Abstract
Carcinoma ex pleomorphic adenoma (CXPA) is a broad category of carcinomas of the salivary glands which includes at least 2 clinically relevant categories; one is referred here as early CXPA (ECXPA), the other as widely invasive CXPA. The former includes several histological patterns ranging from non-invasive/in situ/intraductal/intratubular, early invasive/extratubular/intracapsular and extracapsular (up to 6 mm). The latter includes any CXPA with invasion of >6 mm. The clinical behaviour of ECXPA is not aggressive and tends to overlap that of a pleomorphic adenoma (PA) which makes the histological report of carcinoma contradictory. These early malignant changes in PA are known since the 1970s but it has been the use of immunohistochemical and molecular genetic analysis for HER-2 and TP53 gene in the last decade that has clarified the genuine malignant nature of the cells. HER-2 and TP53 gene and protein are involved in the early stages of malignant transformation of PA. Moreover the immunohistochemical over-expression HER-2, p53 protein and Mib-1 proliferation marker may be useful markers to identify malignant areas in PA.
Keywords: Carcinoma ex pleomorphic adenoma (CXPA), ECXPA, Salivary gland, HER-2 gene and protein, TP53
Background
Carcinoma ex pleomorphic adenoma (CXPA), in the revised WHO classification published in 2005 is defined as: a pleomorphic adenoma in which an epithelial malignancy is derived [1]. The histological diagnosis of pleomorphic adenoma (PA) (benign mixed tumour) is not always straightforward, as benign lesions may display atypical histologic features such as capsular infiltration, hypercellularity, cellular atypia, necrosis and vascular invasion [2, 3] which raises suspicion for malignancy. In addition, some PAs contain genuine cytologically malignant cells, but behave in a benign fashion [4]. A further paradox is the rare occurrence of histologically benign-looking PAs, which metastasize [5]. Thus, the concept of malignancy in PA is much more complex than appears at first sight. This is reflected by the variable incidence for the reported frequency of malignancy in PA. In several large series [1, 6–9] the average was 3.6 % of all salivary gland tumours and 11.7 % of malignancies; overall, malignancy develops in 6.2 % of all PAs (reported range: 1.9–23.3 %). The incidence of malignant change increases with the length of history of the PA, from 1.5 % at 5 years to 10 % after 15 years [1]. By definition CXPA must arise in association with PA which requires histological evidence of co-existent or pre-existing PA. Which PAs will develop malignant changes is difficult to predict. Older patient age, larger size and submandibular origin tend to be associated with malignant transformation [9]. Some authors have also suggested that the presence of marked hyalinisation and increased mitotic activity up to 10× HPF has more probability of malignant change [2].
The recent WHO classification discusses 3 main categories of CXPA based on the degree of invasion of carcinoma beyond the capsule of maternal PA—widely invasive, minimally invasive and non-invasive CXPA. In addition the WHO classification also includes the possibility of myoepithelial carcinoma arising in PA in 50 % of reported myoepithelial carcinomas of salivary glands [1]. The reported 5 year survival rate of CXPA ranges from <20 to >90 % which is too wide to be clinically meaningful [1, 8–10] and suggests that the diagnosis of CXPA may be used as a dustbin diagnosis and therefore of limited clinical value for patient management.
The following review gives an overview of the histological patterns of invasive CXPA but will be mostly focused on morphological, immunohistochemical and molecular analysis of early invasive and non invasive/in situ CXPA.
Widely Invasive Carcinoma Ex Pleomorphic Adenoma
The most common histologic subtype of CXPA is the widely invasive form, in which the malignancy involves the epithelial component. At presentation most patients are men over 60 years old with advanced clinical stage and poor prognosis. Most cases (81.7 %) involve the parotid gland, followed by the submandibular (18 %) and the sublingual (0.3 %); the minor salivary glands, particularly in the palate can also be affected [11]. The typical presentation is of a long history of a salivary gland nodule that suddenly increases in size. A history of a long-standing parotid tumour is not sufficient evidence for a pre-existing PA, whilst a previously excised PA at the site of a carcinoma is acceptable [13].
Pathological Findings
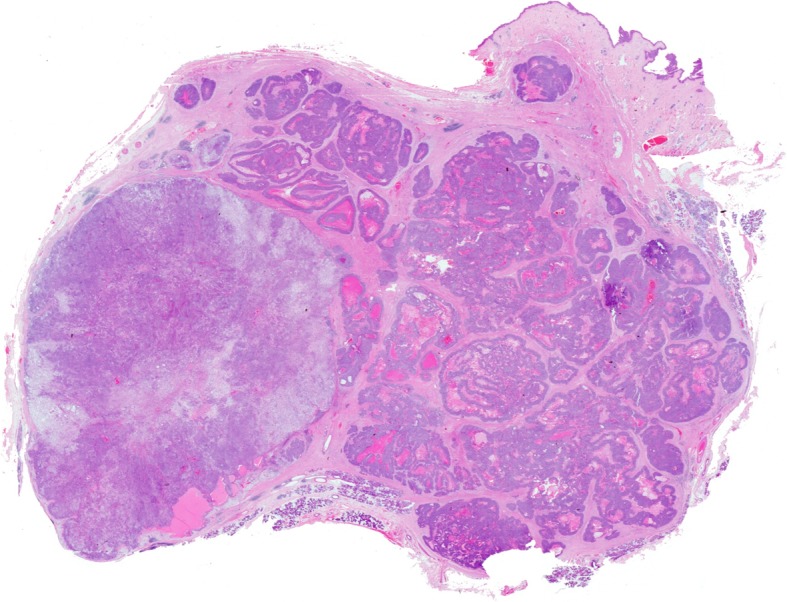
Grossly, widely invasive CXPAs are often larger than a benign PA [1, 9]. They are firm and ill-defined tumours with infiltration into adjacent salivary gland tissue. Gross features of a background PA, which is necessary for the diagnosis (Fig. 1) may require extensive sampling. Histologically the pre-existing PA may be obscured by the carcinoma, or may only show degenerative changes such as scarring, dystrophic calcifications, necrosis and haemorrhage with occasional transitional changes composed of cells with features intermediate between frankly malignant cells and PA cells [9, 12, 13].
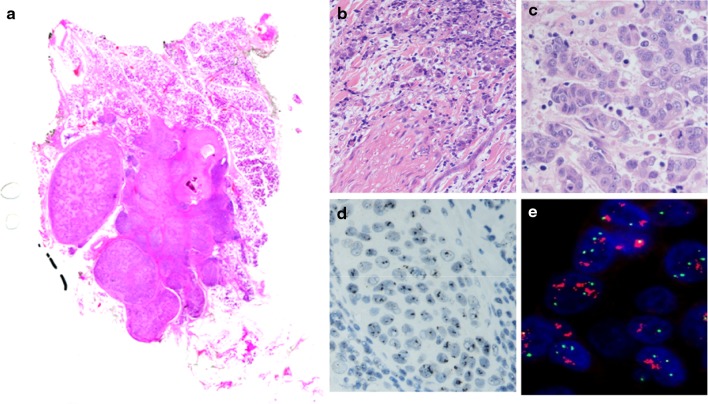
Fig. 1.
Whole mounted section of carcinoma ex Pleomorphic adenoma of parotid gland with perineural infiltration (b) and highly pleomorphic cells with numerous mitoses (c). The maternal PA shows sclerosis and calcifications. HER-2/neu gene amplification detected by Silver (d) and fluorescent (e) in situ hybridization
When the maternal PA is detected, the contrast between PA and carcinoma is obvious and the recognition of widely invasive carcinoma ex PA is usually simple. The malignant cells tend to have pronounced nuclear pleomorphism and increased number of mitoses (Fig. 1). In addition, capsular, perineural (Fig. 1) and vascular invasion are easily identified as well as extension into neighbouring tissues. Several studies show adenocarcinoma NOS and salivary duct carcinoma to be the most frequent histological types [12, 15] but it is not uncommon to find other concurrent differentiation, e.g. squamous, mucoepidermoid, polymorphous low grade adenocarcinoma [6, 7, 9, 16].
Immunohistochemical Findings
The immunohistochemical profile of CXPA shows diffuse and strong expression of pan-cytokeratin (AE1–AE3 and CAM5.2), CK7, CK8, CK18, CK19 and epithelial membrane antigen (EMA). Staining for basal/myoepithelial cell markers such as p63, smooth muscle actin, CK5/6 and CK14 may be focally present in the basal/myoepithelial cells surrounding residual foci of non-invasive CXPA.
Proliferative activity using the monoclonal antibody MIB-1 to the Ki-67 antigen shows higher (35 %) proliferation index in the foci of CXPA which contrasts with the low proliferation index of the maternal PA.
In addition, detection of Androgen Receptor, with p53 and HER-2 protein overexpression can be seen in the malignant component. Immunohistochemistry for HER/2 protein is seen in CXPA as a distinct membrane staining (3+) in cases of high grade CXPA while low grade show moderate (2+), weak or absent (1+/0) membrane staining.
Molecular Genetic Findings
HER-2 gene amplification as detected by in situ hybridization (ISH) is seen in cases of CXPA with high grade morphology and it is restricted to the cytologically malignant luminal cells. Mutation of TP53 gene [14] as detected by gene sequencing is noted in the malignant cells of CXPA.
Myoepithelial Carcinoma (Malignant Myoepithelioma) Arising in PA
Myoepithelial carcinoma is a variant of widely invasive CXPA which may arise de novo, but at least 50 % develop in a pre-existing PA or benign myoepithelioma. Most cases arise in the parotid, but they also occur in the submandibular and minor glands [17–19]. Figure 2 shows an example of myoepithelial carcinoma arising in a PA. The diagnosis usually requires immunohistochemical demonstration of markers such as S-100 protein, wide spectrum keratins, vimentin, alpha smooth muscle actin, calponin and p63 and/or ultrastructural demonstration of both epithelial and smooth muscle differentiation. Histologically the recognition of PA and myoepithelial carcinoma arising in PA is usually more difficult than that of carcinoma composed of cells with epithelial/luminal phenotype because the cell type is similar and the contrast between myoepithelial carcinoma and PA is less marked. More detailed description of myoepithelial CXPA is well-documented in the literature [17–19] and is therefore not elaborated on here (Fig. 3).
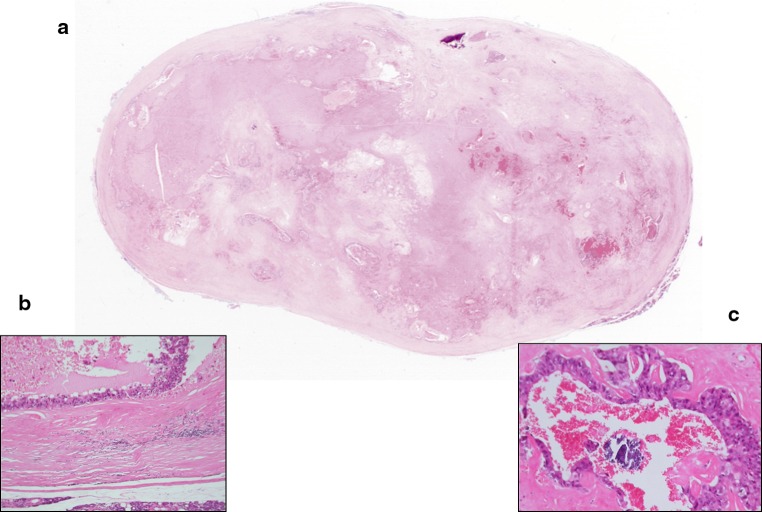
Fig. 2.
Whole mounted section of myoepithelial carcinoma (right) arising in Pleomorphic Adenoma (left) of parotid. Note the multinodular growth pattern with infiltration of the dermis
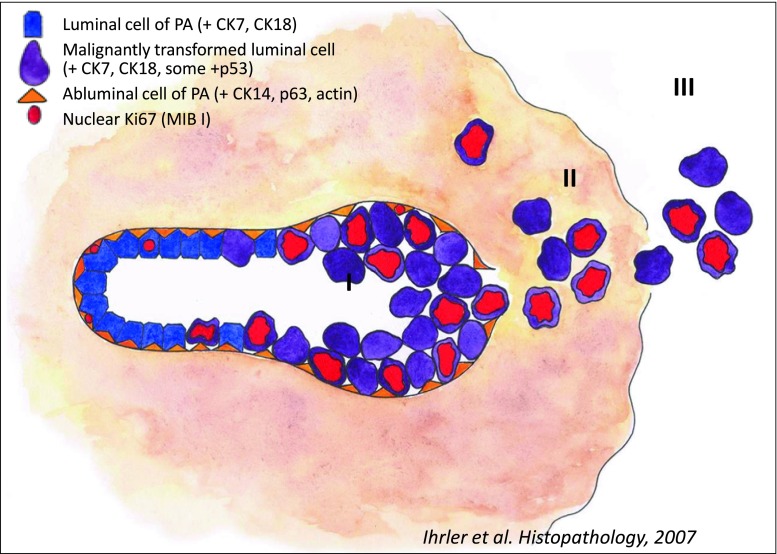
Fig. 3.
Diagram showing progression of carcinoma ex pleomorphic adenoma. I Pre-existing tubules of Pleomorphic Adenoma partly replaced by malignant epithelial cells. II Rupture of basal membrane with extratubular extension but still confined within the capsule of PA. III Extracapsular spread with infiltration of surrounding tissues. Courtesy by Dr. S. Ihrler
Early Carcinoma Ex Pleomorphic Adenoma (ECXPA)
Early malignant changes in PA are sometimes referred to as non-invasive, in situ, intracapsular, intratubular or intraductal CXPA but there is no definite agreement on how to report these cases. In essence ECXPA is a cytologically malignant neoplasm with either no invasion or with a degree of invasion of no more than 6 mm, arising in PA. Its clinical behaviour is not aggressive, and it should be separated from the broad—without further specification of degree of invasion—category of CXPA. A reappraisal of ECXPA has been discussed by Irhler et al. and Weiler et al. in 2007 and 2011 [14, 20], respectively with particular emphasis on both progressive histological changes and the threshold between minimally invasive and frankly invasive [14, 20]. Based on these two recent studies and for the purposes of this chapter the definition of early carcinoma ex pleomorphic adenoma (ECXPA) will be used to include in situ, intratubular, intraductal, intracapsular and invasive up to 6 mm of extracapsular extension. It includes a spectrum of histological features ranging from duct-like structures resembling in situ ductal carcinoma of the breast to micro-invasive DCIS, where there is extraductal spread but still confined to the capsule of the underlying PA, to extension outside the capsule but no more than 6 mm [20].
The clinical significance of ECXPA is still controversial. Because of the excellent prognosis which overlaps that of PA it is still unclear as to whether or not it represents a genuine malignancy or simply pseudomalignant changes. There are, however immunohistochemical and molecular genetic data involving the TP53 and HER-2 gene and their protein expression which are mainly seen in malignant cells [4, 14] suggesting that they are early but genuine malignant changes. The literature in the last two decades has seen an increased number of papers discussing the progression from benign PA to carcinoma where long term follow-up data reported by several investigators have also highlighted that not all CXPA behave in a similar way and that the presence or absence and the degree of invasion beyond the capsule of PA is the best predictor of subsequent clinical behaviour [4, 10, 20, 21].
Pathological Findings of ECXPA
ECXPA are well-circumscribed and small sized (stage T1-2) tumours (Table 4) without invasive growth to adjacent tissue (Figs. 4, 5, 6). Those arising in the deep parotid lobe may be larger in size. Histologically the overall appearance is more reminiscent of PA rather than of a carcinoma. However within the maternal PA which is usually identified in all cases there are cytologically malignant cells within the pre-existing ducts of PA, surrounded by small bland looking actin/CK14/p63 positive myoepithelial cells. These ducts show central necrosis giving a comedo-like appearance and papillary and cribriform architecture resembling intraductal carcinoma of the breast.
Table 4.
Macroscopic and microscopic features of ECXPA
| T Stage: mostly T1–T2 |
| Circumscription: well circumscribed |
| Clear evidence of PA: macroscopically and histologically unequivocal |
| Tubules replaced by atypical cells |
| Presence/absence of surrounding myoepithelial cells |
| DCIS-like patterns: solid, cribriform, papillary, mixed |
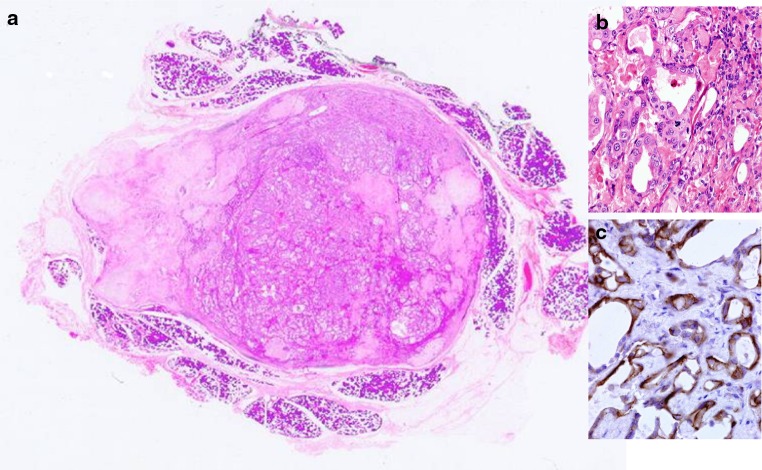
Fig. 4.
Whole mounted section of Early Carcinoma Ex Pleomorphic adenoma (ECXPA). The nodule is well circumscribed and there is no extension into surrounding tissues. Calcifications and haemorrhage is seen. The viable areas consist of ducts lined by atypical cells b with early cribriform architecture c
Fig. 5.
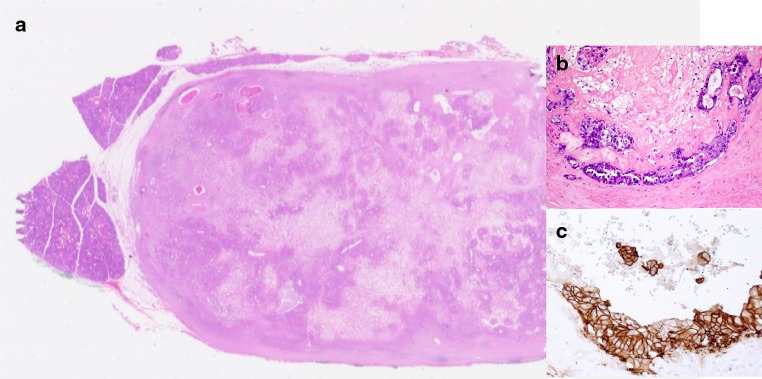
Another example of ECXPA where the atypical cells show strong membrane staining for HER-2/neu (score 3+). (Immunohistochemical staining, original magnification ×360)
Fig. 6.
ECXPA. Whole mounted section a. Atypical epithelial proliferation replacing ducts of PA b. Moderate membrane staining for HER-2/neu (score 2+) in atypical cells of carcinoma ex pleomorphic adenoma. Note absence of staining in benign component. c (immunohistochemical staining, original magnification ×80)
When extra-tubular invasion appears the features overlap those of widely invasive CXPA described earlier.
Molecular Genetic Findings
The molecular genetic findings of ECXPA are similar to widely invasive CXPA described above.
Concept of ECXPA: Historical Review
Historically (see Table 1) the concept of ECXPA has been noted since 1977 by LiVolsi and Perzin who were the first authors who raised the issue of non-invasive CXPA [6]. In 6/47 cases of CXPA there was no evidence of invasion. The clinical behaviour in these patients was similar to PA with no local recurrence or distant metastases suggesting the non-aggressive nature of these tumours. In 1984 Tortoledo et al. [22] reported 16 patients with extracapsular invasion ranging from 9–20 mm who died of their disease; while none of 16 patients with extracapsular invasion less than 8 mm did . Brandwein et al. [10] reported 12 patients with capsular invasion of ≤1.5 mm. None of patients developed local recurrences or metastases within a follow-up period of up to 13 years. The study did not include patients with extracapsular invasion of more than 1.5 mm. This threshold has been adopted by the WHO classification of Salivary Gland Tumours [1] to distinguish between minimally invasive (with favourable prognosis) and frankly invasive (with poor prognosis) CXPA (see Table 2) but there are at least 4 recent studies suggesting a higher threshold of 4–6 mm [20, 21, 23, 24]. Olsen and Lewis in 2001 reported a total of 73 patients of CXPA. Two patients with 2 and 3 mm extracapsular invasion had no progression [21]. There was only one study where lymph node metastasis developed in a non-invasive CXPA. However this case of metastasizing non-invasive (intracapsular) CXPA may be a result of undersampling of invasive areas. This is also a well-known occurrence in the breast where cases with “DCIS only” may show metastasis. In 2005 Di Palma et al. [4] reported a series of 11 cases of non-invasive (intracapsular) CXPA where the terminology was discussed and the importance of separating non/early invasive CXPA from widely invasive CXPA was emphasised. The authors suggested that intracapsular CXPA may represent an early stage in the development of widely invasive carcinoma and speculated that HER-2 overexpression might help to demonstrate that ECXPA is a genuinely malignant process (Fig. 7).
Table 1.
Early carcinoma ex pleomorphic adenoma
| Authors | Years | No. of cases | Development of recurrence/metastases |
|---|---|---|---|
| Livosi and Perzin [6] | 1977 | 6/47 | None |
| Brandwein et al. [10] | 1997 | 12 | None |
| Olsen et al. [8] | 2001 | 2/73 | None |
| Felix et al. [25] | 2002 | 1 | Lymph node metastasis |
| Di Palma et al. [4] | 2005 | 11 | None |
| Altemani et al. [32] | 2005 | 10 | None |
| Ihrler et al. [14] | 2007 | 8/19 | None |
| Katabi et al. [23] | 2010 | 13 | 3 developed metastasesa |
| Weiler et al. [20] | 2011 | 8/19 | None |
| Hashimoto [24] | 2012 | 13/31 | None |
aNot indicated if intracapsular/minimally invasive and degree of invasion
Table 2.
Classification of Malignant Mixed Tumours WHO [1]
| Carcinoma ex pleomorphic adenoma |
| Non-invasive (in situ, intracapsular) |
| Minimally invasive (≤1.5 mm) Invasive (>1.5 mm) |
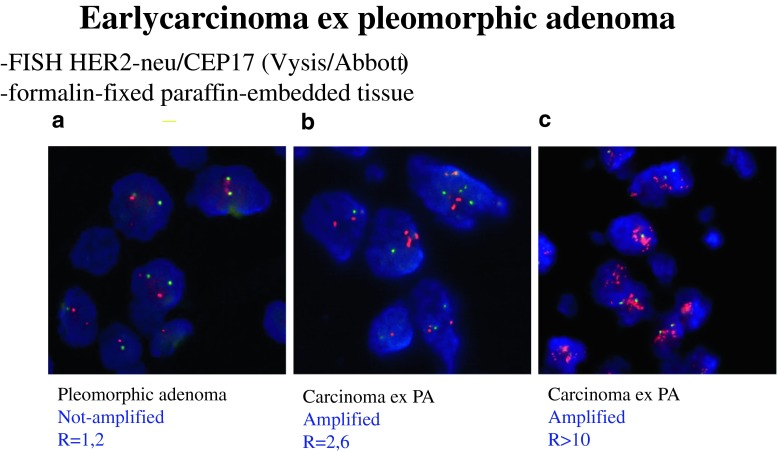
Fig. 7.
ECXPA. a No amplification of HER-2/neu gene in PA, b and c low and high level of amplification, with 2.6 and >10 mean number of gene copies per nucleus (FISH)
In a previous study of salivary duct carcinoma which included cases that had arisen from a PA (i.e. Carcinoma ex pleomorphic adenoma) a group of researchers found immunohistochemical overexpression of HER-2 in all cases of SDC, and also 30 % of them showed amplification of HER-2 gene by means of fluorescence in situ hybridization (FISH) analysis [25]. The authors suggested that strong immunocytochemical overexpression of HER-2 could be a useful marker to identify salivary duct carcinoma arising from PA.
In immunohistochemistry, overexpression of HER-2 protein was identified as distinct membrane staining in most carcinoma cells in all cases of intracapsular/non-invasive CXPA, while only approximately 20 % of the cases revealed an amplification of HER-2 gene by means of in situ hybridization analysis [4, 24]. Indeed the HER2 phenotype is further confirmed by the application of molecular classification of breast carcinoma to in situ and invasive salivary duct carcinomas [26, 27]. The presence of high grade ductal carcinoma in situ arising in a PA [4] as well as arising de novo [26] indicates that the HER-2 is involved in the early events in the cases of malignant transformation of PA with high grade phenotype. A similar phenomenon occurs in the breast [28], where high grade ductal carcinoma in situ (DCIS) which overexpresses HER-2 often progresses to HER-2 positive invasive ductal carcinoma. In contrast HER-2 negative low/intermediate grade DCIS often progress to low grade, HER-2 negative invasive ductal carcinoma. This suggests that HER-2 may be a useful marker to identify malignant transformation in PA by means of immunohistochemistry for HER-2 protein which is currently the most attractive method for evaluation of its expression due to factors such as cost, convenience and biological relevance. However considerable and well documented variations in IHC staining are caused by differences in sensitivity of antibodies, in interpretation by different observers, or in processing of tissues studied. Our immunohistochemical and fluorescent in situ hybridization (FISH) study on salivary duct carcinoma [25] has demonstrated that commercially available antibodies for HER-2 protein may produce different degree of membranous staining leading to positive (3+), inconclusive (2+) and negative (0/1+) scores in the same case. These results are similar to those described in breast carcinomas where appropriate formalin fixation also plays a role in the variations of immunohistochemical results [25].
In situ hybridization has been shown to correlate well with protein overexpression in IHC and have similar predictive and prognostic significance. The goal of HER-2 testing in salivary gland carcinomas is on one hand to assist in the diagnosis of ECXPA, and on the other hand to identify patients eligible for Trastuzumab therapy if they develop metastatic disease [29]. At the 24th European Congress of Pathology, held in Prague in September 2012 Di Palma et al. presented a case of CXPA successfully treated with Trastuzumab and radiotherapy [30]. The possibility of assessing HER-2 gene amplification by newly developed in situ hybridization techniques which can be assessed by light microscopy may encourage the assessment of HER-2 status of salivary gland carcinomas in routine diagnostic reporting [24, 27]. There are other molecular genetic studies corroborating the true malignant nature of ECXPA. Irhler et al. have analyzed the P53 oncogene in a series of 19 cases of CXPA where the histologic stages of malignant transformation have been described in detail [14]. In this study mutation of the p53 gene as detected by gene sequencing from laser microdissected samples of CXPA was found to be implicated in the malignant transformation in 37 % (7/19) of the cases of intraductal carcinoma. In addition p53 overexpression (assessed according to staining of intensity from 0 to 3 and percentage of positive cells) was found in 11/19 cases. If both demonstration of mutant TP53 gene and accumulation of p53 protein are taken into account it can be stated that most ECXPA are associated with dysfunctional p53 status. The authors suggest that intraductal carcinoma represents the pre-invasive stage (Table 3) of most, low and high grade, CXPA with luminal phenotype but data reported in literature about histological features of pre-invasive stages of myoepithelial and other specific types of CXPA are not well-documented (Table 4).
Table 3.
Proposed classification of early carcinoma ex pleomorphic adenoma (ECXPA)
| Non Invasive/in situ/intratubular/intraductal (DCIS-like pattern) |
| Invasive but still intracapsular (DCIS with microinvasion-like pattern) |
| Minimally Invasive <1.5 mm |
| Invasive up to 6 mm |
The presence of intraductal carcinoma in the majority of cases (15/19) indicates that this is an early stage of malignant transformation in most CXPA. Immunohistochemistry for CK14 and p63 is necessary to confirm the intraductal/in situ nature of the CXPA. The absence of CK14 and p63 positive myoepithelial/basal cells may be used to detect progression of carcinoma with rupture of the basal membrane with extraductal but still intracapsular extension of carcinoma with further progression to extracapsular spread of varying degrees. Table 3 shows a proposed classification of ECXPA based on the recent papers reported in literature where the basal membrane of pre-existing tubules of PA, replaced by carcinoma, is used for in situ/intraductal/intratubular CXPA [4].
Katabi et al. in 2010 reported 13 cases of intracapsular and minimally invasive CXPA (≤1.5 mm). In 3/13 cases, patients developed metastatic disease. However, in the study it was not indicated if the 3 cases were intracapsular or minimally invasive and the exact degree of invasion in mm was not given. Weiler, in 2011 described a total of 41 patients with CXPA. Eight patients with extracapsular invasion of up to 6 mm had no disease progression while 12/17 patients with extracapsular invasion of 8 mm or more died of disease.
Immunohistochemistry for Mib-1 [4] and androgen receptor (AR) are suggested as useful tools to recognise microscopic foci of CXPA. However AR should not be used in isolation as up to 10 % of benign PA express nuclear staining for AR which may lead to an over-diagnosis of CXPA [31].
Researchers who have characterized the immunoprofile of the PA cells that undergo malignant transformation in CXPA identified two types of carcinomas arising in PA. The most common was composed of CK7, CK8, CK19 positive cells comparable to the luminal cells of PA and the least common were both luminal and CK14 positive myoepithelial/basal cells [32].
Conclusion
In this review we propose a classification of carcinoma ex pleomorphic adenoma (CXPA) in two prognostically relevant categories: widely invasive and early CXPA. The widely invasive CXPA is the most common and well recognised type. The ECXPA includes a spectrum of histological features that encompasses intraductal—DCIS like, microinvasive/intracapsular and, invasive with degree of invasion outside the capsule of no more than 6 mm (see Table 3). This classification of ECXPA allows an accurate definition of carcinoma in situ, when it is surrounded by basal membrane, microinvasive outside the basal membrane but still confined to the capsule of PA and early invasive when the extension outside the capsule of PA is no more than 6 mm. (Table 3).
It also clarifies that it is the basal membrane that distinguishes between in situ and invasive carcinoma not the capsule of PA. The new threshold of 6 mm also identifies patients with good prognosis.
References
- 1.Gnepp DR, Brandwein-Gensler MS, El-Naggar AK, Nagao T. Carcinoma ex pleomorphic adenoma. In: Barnes EL, Everson JW, El-Naggar AK, editors.WHO classification of tumors, vol. 9. 2005. p.242–3.
- 2.Auclair PL, Ellis GL. Atypical features in salivary gland mixed tumors: their relationship to malignant transformation. Mod Pathol. 1996;9:652–657. [PubMed] [Google Scholar]
- 3.Skálová A, Altemani A, Di Palma S, Simpson R, Hostička L, Andrle P, Laco J, Toner M, Vozmitsel M, Szakacs S, Kazakov D, Kinkor Z, Michal M. Pleomorphic adenoma of the salivary glands with intravascular tumor deposits, A diagnostic pitfall. Am J Surg Pathol. 2012;36:1674–1682. doi: 10.1097/PAS.0b013e3182690afe. [DOI] [PubMed] [Google Scholar]
- 4.Di Palma S, Skálová A, Vanìèek T, Simpson RH, Stárek I, Leivo I. Non-invasive (intracapsular) carcinoma ex pleomorphic adenoma: recognition of focal carcinoma by HER2/neu and MIB1 immunohistochemistry. Histopathology. 2005;46:144–152. doi: 10.1111/j.1365-2559.2005.02058.x. [DOI] [PubMed] [Google Scholar]
- 5.Wenig BM, Hitchcock CL, Ellis GL, Gnepp DR. Metastasizing mixed tumour of salivary glands: a clinicopathologic and flow cytometric analysis. Am J Surg Pathol. 1992;16:845–858. doi: 10.1097/00000478-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 6.LiVolsi VA, Perzin KH. Malignant mixed tumors arising in salivary glands. I. Carcinomas arising in benign mixed tumors: a clinicopathologic study. Cancer. 1977;39(5):2209–2230. doi: 10.1002/1097-0142(197705)39:5<2209::AID-CNCR2820390540>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Nagao K, Matsuzaki O, Saiga H, Sugano I, Shigematsu H, Kaneko T, Katoh T, Kitamura T. Histopathologic studies on carcinoma in pleomorphic adenoma of the parotid gland. Cancer. 1981;48:113–121. doi: 10.1002/1097-0142(19810701)48:1<113::AID-CNCR2820480122>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Gnepp DR. Malignant mixed tumors of the salivary glands: a review. Pathol Annu. 1993;28(1):279–328. [PubMed] [Google Scholar]
- 9.Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001;32:596–604. doi: 10.1053/hupa.2001.25000. [DOI] [PubMed] [Google Scholar]
- 10.Brandwein M, Huvos AG, Dardick I, Thomas MJ, Theise ND. Non-invasive and minimally invasive carcinoma ex mixed tumor: a clinicopathologic and ploidy study of 12 patients with major salivary tumors of low (or no?) malignant potential. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:655–664. doi: 10.1016/S1079-2104(96)80071-0. [DOI] [PubMed] [Google Scholar]
- 11.Waldron CA, el Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323–333. doi: 10.1016/0030-4220(88)90240-X. [DOI] [PubMed] [Google Scholar]
- 12.Di Palma S. Malignancy in pleomorphic adenoma (malignant mixed tumour) of salivary glands. In: Gale N, Luzar B editors. Proceedings in head and neck pathology. Institute of Pathology Faculty of Medicine, University of Lubljana, Lubljana 4–6 September 2003, pp 92–8.
- 13.Ellis GL, Auclair RL (2008) Tumours of the salivary glands. Washington, DC: Armed Forces Institute of Pathology 9:259–69.
- 14.Ihrler S, Weiler C, Hirschmann A, et al. Intraductal carcinoma is the precursor of carcinoma ex pleomorphic adenoma and is often associated with dysfunctional p53. Histopathology. 2007;51:362–371. doi: 10.1111/j.1365-2559.2007.02736.x. [DOI] [PubMed] [Google Scholar]
- 15.Brandwein-Gensler MS, Skalova A, Nagao T. Salivary duct carcinoma. WHO classification of tumours. In: Barnes EL, Eveson JW, El-Naggar 2005;9:236–37.
- 16.Spiro RH, Huvos AG, Strong EW. Malignant mixed tumor of salivary origin: a clinicopathologic study of 146 cases. Cancer. 1977;39:388–396. doi: 10.1002/1097-0142(197702)39:2<388::AID-CNCR2820390204>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Di Palma S, Pilotti S, Rilke F. Malignant myoepithelioma of the parotid arising in a pleomorphic adenoma. Histopathology. 1991;19:273–275. doi: 10.1111/j.1365-2559.1991.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 18.Alos LL, Cardesa A, Bombi JA, Mallofre C, Cuchi A, Traserra J. Myoepithelial tumors of salivary glands: a clinicopathologic, immunohistochemical and flow-cytometric study. Semin Diagn Pathol. 1996;13:138–147. [PubMed] [Google Scholar]
- 19.Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761–774. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Weiler C, Zengel P, van der Wal JE, Guntinas-Lichius O, Schwarz S, Harrison JD, Kirchner T, Ihrler S, et al. Carcinoma ex pleomorphic adenoma with special reference to the prognostic significance of histological progression: a clinicopathological investigation of 41 cases. Histopathology. 2011;59:741–750. doi: 10.1111/j.1365-2559.2011.03937.x. [DOI] [PubMed] [Google Scholar]
- 21.Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck. 2001;23:705–712. doi: 10.1002/hed.1100. [DOI] [PubMed] [Google Scholar]
- 22.Tortoledo ME, Luna MA, Batsakis JG. Carcinomas ex pleomorphic adenoma and malignant mixed tumours. Histomorphologic indexes. Arch Otolaryngol. 1984;110:172–176. doi: 10.1001/archotol.1984.00800290036008. [DOI] [PubMed] [Google Scholar]
- 23.Katabi N, Gomez D, Klimstra DS, Carlson DL, Lee N, Ghossein R. Prognostic factors of recurrence in salivary carcinoma ex pleomorphic adenoma, with emphasis on the carcinoma histologic subtype: a clinicopatholgic study of 43 cases. Hum Pathol. 2010;41:927–934. doi: 10.1016/j.humpath.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto K, Yamamoto H, Shiratsuchi H, et al. HER-2/neu gene amplification in carcinoma ex pleomorphic adenoma in relation to progression and prognosis: a chromogenic in situ hybridization study. Histopathology. 2012;60:E131–E142. doi: 10.1111/j.1365-2559.2012.04201.x. [DOI] [PubMed] [Google Scholar]
- 25.Skálová A, Stárek I, Vanecek T, Kucerová V, Plank L, Szépe P, Di Palma S, Leivo I. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in situ hybridization and immunohistochemistry. Histopathology. 2003;42(4):348–356. doi: 10.1046/j.1365-2559.2003.01600.x. [DOI] [PubMed] [Google Scholar]
- 26.Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology. 2008;53:416–425. doi: 10.1111/j.1365-2559.2008.03135.x. [DOI] [PubMed] [Google Scholar]
- 27.Di Palma S, Simpson R, Marchio C, Skalova A, Ungari M, Sandison A, Whitaker S, Parry S, Reis-Filho J. Pure salivary duct carcinomas can be classified into luminal, HER2 and basal-like phenotypes. Histopathology. 2012;61:629–643. doi: 10.1111/j.1365-2559.2012.04252.x. [DOI] [PubMed] [Google Scholar]
- 28.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 29.Sharon E, Kelly RJ, Szabo E. Sustained response of carcinoma ex pleomorphic adenoma treated with trastuzumab and capecitabine. Head Neck Oncol. 2010;26:2–12. doi: 10.1186/1758-3284-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Palma S, Whitaker S, Potter K, Pitkin L. Carcinoma ex pleomorphic adenoma successfully treated with trastuzumab and radiotherapy. Poster 021 presented at the 24th European congress of pathology. Prague. 2012. 8–12 September, 2012.
- 31.De Roche TC, Hoschar AP, Hunt JL. Immunohistochemical evaluation of androgen receptor, HER2/neu, and p53 in benign pleomorphic adenomas. Arch Pathol Lab Med. 2008;132(12):1907–1911. doi: 10.5858/132.12.1907. [DOI] [PubMed] [Google Scholar]
- 32.Altemani A, Martins MT, Freitas L, Soares F, Araujo NS, Araujo VC. Carcinoma ex pleomorphic adenoma (CXPA): immunoprofile of the cells involved in carcinomatous progression. Histopathology. 2005;46:635–641. doi: 10.1111/j.1365-2559.2005.02157.x. [DOI] [PubMed] [Google Scholar]