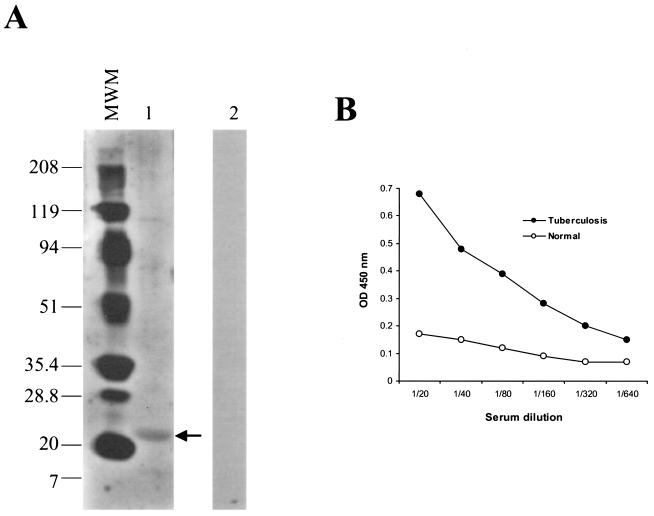
To investigate the recognition of U1 by the immune system of patients with tuberculosis, Western blot analysis was initially carried out using a pool of eight human sera from patients with active pulmonary tuberculosis. Figure 4A illustrates the results and indicates that a band of 22 kDa was recognized by this pool of serum, thus confirming that serum of human patients with pulmonary tuberculosis recognizes the recombinant molecule. No bands were seen in the blot probed with a serum pool from normal individuals. To further confirm the immunological recognition of U1 by serum of tuberculosis patients, their serum pool was tested with the antigen in an ELISA format. Figure 4B shows the results and indicates that high titers of anti-U1 antibody were present in the serum pool of these individuals. In contrast, the normal, control serum pool showed practically no recognition of U1.
FIG. 4.
Recognition of U1 by sera from patients with pulmonary tuberculosis. The recognition of U1 by a pool of sera from patients with confirmed pulmonary tuberculosis was tested using Western blot analyses (A) and ELISA (B). For the Western blot analyses, the recombinant antigen (lane 1) was submitted to electrophoresis under reducing conditions in a 4 to 20% gradient gel and transferred to a nitrocellulose membrane, followed by probing with the pool of human sera from patients with pulmonary tuberculosis. Numbers on the left are the molecular weights of the markers (MWM) expressed in thousands. The arrow points to a band of ∼22 kDa identified by the patient's sera. A separated membrane blotted with U1 was probed with a pool of normal sera. No bands were seen (lane 2). For the ELISA, the standard approach was used. Plates were coated with 200 ng of U1, blocked, and incubated with various dilutions of either a pool of sera from patients with tuberculosis or a pool of sera from normal human PPD-negative individuals. After incubation for 1 h, plates were washed and wells were incubated with protein A-horseradish peroxidase conjugate. Plates were washed followed by incubation with the substrate-chromogen mixture. Reactions were blocked with H2SO4, and plates were read and plotted as OD at 450 nm.
Usefulness of U1 as a possible serological antigen for the diagnosis of tuberculosis.
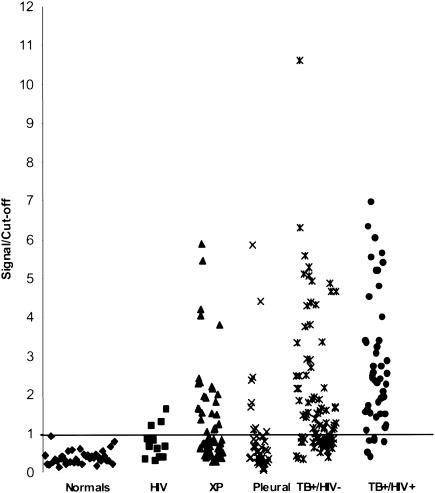
To begin the evaluation of the possible utility of U1 as a serological diagnostic tool for tuberculosis, a small clinical investigation was performed. Sera were selected from several patient groups and from normal individuals and were evaluated by conventional ELISA. The following groups were evaluated: (i) patients (n = 83) with pulmonary tuberculosis (smear positive) in the absence of coinfection with HIV; (ii) patients (n = 47) with pulmonary tuberculosis coinfected with HIV; (iii) HIV patients (n = 12) with no signs of tuberculosis; (iv) patients (n = 54) with an extrapulmonary form of tuberculosis (excluding pleural tuberculosis); (v) patients (n = 37) with pleural tuberculosis only; and (vi) thirty-seven sera from healthy PPD-negative individuals (normal). The results are expressed in Fig. 5 and show that none of the sera from normal individuals reacted with U1. In contrast, U1 was recognized by the majority of the sera from patients with pulmonary tuberculosis regardless of coinfection with HIV. Sera from 9 of 12 patients with HIV and with no clinical signs of tuberculosis were negative with U1. However, 3 of these 12 sera gave borderline ELISA signals (signal/cutoff ≤ 1.75) with the recombinant antigen. In addition, the reactivity with U1 was compared that with Mtb81, an antigen known to be recognized by the majority of the sera from HIV patients coinfected with tuberculosis. The results are expressed in Table 1 and point to a 60.2% reactivity for U1 and 44.6% for Mtb81 for patients with tuberculosis only. Interestingly, the sera of tuberculosis-HIV-coinfected patients recognized both antigens equally. Also interesting was the observation that some of the latter sera recognized only one of the two antigens. The significance of this finding is that these two antigens can complement each other for the diagnosis of tuberculosis in HIV-coinfected patients. Indeed, using this approach, 93.6% of these patients could be identified (Table 1).
FIG. 5.
Serological reactivity of U1 with sera from tuberculosis patients with and without HIV coinfection. Sera from 37 healthy PPD-negative individuals (Normals), from 12 patients with HIV infection and no clinical signs of tuberculosis (HIV), from 54 patients with extrapulmonary tuberculosis (XP), from 40 patients with pleural tuberculosis (Pleural), from 83 patients with tuberculosis and no signs of coinfection with HIV (TB+/HIV-), and from 47 patients coinfected with tuberculosis and HIV (TB+/HIV+) were evaluated for reactivity with U1 using standard ELISA. Results are plotted as signal/cutoff, which represents the ratio of the OD at 450 nm given by the patients' sera divided by the mean OD at 450 nm plus 3 standard deviations given by the sera of normal individuals.
TABLE 1.
Comparative reactivities of U1 and Mtb81 antigens with sera from patients with tuberculosis only and from patients with tuberculosis and HIV coinfection
| Antigen | % Positive sera for patient groupa
|
|
|---|---|---|
| Tuberculosis only (n = 83) | Tuberculosis and HIV (n = 47) | |
| Mtb81 | 44.6 | 87.2 |
| U1 | 60.2 | 87.2 |
| Mtb81+U1 | 66.3 | 93.6 |
Percent positive sera (OD of patient serum/OD of normal control serum plus 3 standard deviations). n, no. of samples tested.
DISCUSSION
The serological diagnosis of tuberculosis has been traditionally regarded as a nonreliable practice, mostly because of the low sensitivity and specificity of the tests. Moreover, none of the tests thus far described can discriminate latent tuberculosis from tuberculosis disease. Aside from the intrinsic complexity of the immunological and physiopathological characteristics of tuberculosis (chronic disease that induces predominantly cellular immunity and weak humoral immunity), one possible explanation for the general lack of success of most serological tests is the complex mixture of crude antigens that has been used in these tests (7, 14). More recently, however, a series of highly purified M. tuberculosis recombinant antigens have been described and evaluated as serological markers of the disease. Some of these antigens have been more extensively evaluated and shown to have relatively high sensitivity and specificity for the identification of patients with pulmonary tuberculosis (4, 8, 10, 15, 20, 23, 25, 29). These findings have revived the concept that serological tests for the diagnosis of tuberculosis, as for most infectious diseases, are indeed feasible.
The present studies describe a novel M. tuberculosis recombinant antigen that can be an important aid in improving the sensitivity and specificity of serological tests for tuberculosis. The direct approach of the strategy used to identify this novel antigen revealed an M. tuberculosis protein that is released by the microorganisms during the early phase of the infectious process and is eliminated in the urine of the infected host. The identification of this molecule was made possible because of both the recent technological progress in sequencing proteins at the fentomole levels and the definition and database availability of the entire M. tuberculosis genome. The ultra-high sensitivity of MS/MS in sequencing proteins was used to sequence silver-stained polyacrylamide gel fragments containing protein bands on an SDS-polyacrylamide gel unique to the urine of M. tuberculosis-infected C57BL/mice and absent in the urine of normal uninfected syngeneic mice. Interestingly, several of these bands contained sequences with strong matches with mouse proteins such as protrombin precursor, prostaglandin-H2, D-isomerase, C3 convertase activator, tyrosine protein kinase receptor, cathepsin E, alpha-1 antitrypsin, serum albumin, glycan-1, alpha-amylase, and interleukin-2 receptor alpha chain. The anomalous presence of autologous proteins in the urine of these mice may be a reflection of glomerular and/or tubular dysfunction caused by the infectious process. However, although several mouse protein sequences were found in the urine of infected animals, the sequence of a band of ∼16 kDa revealed a peptide sequence that strongly matched the deduced sequence of a 16.8-kDa hypothetic thiol peroxidase M. tuberculosis protein. Cloning and expression of the gene encoding this protein gave rise to a highly purified and soluble recombinant molecule, which we called M. tuberculosis U1. Moreover, Western blot analyses using either a specific rabbit anti-recombinant protein or a pool of human sera from patients with pulmonary tuberculosis clearly revealed that U1 was indeed a true M. tuberculosis protein that is present in the culture filtrates of these organisms.
The possible utility of U1 as a diagnostic tool was confirmed in serological studies using ELISA. A panel of sera from several tuberculosis patients with different forms of tuberculosis specifically recognized the recombinant molecule, including sera from patients coinfected with HIV. The reactions of all sera from normal individuals (54 donors) with U1 were either below detection or with optical density (OD) signals of ≤0.2. It is interesting that the criterion for defining positive and negative reactions used in these studies was the use of an arbitrarily stringent cutoff. This critical point was calculated using the average of the OD given by a panel of 54 sera from normal individuals plus three standard deviations (20). Using this criterion, the sensitivity of the test revealed that among the tuberculosis patients not coinfected with HIV, approximately 60% of the sera from patients with pulmonary tuberculosis were specifically identified by U1. Interestingly and importantly, the antigen could recognize 87.2% of the tuberculosis patients coinfected with HIV. It is important to emphasize that out of 12 sera samples from patients with HIV infection and no clinical signs of tuberculosis, 9 were negative and only 3 were borderline positive. These results further support the specificity of U1 antigen. However, the ability of the antigen to detect patients with either pleural tuberculosis or other extrapulmonary forms of tuberculosis was surprisingly weaker (40.3 and 23.6%, respectively). However, the high level of sensitivity of U1 for the serological diagnosis of pulmonary tuberculosis, although not high enough to be used alone in routine tests for the diagnosis of this disease, is of great interest, because few recombinant proteins have been reported to perform this well. Among the described molecules, only the 38-kDA antigen has been shown to have sensitivity equal to that of U1 for the diagnosis of pulmonary tuberculosis in the absence of coinfection with HIV (12). However, the 38-kDa antigen has poor sensitivity for the diagnosis of the latter cases of the disease (7, 14). Therefore, because U1 has a high sensitivity to detect tuberculosis-HIV coinfection, this antigen can be an important aid in complementing the reactivity of the 38-kDa antigen and consequently can increase the overall sensitivity of the serological test for the diagnosis of tuberculosis. Moreover, such complementation may also occur for patients with tuberculosis in the absence of HIV coinfection. This possibility, however, needs to be further confirmed. It is interesting that U1 and Mtb81 have similar sensitivities in identifying patients with tuberculosis who are coinfected with HIV. However, U1 has higher sensitivity than Mtb81 for detection of tuberculosis only (60 versus 44%). Moreover, U1 is easily expressed and purified as a recombinant protein. In addition, this molecule is highly soluble in PBS, and it is stable. In contrast, Mtb81 appears to be an unstable molecule.
At this point we do not have strong data to explain the high sensitivity of U1 in identifying tuberculosis patients coinfected with HIV. However, an interesting possibility can be drawn from the mouse experiments. In this model, because U1 was found in the urine of mice early after infection of the animals with M. tuberculosis, it appears that this protein is abundantly released in vivo by the actively dividing mycobacteria. A similar scenario can therefore be extrapolated for tuberculosis patients coinfected with HIV. It is generally accepted that the initial breakdown of the immune system caused by the viral infection leads to reactivation of primary tuberculosis in many individuals. Consequently, during this phase it is possible, similar to the course of infection in mice, that U1 is abundantly released and causes restimulation of the immune response and production of high titers of immunoglobulin G antibodies that persist for months in the patient's serum. Although as yet circumstantial, this is an attractive possibility to explain the high sensitivity of U1 in identifying tuberculosis patients coinfected with HIV.
In conclusion, the presence of a molecule like U1 in the urine of infected mice, at a time point that coincides with the logarithmic multiplication of M. tuberculosis in vivo, suggests that this molecule is a promising marker candidate for active tuberculosis.
Finally, it is interesting that recent results from our laboratories indicate that the urine of a patient with pulmonary tuberculosis contains a protein of ∼16 kDa in mass that is detected by Western blot analyses using the rabbit anti-U1 antiserum described above. These results suggest that an antigen detection assay can in principle be developed for the diagnosis of tuberculosis. Studies are in progress to investigate this interesting possibility.
Acknowledgments
We thank Yasir Skeiky for invaluable discussions, Roberto Badaró (Federal University of Bahia, Salvador, Bahia, Brazil) and Virmondes Rodrigues-Júnior (Medical School of TriÂngulo Mineiro, Uberaba, Minas Gerais, Brazil) for kindly providing the human serum samples used in these studies.
This work was supported by the following grants from the National Institutes of Health: AI 43528 to A. Campos-Neto and AI 44373 to S. G. Reed.
REFERENCES
- 1.American Thoracic Society. 1981. The tuberculin skin test. Am. Rev. Respir. Dis. 124:356-363. [Google Scholar]
- 2.American Thoracic Society and Centers for Disease Control. 1991. Diagnostic standards and classification of tuberculosis. Am. Rev. Respir. Dis. 142:725-735. [DOI] [PubMed] [Google Scholar]
- 3.Bothamley, G. H. 1995. Serological diagnosis of tuberculosis. Eur. Respir. J. Suppl. 20:676s-688s. [PubMed] [Google Scholar]
- 4.Brusasca, P. N., R. Colangeli, K. P. Lyashchenko, X. Zhao, M. Vogelstein, J. S. Spencer, D. N. McMurray, and M. L. Gennaro. 2001. Immunological characterization of antigens encoded by the RD1 region of the Mycobacterium tuberculosis genome. Scand. J. Immunol. 54:448-452. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Neto, A., V. Rodrigues-Junior, D. B. Pedral-Sampaio, E. M. Netto, P. J. Ovendale, R. N. Coler, Y. A. Skeiky, R. Badaro, and S. G. Reed. 2001. Evaluation of DPPD, a single recombinant Mycobacterium tuberculosis protein as an alternative antigen for the Mantoux test. Tuberculosis (Edinburgh) 81:353-358. [DOI] [PubMed] [Google Scholar]
- 6.Campos-Neto, A., L. Soong, J. L. Cordova, D. Sant'Angelo, Y. A. W. Skeiky, N. H. Ruddle, S. G. Reed, Jr., C. A. Janeway, and D. McMahon-Pratt. 1999. Cloning and expression of a Leishmania donovani instructed by a peptide isolated from major histocompatibility complex class II molecules of infected macrophages. J. Exp. Med. 182:1423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, E. D., L. Heifets, and M. D. Iseman. 2000. Immunologic diagnosis of tuberculosis: a review. Tuber. Lung Dis. 80:131-140. [DOI] [PubMed] [Google Scholar]
- 8.Chiang, I. H., J. Suo, K. J. Bai, T. P. Lin, K. T. Luh, C. J. Yu, and P. C. Yang. 1997. Serodiagnosis of tuberculosis. A study comparing three specific mycobacterial antigens. Am. J. Respir. Crit. Care Med. 156:906-911. [DOI] [PubMed] [Google Scholar]
- 9.Coler, R. N., Y. A. Skeiky, P. J. Ovendale, T. S. Vedvick, L. Gervassi, J. Guderian, S. Jen, S. G. Reed, and A. Campos-Neto. 2000. Cloning of a Mycobacterium tuberculosis gene encoding a purified protein derivative protein that elicits strong tuberculosis-specific delayed-type hypersensitivity. J. Infect. Dis. 182:224-233. [DOI] [PubMed] [Google Scholar]
- 10.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 12.Espitia, C., I. Cervera, R. Gonzalez, and R. Mancilla. 1989. A 38-kD Mycobacterium tuberculosis antigen associated with infection. Its isolation and serologic evaluation. Clin. Exp. Immunol. 77:373-377. [PMC free article] [PubMed] [Google Scholar]
- 13.Ginsberg, A. M. 1998. The tuberculosis epidemic. Scientific challenges and opportunities. Public Health Rep. 113:128-136. [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta, S., R. Bhatia, and K. K. Datta. 1995. Serodiagnosis of tuberculosis. J. Commun. Dis. 27:208-214. [PubMed] [Google Scholar]
- 15.Gupta, S., S. Kumari, J. N. Banwalikar, and S. K. Gupta. 1995. Diagnostic utility of the estimation of mycobacterial antigen A60 specific immunoglobulins IgM, IgA and IgG in the sera of cases of adult human tuberculosis. Tuber. Lung Dis. 76:418-424. [DOI] [PubMed] [Google Scholar]
- 16.Gygi, S. P., G. L. Corthals, Y. Zhang, Y. Rochon, and R. Aebersold. 2000. Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology. Proc. Natl. Acad. Sci. USA 97:9390-9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas, F., and S. S. Haas. 1996. The origins of Mycobacterium tuberculosis and the notion of its contagiousness, p. 3-19. In W. R. Rom and S. M. Garay (ed.), Tuberculosis. Little, Brown & Company, Boston, Mass.
- 18.Hendrickson, R. C., J. F. Douglass, L. D. Reynolds, P. D. McNeill, D. Carter, S. G. Reed, and R. L. Houghton. 2000. Mass spectrometric identification of mtb81, a novel serological marker for tuberculosis. J. Clin. Microbiol. 38:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopewell, P. C. 1992. Impact of human immunodeficient virus infection on the epidemiology, clinical features, management and control of tuberculosis. Clin. Infect. Dis. 18:540-547. [DOI] [PubMed] [Google Scholar]
- 20.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huebner, R. E., M. F. Schein, and J. B. Bass, Jr. 1993. The tuberculin skin test. Clin. Infect. Dis. 17:968-975. [DOI] [PubMed] [Google Scholar]
- 22.Kochi, A. 1991. The global tuberculosis situation and the control strategy of the World Health Organization. Tubercle 72:1-6. [DOI] [PubMed] [Google Scholar]
- 23.Lodes, M. J., D. C. Dillon, R. Mohamath, C. H. Day, D. R. Benson, L. D. Reynolds, P. McNeill, D. P. Sampaio, Y. A. Skeiky, R. Badaro, D. H. Persing, S. G. Reed, and R. L. Houghton. 2001. Serological expression cloning and immunological evaluation of MTB48, a novel Mycobacterium tuberculosis antigen. J. Clin. Microbiol. 39:2485-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantoux, M. C. 1912. La vioe intradermique en tuberculinatherapie. Presse Med. 20:146-148. [Google Scholar]
- 25.Moran, A. J., J. D. Treit, J. L. Whitney, B. Abomoelak, R. Houghton, Y. A. Skeiky, D. P. Sampaio, R. Badaro, and F. E. Nano. 2001. Assessment of the serodiagnostic potential of nine novel proteins from Mycobacterium tuberculosis. FEMS Microbiol. Lett. 198:31-36. [DOI] [PubMed] [Google Scholar]
- 26.Netto, E. M., C. Dye, and M. C. Raviglione. 1999. Progress in global tuberculosis control 1995-1996, with emphasis on 22 high-incidence countries. Global Monitoring and Surveillance Project. Int. J. Tuberc. Lung Dis. 3:310-320. [PubMed] [Google Scholar]
- 27.Okwera, A., C. Whalen, F. Byekwaso, M. Vjecha, J. Johnson, R. Huebner, R. Mugerwa, and J. Ellner. 1994. Randomised trial of thiacetazone and rifampicin-containing regimens for pulmonary tuberculosis in HIV-infected Ugandans. The Makerere University-Case Western University Research Collaboration. Lancet 344:1323-1328. [DOI] [PubMed] [Google Scholar]
- 28.Orme, I. M. 1987. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J. Immunol. 138:293-298. [PubMed] [Google Scholar]
- 29.Raja, A., U. D. Ranganathan, R. Bethunaickan, and V. Dharmalingam. 2001. Serologic response to a secreted and a cytosolic antigen of Mycobacterium tuberculosis in childhood tuberculosis. Pediatr. Infect. Dis. J. 20:1161-1164. [DOI] [PubMed] [Google Scholar]
- 30.Raviglione, M. C., J. P. Narain, and A. Kochi. 1992. HIV-associated tuberculosis in developing countries: clinical features, diagnosis and treatment. Bull. W. H. O. 70:515-526. [PMC free article] [PubMed] [Google Scholar]
- 31.Raviglione, M. C., D. E. Snider, Jr., and A. Kochi. 1995. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273:220-226. [PubMed] [Google Scholar]
- 32.Sudre, P., G. ten Dam, and A. Kochi. 1992. Tuberculosis: a global overview of the situation today. Bull. W. H. O. 70:149-159. [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson, R. J., K. Haslov, R. Rappuoli, F. Giovannoni, P. R. Narayanan, C. R. Desai, H. M. Vordermeier, J. Paulsen, G. Pasvol, J. Ivanyi, and M. Singh. 1997. Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent. J. Clin. Microbiol. 35:553-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. 1994. The current global situation of the HIV/AIDS pandemic. Wkly. Epidemiol. Rec. 69:191-192.7917878 [Google Scholar]
- 35.Yates, J. R., III, J. K. Eng, A. L. McCormack, and D. Schieltz. 1995. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal. Chem. 67:1426-1436. [DOI] [PubMed] [Google Scholar]