Abstract
Sclerosing polycystic adenosis (SPA) is a rare condition of salivary glands. The most common site is the parotid gland (80 % of cases). SPA shows no gender predilection and occurs over a wide age spectrum (9–84 years). SPA is mostly unifocal, but may rarely be multifocal. Histologically, SPA are sharply circumscribed mostly unencapsulated lesions composed of acinar and ductal components with variable cytomorphological characteristics, including foamy, vacuolated, apocrine, mucous, clear/ballooned, squamous, columnar and oncocyte-like cells. Characteristic for SPA is the presence of large acinar cells with abundant eosinophilic cytoplasmic granules. The stroma is densely collagenized, frequently harbouring a variably intense chronic inflammatory infiltrate and may contain fat. Rarely the stroma is myxoid. Some degree of intraductal epithelial proliferations have been reported in at least 50 % of cases. The proportion of cases with epithelial proliferations that fulfill criteria for high-grade ductal carcinoma in-situ is <10 %. Immunohistochemically, both ductal and acinar cells are positive for broad spectrum cytokeratins. There is variable immunoreactivity for epithelial membrane antigen and S-100 protein. CEA, p53 and HER2 is reportedly negative. Gross cystic disease fluid protein-15 is strongly expressed in the acinar component. There is consistent but variable expression of estrogen and progesterone receptors. The proliferative index (Ki-67) is low (1–2 %) in the benign (acinar and ductal) components. Using HUMARA methodology (non-random inactivation of X-chromosomes), six cases with atypical epithelial proliferations have been shown to be clonal processes. Recurrences have been reported in up to 19 % of cases.
Keywords: Sclerosing polycystic adenosis, Salivary gland, Carcinoma, Dysplasia, Adenosis, Hyperplasia
Introduction
Sclerosing polycystic adenosis (SPA) is a rare tumorous condition of salivary glands with a characteristic combination of histological features, some of which are reminiscent of histopathological changes that occur in the mammary gland (fibrocystic disease/sclerosing adenosis and intraductal epithelial proliferations of various types). SPA was first described by Smith et al. [1] in a series comprising 9 cases. Since then, just over 50 cases in a few, mostly small series and several case reports, in a total of 19 original studies, have been published [2–20]. Apart from the seminal paper by Smith et al., the largest series to date was published by Gnepp et al. [5] and comprises 16 cases.
Epidemiology and Clinical Features
There are no reliable data on the incidence of SPA in the literature. However, the rarity of SPA is underscored by the paucity of reported cases and that all but two original publications are either case reports or small series (of maximal three cases) in conjunction with the fact that the largest series was a collaborative effort by several experienced Head and Neck pathologists (who collectively must have seen a substantial number of salivary gland lesions). Regarding location, by far the most common site is the parotid gland (at least 80 % of cases) with much less frequent occurrence in the submandibular gland. SPA seems to occur with roughly equal frequency in men and women and occurs over a wide age spectrum: 9–84 years. Reportedly, the mean age at presentation is around 40 years. Symptoms are non-specific, i.e. those of a slow growing painless mass, although a few cases with some reported pain are on record [1, 2]. Authors have claimed to have detected SPA also in minor salivary glands [5, 7, 9, 10, 21]. Based on the verbal descriptions and the photomicrographs provided, it is this author’s view that most of these lesions represent de facto SPA, except for the cases presented by Swelam et al. (who also claim to have identified Epstein Barr virus in their lesions) and Meer and Altini [9, 21].
Sclerosing polycystic adenosis is most commonly seen as an isolated pathologic finding in the salivary gland, but cases associated with recurrent pleomorphic adenoma, oncocytoma, a combined sebaceous lymphadenoma-Warthin’s tumor, and a pure Warthin’s tumor are on record [5, 15]. Similarly, SPA is most often seen as a single lesion, but rare cases with multifocality have been documented [5, 19]. The reported size range of SPA is between 0.7 and 12 cm.
Histopathology
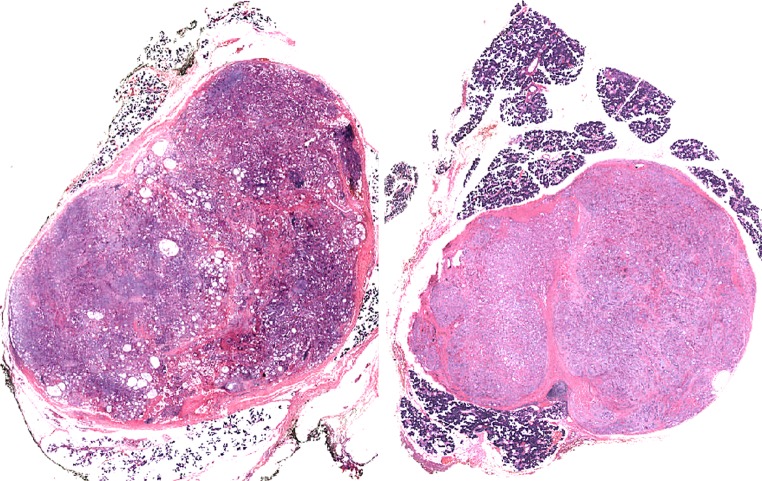
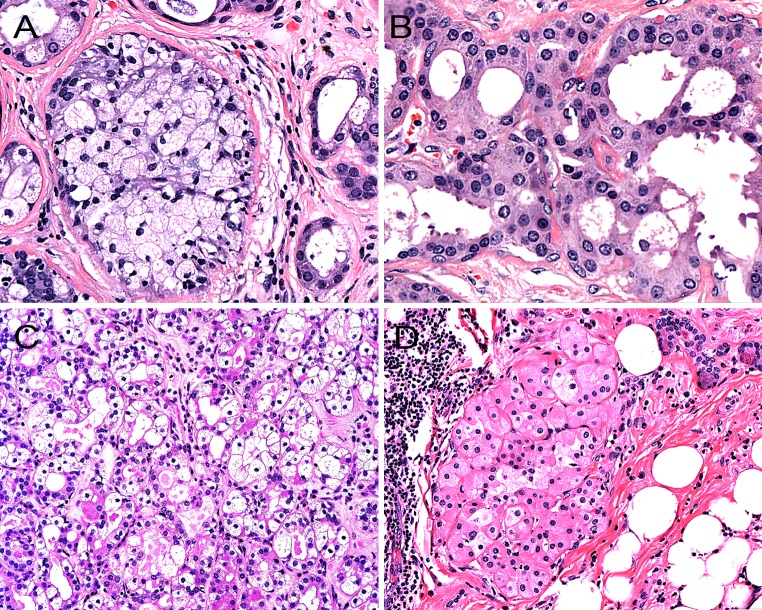
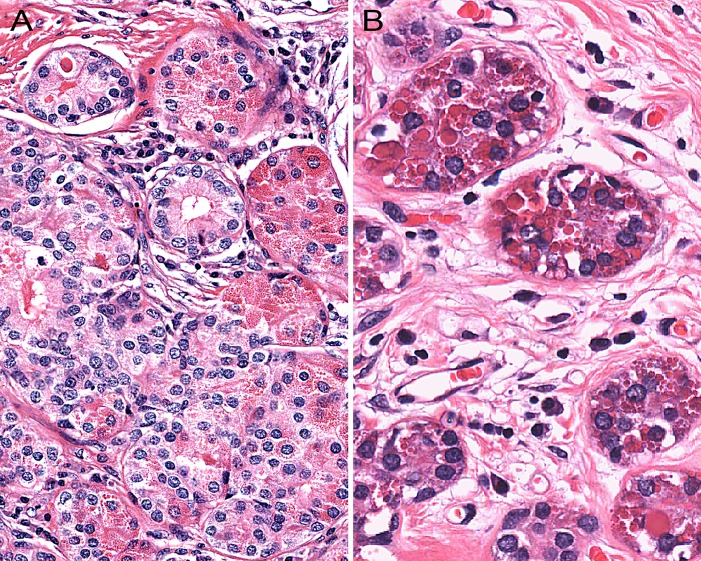
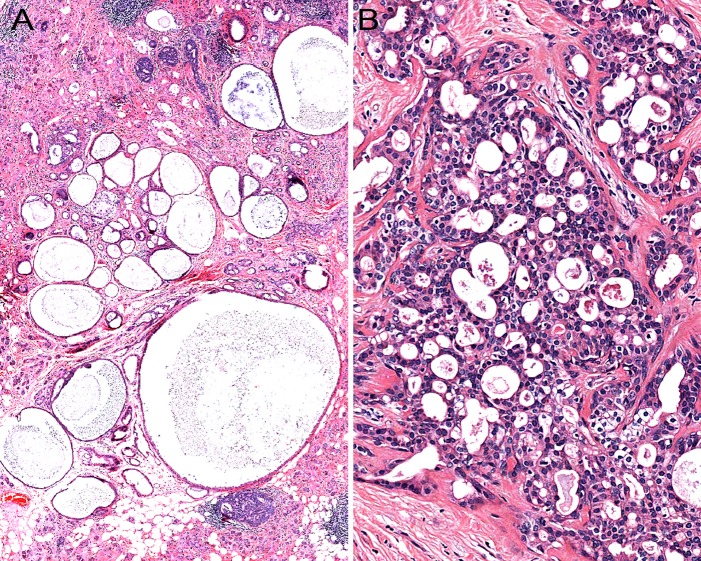
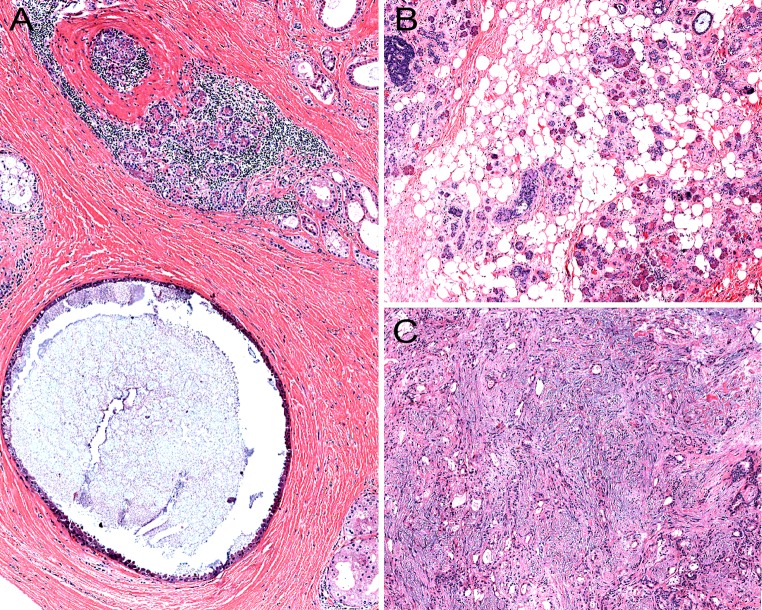
SPAs are sharply-circumscribed round to oval nodular lesions (Fig. 1). The lesions are frequently unencapsulated, but may have a more or less complete pseudocapsule and are composed of acinar and ductal components with variable cytomorphological characteristics, including foamy, vacuolated (sebocyte-like), apocrine, mucous, clear/“ballooned”, squamous, columnar and oncocyte-like cells (Fig. 2). Highly characteristic for SPA is the presence of large acinar cells with abundant eosinophilic (PAS-positive, diastase resistant) cytoplasmic granules of varying sizes. Not uncommonly, these granules may acquire a very large size and appear as intracytoplasmic globules (Fig. 3). The focal presence of acinar cells with serous differentiation as an inherent component of SPA has received little attention in the literature (compared to the acinar—lesional cells with the eye-catching large eosinophilic globules). This author has seen a case with acini composed of distinctly serous cells within the center of a case of SPA, juxtaposed to the more commonly occurring and characteristic eosinophilic, granulated acinar cells. In most cases, a variably prominent, cystic component is present (Fig. 4a). The lumina of these cysts/cystically-dilated ducts frequently contain PAS-positive, diastase-resistant secretory material. The cysts may be lined by flattened, apocrine, or vacuolated cells. Wholly or partly, the epithelial cells lining the cysts may be denuded and replaced by foamy macrophages. In some cases, however, the cystic component may be inconspicuous (“paucicystc SPA”) [19]. In some cases, dilated ducts with intraluminal cribriform arrangements composed of cells with secretory activity may be encountered. This may create a pattern vaguely reminiscent of that seen in mammary analogue secretory carcinoma (Fig. 4b). Most commonly, the cellular components in SPA are embedded in a sharply-delineated, dense, sclerotic-collagenous stroma (Fig. 5). The stroma may on occasion form hyalinized hypocellular nodules/plaque-like structures that may or may not contain residual epithelial cellular remnants and/or vacuolated foam cell-type macrophages. These scar-like structures may display clefts of the hyalinized sclerotic collagen similar to those seen in silicotic nodules. In many cases, a stromal lipocytic component is present and, in some cases, there may be a component of periductal stromal hyalinization. In addition, the stroma may also display a distinctly myxoid quality (Fig. 5). This is a feature that has only infrequently been highlighted in the literature. Commonly, the stroma harbours a variably intense, frequently nodular chronic inflammatory infiltrate which may contain bona fide lymphoid follicles with germinal centers.
Fig. 1.
a, b Low-power histological sections of two cases of sclerosing polycystic adenosis of major salivary glands. Both lesions are round to oval with sharply circumscribed borders
Fig. 2.
The acinar and ductal epithelial components in sclerosing polycystic adenosis may show a range of variable cytomorphological features, including foamy-vacuolated (a), apocrine (b), clear- (c) and oncocyte-like cells (d)
Fig. 3.
Highly characteristic for sclerosing polycystic adenosis is the presence of acinar cells with abundant eosinophilic cytoplasmic granules. The size of the granules ranges from fine (a) to very large and, in the latter case, they appear as intracytoplasmic globules (b)
Fig. 4.
a In most cases of sclerosing polycystic adenosis, a variably prominent, dilated ductal—cystic component is present. b Occasionally, sclerosing polycystic adenosis may harbour dilated ducts with cribriform arrangement composed of cells with intraluminal secretory activity creating a pattern vaguely reminiscent of that seen in mammary analogue secretory carcinoma
Fig. 5.
Most commonly, the stroma in sclerosing polycystic adenosis is composed of dense, sclerotic collagen (a). In many cases, a stromal lipocytic component is present (b); in addition, the stroma may also display a distinctly myxoid quality (c)
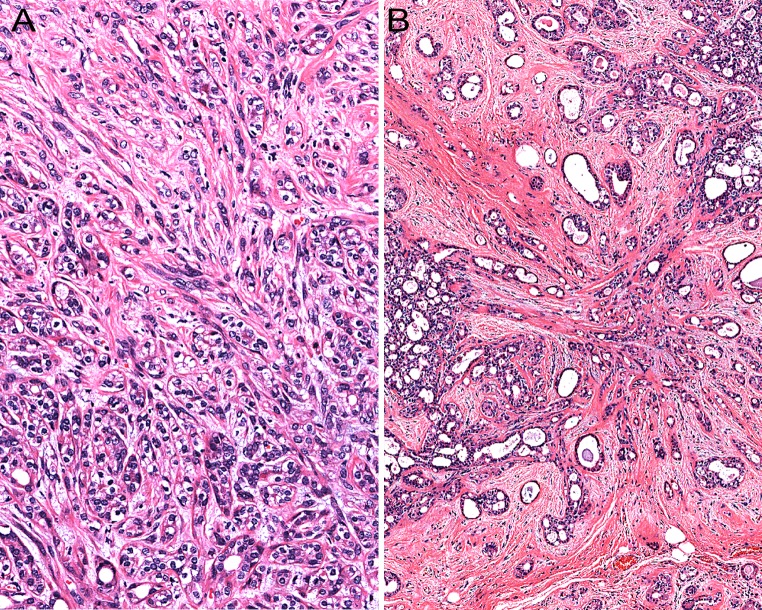
The acinar component may or may not show preservation of the lobular architecture. In the latter case, a sclerosing adenosis-like histopathological pattern is encountered and when this is associated with a stroma-induced distortion of the acini/ductules, the process may acquire an infiltrative appearance (Fig. 6). Moreover, as described in a few cases by Gnepp et al. [5], stromal distortion may lead to the formation of radial scar-like structures. This phenomenon has also been encountered by this author (Fig. 6).
Fig. 6.
The acinar component may be associated with stromal distortion which may impart an infiltrative appearance (a). Stromal distortion may also lead to the formation of radial scar-like structures (b)
In occasional cases, foci of basement membrane-like material may occur associated with cribriform epithelial proliferations imparting a collagenous spherulosis-like appearance.
Sclerosing polycystic adenosis frequently harbors intraductal epithelial proliferations which frequently have been labeled as dysplastic. In some cases, investigators have found that the degree of cellular atypia and structural changes have reached their threshold for ductal carcinoma in situ (DCIS) [2, 4, 5, 8, 12, 13]. Given the fact that these assessments are plagued by a significant degree of subjectivity (especially when dealing with milder, presumably “low-grade” dysplastic epithelial proliferations), the frequency of this phenomenon is difficult to ascertain. Some degree of intraductal epithelial proliferation in SPA has been reported in at least 50 % of cases. However, the proportion of cases of SPA with epithelial proliferation that fulfill reasonably accepted criteria for high-grade malignancy/DCIS (e.g., significant nuclear pleomorphism, intraluminal necrosis, increased mitotic activity), seems not to exceed 10 % of reported cases. There are no formal and detailed studies on a large number of cases of SPA focusing on the histopathological and/or immunohistochemical features of the spectrum of intraductal proliferations that may occur in SPA. Moreover, no investigator has undertaken the task to systematically compare the similarities and dissimilarities between intraductal epithelial proliferations in SPA compared to those which much more commonly occur in the mammary gland. One major problem in establishing a diagnosis of “dysplastic epithelial proliferation” or low-grade DCIS in the context of salivary gland pathology in general, and in SPA in particular, is that in contrast to the situation in the breast, this phenomenon, as referred to above, is not well-studied and that widely-accepted and reliable criteria are lacking. Moreover, there is no reason a priori to postulate that various categories of epithelial proliferations, e.g., hyperplasia, atypical hyperplasia and low-grade DCIS, in SPA are morphologically identical or biologically similar to those in the breast. In mammary pathology, there is a sharp distinction between intraductal hyperplasia (“usual type”; UDH) on the one hand and atypical hyperplasia (ADH) and low-grade DCIS, on the other hand. There is substantial evidence for the idea that UDH is a proliferation of primitive, uncommitted, pluripotent mammary epithelial cells with little or no tendency to luminal differentiation, whereas ADH and DCIS develop from cells that are committed to luminal differentiation. This is reflected both in the different histopathological patterns and cytological features exhibited by UDH on the one hand and ADH/low-grade DCIS on the other. UDH typically displays architectural features, such as crescent-shaped masses, irregular fenestrations, streaming arrangement of cells, uneven distribution of nuclei, maturation and cytological (non-uniform) features, including variability of nuclear shapes with folds, notches, grooves and inclusions and variable chromatin texture; in contrast, ADH/low-grade DCIS forms cribriform spaces with distinct luminal differentiation of the neoplastic cells that are evenly distributed and with nuclei polarized away from the lumina with a cytoplasmic compartment towards the luminal spaces and have uniform nuclear features including smooth contours and nuclear hyperchromasia, not infrequently with finely-dispersed chromatin. Moreover, these differences are (in the breast) reflected in the different types of cytokeratins expressed by the lesional cells such that UDH tends to express basal cytokeratins (CK); 5/6 and 14, while low-grade DCIS are negative on IHC for these intermediate filaments and express “luminal” cytokeratins like CK7, 8, 18 and 19, which are conversely negative in UDH [22–24]. That there is a difference in perception (in relation to mammary intraductal proliferations) of what constitutes low-grade DCIS can be inferred from a statement on this topic by some investigators [12]. In this paper, the authors state: “In every case, nuclearpleomorphism was noted, ranging in severity from mild up to severe, then amounting to low-grade ductal carcinoma in situ”. It should be noted that nuclear pleomorphism in mammary intraductal proliferations is a feature that is attributed to either UDH or high-grade DCIS, but not ADH or low-grade DCIS, which are composed of a rather monotonous population of lesional cells.
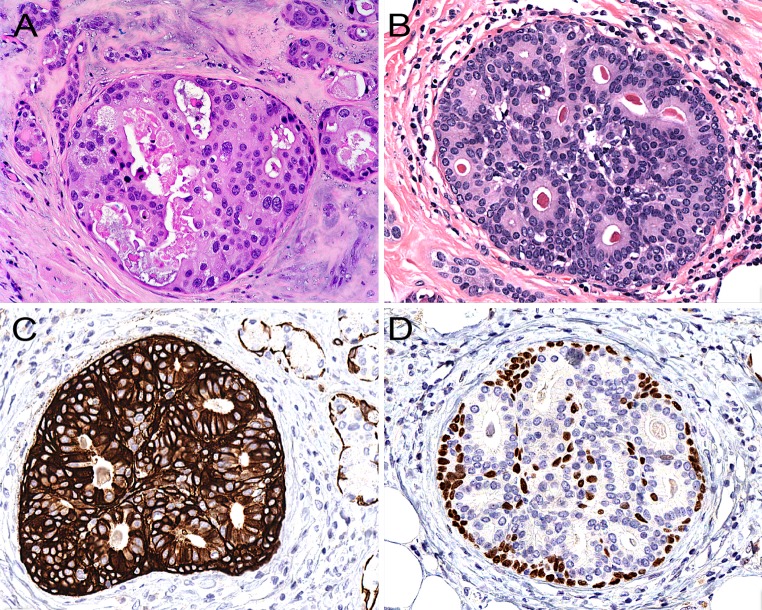
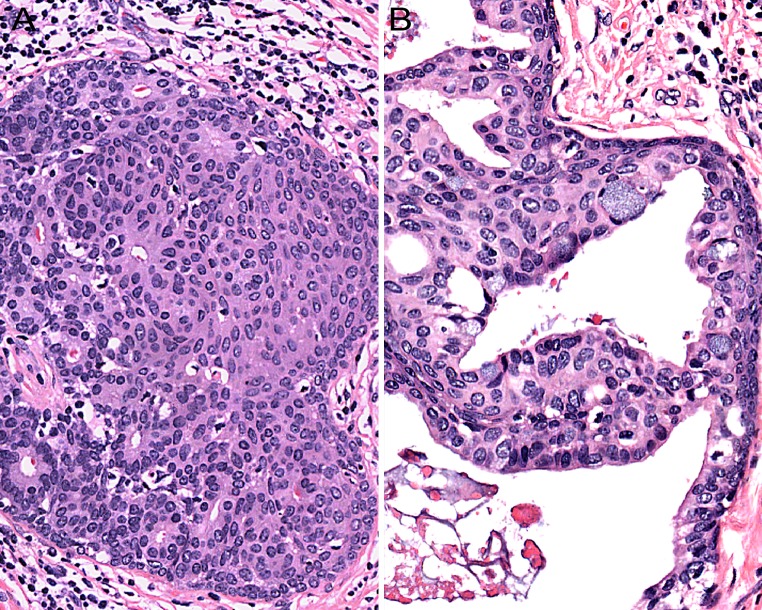
When SPA contains intraductal proliferations that are obviously histologically malignant, the diagnosis is easy (Fig. 7a). However, when SPA harbours “low-grade” intraductal proliferations which display distinct “luminal differentiation” akin to what is stated above regarding mammary ADH/low-grade DCIS (Fig. 7b), strong expression of both CK 7 and CK 5/6 may be encountered (Fig. 7c). Another distinguishing immunohistochemical feature between ADH/low-grade DCIS, as defined in the breast, in comparison to morphologically similar processes, i.e. cytologically low-grade intraductal proliferations with luminal/glandular differentiation in SPA, is that in contrast to what is seen in the mammary gland, these proliferations (in SPA) may show participation of p63-positive (non-squamoid) cells within the intraluminal cell-mass (Fig. 7d). This feature would in the breast point towards a benign, non-neoplastic proliferation. However, this may not necessarily be true for salivary glands (rather the contrary) where well-established malignant entities, e.g., epithelial-myoepithelial carcinoma and adenoid cystic carcinoma, are typified by a dual-cell population of which one is consistently positive for p63. The expression of p63 by some of the cells in an intraductal proliferation in SPA should (of course) be separated from cases where the intraductal proliferation harbours some cells that display glandular/luminal features and others with a squamoid appearance (Fig. 8). In addition, in some instances, intraluminal proliferations in SPA may show a pure squamoid phenotype. Occasionally, some ducts in SPA may display a cellular proliferation with “mucoepidermoid features”, i.e., with squamoid/intermediate cells intermingled with mucocytes (Fig. 8).
Fig. 7.
a When intraductal proliferations in sclerosing polycystic adenosis display obvious nuclear pleomorphism and luminal necrosis, the diagnosis of ductal carcinoma in situ is easy. b A “low-grade” cribriform intraductal proliferation with distinct luminal differentiation similar to mammary atypical ductal hyperplasia/low-grade ductal carcinoma in situ. c The lesional cells are frequently strongly positive for cytokeratin 5/6, and some cells may also be positive for p63 (d), a feature that differs from ADH/low-grade DCIS in the mammary gland
Fig. 8.
a Some intraductal proliferations in sclerosing polycystic adenosis may show both glandular/luminal and squamous features. b Occasionally, some ducts in sclerosing polycystic adenosis may display a cellular proliferation with “mucoepidermoid features”, i.e., with squamoid/intermediate cells intermingled with mucocytes
Cytopathology
The cytopathological/fine needle aspiration (FNA) features of SPA have been characterized or commented on in seven case reports [3, 4, 6, 8, 11, 17, 19] and in one series (4 out of 16 patients) [5]. The investigators have reported the presence of “syncytial epithelial ducts with apocrine changes”, oncocytic cells in a frequently “cystic-type background”, i.e., foamy histiocytes and proteinaceous material. Sebocyte-like (vacuolated) cells have been described by Gnepp et al. and Kloppenborg et al. [5, 17]. Fulcinetti et al. [4] claim to have identified bona-fide sebocytes (“sebaceous cells”) in their case. However, although they nicely illustrate the presence of vacuolated cells, the authors state that these cells were “fat-laden”, but no fat-stain was performed and no immunocytochemical evidence of sebaceous differentiation (adipophilin) was provided. These authors conclude that sheets of cells composed of apocrine cells and sebaceous (e.g., vacuolated/sebocyte-like) cells is a finding highly suggestive of SPA. Moreover, as emphasized by Fulcinetti et al., the suspicion of SPA should be even stronger if the above cytopathological findings are seen in conjunction with evidence of an intraductal epithelial proliferation, which in their case focally-displayed “a moderate degree of cell atypia”, with disturbance of cell polarity, “anisonucleosis and anisocytosis”. Imamura et al. [8] point out that the presence of lymphoplasmacytic cells in the background in association with apocrine-type cells (which may be mistaken for oncocytic cells) and ductal structures may lead to an erroneous diagnosis of Warthin’s tumor.
In the case published by Perrottino et al., the authors briefly state that “cytology suggested pleomorphic adenoma”, with no verbal descriptions or photomicrographs of the cytopathological findings provided. A more elaborate description is given by Etit et al. [3] who identified sheets and aggregates of epithelial cells with moderate to abundant “finely granular oncocytic cytoplasm”. These cells had round to oval nuclei with even chromatin and indistinct nucleoli. The authors also report few flat sheets of cells having a “squamoid” appearance, some cells that formed glandular structures, markedly vacuolated cells “reminiscent of sebaceous differentiation”, and cells featuring apical cytoplasmic snouts. Petersson et al. [19] reported epithelial cells in acinar and trabecular arrangements with focal mild nuclear atypia and ample granular to microvacuolated cytoplasm with a small number of lymphoid cells and naked nuclei in the background.
In the series by Gnepp et al. [5], 4 cases with FNA findings were commented on (cases 1, 2, 6 and 15). These cases had a cytological diagnosis of: “Oncocytes and myoepithelial cells consistent with Warthin’s tumor”, “pleomorphic adenoma”, “features suggestive of a neoplasm, ?mucoepidermoid” and “epithelial neoplasm suggestive of low-grade carcinoma”, respectively.
Thus, taking all into account, it appears that the FNA-diagnosis of SPA is challenging. This is partly due to the rarity of SPA, but also to the protean variation/range of light microscopic morphologies/appearances that the cells of SPA characteristically display.
Immunohistochemistry
Both ductal and acinar cells are positive for broad spectrum cytokeratins (AE1/AE3 and Cam5.2). Variable immunoreactivity for EMA, S-100 protein, and antimitochondrial antibody has been reported, whereas CEA, p53 and HER2 have been negative. GCDFP-15 is usually strongly expressed in the acinar component [12, 13] (Fig. 9). Weak positivity for GCDFP-15 may also be seen in the dysplastic or DCIS components. Consistent but variable expression of ER and PR is present in the acinar and ductal cells. ER and PR may or may not be present in the dysplastic/in situ intraductal proliferations [12, 19]. In one case report, ER was expressed by 10 % of the cells in the high-grade DCIS component [19].
Fig. 9.
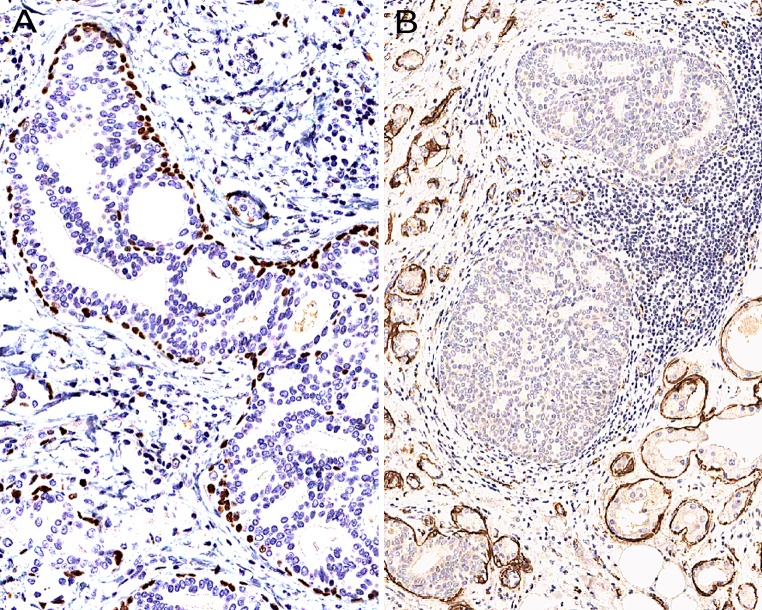
Low-grade, cribriform intraductal proliferations in SPA are rimmed by a continuous layer of p63-positive cells (a) which on occasion are completely negative for SMA (b)
The proliferative activity (Mib-1/Ki-67) is low (1, 2 %) in the benign (both ductular and adenosis) components, whereas significantly increased proliferation may be encountered in an associated high-grade DCIS [13, 19].
P63—, smooth muscle actin—(SMA) and calponin-positive myoepithelial cells are present in the periphery of the acini and ducts in SPA. These myoepithelial cells are also present, albeit occasionally somewhat discontinuously, around a DCIS-component. The immunohistochemical identification of their presence is a useful feature in distinguishing a true invasive carcinoma in an adenosis-component (with loss of the lobular architecture) or in conjunction with DCIS, since both these processes may be associated with stroma-induced distortion of the architecture, as previously described [5, 12, 19, 25].
In contrast to other investigators who have reported that the myoepithelial cells surrounding “ducts filled with hyperplastic and dysplastic epithelium” (Skalova et al. [12, 13] and Gnepp et al. [5]) (“ductal and acinar structures, whether or not they contained normal-appearing, mildly or severely atypical cells”), have been positive for SMA, this author has identified a peculiar immunohistochemical finding in a case of SPA where some of the low-grade, cribriform intraductal proliferations were rimmed by a continuous layer of p63-positive myoepithelial cells that were completely negative for SMA (Fig. 9).
Ultrastructure
The ultrastructural features of SPA have only been investigated in one previous study [13]. Skalova et al. reported that the epithelial cells demonstrated secretory features and desmosomes. Some cells contained numerous electron dense granules which were interpreted as zymogen granules. In addition, fewer, irregular electron lucent granules were seen. Rough endoplasmic reticulum was frequently prominent within the space between the secretory granules. Apocrine cells displayed microvilli and the ballooned/clear cells had large cytoplasmic vacuoles.
Etiopathogenesis
No conclusive evidence on the etiology and/or pathogenesis of SPA has been presented. The nature of this lesion was initially believed to be reactive/inflammatory [1]. However, using HUMARA methodology, Skalova et al. could establish clonality (non-random inactivation of X-chromosomes) in six out of six successfully analyzed (female) cases, providing evidence that SPA may in reality be a neoplastic process. However, all these cases displayed areas with epithelial atypia (“nuclear pleomorphism, ranging in severity from mild up to severe”) [12]. Hence, it is still an open question whether cases of SPA without atypical epithelial proliferations also are clonal processes. Given the fact that apocrine adenosis in the breast [26] and one adenosis tumor of the anogenital glands [27] (with no dysplastic epithelial components) have been shown to be monoclonal proliferations, a monoclonal nature of SPA without atypical/dysplastic intraductal proliferations, seems to be a plausible (but unproven) assumption.
Differential Diagnosis
The differential diagnostic considerations for SPA have been discussed and commented on by previous authors [1, 5, 13, 25] and include acinic cell carcinoma (ACC), polycystic (dysgenetic) disease, sclerotic sialadenitis, and salivary duct carcinoma, including the low-grade variant and adenocarcinoma, NOS. Given the predominance of cells with acinar differentiation and mild nuclear abnormalities, the differential diagnosis of an ACC on FNAC may be problematic. Similarly, the presence of a “dirty”/cystic background containing lymphoid cells in conjunction with cells showing oncocyte (-like) features may raise the possibility of a Warthin’s tumor. In contrast, the histological features of SPA are highly-characteristic and awareness of these allows for a diagnosis of this lesion based on hematoxylin and eosin stained sections alone. However, an immunohistochemical study focusing on myoepithelial cells may be necessary to reveal the pseudoinfiltrative nature of some areas affected by stromal distortion, as described above.
Clinical Behaviour
Recurrences, sometimes multiple, have been reported quite frequently (19 % as summarized by Gnepp et al.). The recurrences typically occur after a long time-span (up to 22 years). The true recurrence rate of SPA is difficult to ascertain based on the limited cases with adequate follow-up as highlighted in Table 1 in the paper by Meer and Altini [9]. The proposed mechanism behind recurrences is either incomplete surgical resection and/or multifocal disease [5]. A rather spectacular (second) recurrence where the tumor measured almost 12 cm was presented by Gnepp et al. [5]. To date, no case of invasive carcinoma in SPA either with or without metastatic disease has been documented in the literature. However, this author knows of one case with invasive carcinoma ex SPA where the carcinoma was infiltrating into the surrounding salivary gland parenchyma (personal communication, Dr. Michal Michal, Czech Republic). Based on this, it seems plausible that a metastasizing case of carcinoma ex SPA will be documented at some point in time.
Acknowledgments
The author wishes to thank Professor Ken Berean, Department of Pathology, Surrey Memorial Hospital, University of British Columbia, Canada, for kindly providing some of the material that was used for the photomicrographs.
References
- 1.Smith BC, Ellis GL, Slater LJ, et al. Sclerosing polycystic adenosis of major salivary glands. A clinicopathologic analysis of nine cases. Am J Surg Pathol. 1996;20:161–170. doi: 10.1097/00000478-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bharadwaj G, Nawroz I, O’Regan B. Sclerosing polycystic adenosis of the parotid gland. Br J Oral Maxillofac Surg. 2007;45:74–76. doi: 10.1016/j.bjoms.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Etit D, Pilch BZ, Osgood R, et al. Fine-needle aspiration biopsy findings in sclerosing polycystic adenosis of the parotid gland. Diagn Cytopathol. 2007;35:444–447. doi: 10.1002/dc.20671. [DOI] [PubMed] [Google Scholar]
- 4.Fulciniti F, Losito NS, Ionna F, et al. Sclerosing polycystic adenosis of the parotid gland: report of one case diagnosed by fine-needle cytology with in situ malignant transformation. Diagn Cytopathol. 2010;38:368–373. doi: 10.1002/dc.21228. [DOI] [PubMed] [Google Scholar]
- 5.Gnepp DR, Wang LJ, Brandwein-Gensler M, et al. Sclerosing polycystic adenosis of the salivary gland: a report of 16 cases. Am J Surg Pathol. 2006;30:154–164. doi: 10.1097/01.pas.0000186394.64840.1d. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Jain R, Singh S, et al. Sclerosing polycystic adenosis of parotid gland: a cytological diagnostic dilemma. Cytopathology. 2009;20:130–132. doi: 10.1111/j.1365-2303.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 7.Gurgel CA, Freitas VS, Ramos EA, et al. Sclerosing polycystic adenosis of the minor salivary gland: case report. Braz J Otorhinolaryngol. 2010;76:272. doi: 10.1590/S1808-86942010000200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imamura Y, Morishita T, Kawakami M, et al. Sclerosing polycystic adenosis of the left parotid gland: report of a case with fine needle aspiration cytology. Acta Cytol. 2004;48:569–573. doi: 10.1159/000326420. [DOI] [PubMed] [Google Scholar]
- 9.Meer S, Altini M. Sclerosing polycystic adenosis of the buccal mucosa. Head Neck Pathol. 2008;2:31–35. doi: 10.1007/s12105-008-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noonan VL, Kalmar JR, Allen CM, et al. Sclerosing polycystic adenosis of minor salivary glands: report of three cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:516–520. doi: 10.1016/j.tripleo.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Perottino F, Barnoud R, Ambrun A, et al. Sclerosing polycystic adenosis of the parotid gland: diagnosis and management. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127:20–22. doi: 10.1016/j.anorl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Skalova A, Gnepp DR, Simpson RH, et al. Clonal nature of sclerosing polycystic adenosis of salivary glands demonstrated by using the polymorphism of the human androgen receptor (HUMARA) locus as a marker. Am J Surg Pathol. 2006;30:939–944. doi: 10.1097/00000478-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Skalova A, Michal M, Simpson RH, et al. Sclerosing polycystic adenosis of parotid gland with dysplasia and ductal carcinoma in situ. Report of three cases with immunohistochemical and ultrastructural examination. Virchows Arch. 2002;440:29–35. doi: 10.1007/s004280100481. [DOI] [PubMed] [Google Scholar]
- 14.Mackle T, Mulligan AM, Dervan PA, et al. Sclerosing polycystic sialadenopathy: a rare cause of recurrent tumor of the parotid gland. Arch Otolaryngol Head Neck Surg. 2004;130:357–360. doi: 10.1001/archotol.130.3.357. [DOI] [PubMed] [Google Scholar]
- 15.Tokyol C, Aktepe F, Hasturk GS, et al. Sclerosing polycystic adenosis of the parotid gland presenting with a Warthin tumor. Kulak Burun Bogaz Ihtis Derg. 2012;22:288–292. doi: 10.5606/kbbihtisas.2012.055. [DOI] [PubMed] [Google Scholar]
- 16.Kim BC, Yang DH, Kim J, et al. Sclerosing polycystic adenosis of the parotid gland. J Craniofac Surg. 2012;23:e451–e452. doi: 10.1097/SCS.0b013e318262d2a5. [DOI] [PubMed] [Google Scholar]
- 17.Kloppenborg RP, Sepmeijer JW, Sie-Go DM, et al. Sclerosing polycystic adenosis: a case report. B-Ent. 2006;2:189–192. [PubMed] [Google Scholar]
- 18.Eliot CA, Smith AB, Foss RD. Sclerosing polycystic adenosis. Head Neck Pathol. 2012;6:247–249. doi: 10.1007/s12105-011-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersson F, Tan PH, Hwang JS. Sclerosing polycystic adenosis of the parotid gland: report of a bifocal, paucicystic variant with ductal carcinoma in situ and pronounced stromal distortion mimicking invasive carcinoma. Head Neck Pathol. 2011;5:188–192. doi: 10.1007/s12105-011-0242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beato Martinez A, Moreno Juara A, Candia Fernandez A. Sclerosing polycystic adenosis of the submandibular gland. Acta Otorrinolaringol Esp. [DOI] [PubMed]
- 21.Swelam WM. The pathogenic role of Epstein-Barr virus (EBV) in sclerosing polycystic adenosis. Pathol Res Pract. 2010;206:565–571. doi: 10.1016/j.prp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Boecker W, Moll R, Dervan P, et al. Usual ductal hyperplasia of the breast is a committed stem (progenitor) cell lesion distinct from atypical ductal hyperplasia and ductal carcinoma in situ. J Pathol. 2002;198:458–467. doi: 10.1002/path.1241. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix-Triki M, Mery E, Voigt JJ, et al. Value of cytokeratin 5/6 immunostaining using D5/16 B4 antibody in the spectrum of proliferative intraepithelial lesions of the breast. A comparative study with 34betaE12 antibody. Virchows Arch. 2003;442:548–554. doi: 10.1007/s00428-003-0808-0. [DOI] [PubMed] [Google Scholar]
- 24.Otterbach F, Bankfalvi A, Bergner S, et al. Cytokeratin 5/6 immunohistochemistry assists the differential diagnosis of atypical proliferations of the breast. Histopathology. 2000;37:232–240. doi: 10.1046/j.1365-2559.2000.00882.x. [DOI] [PubMed] [Google Scholar]
- 25.Gnepp DR. Sclerosing polycystic adenosis of the salivary gland: a lesion that may be associated with dysplasia and carcinoma in situ. Adv Anat Pathol. 2003;10:218–222. doi: 10.1097/00125480-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Endoh Y, Tamura G, Kato N, et al. Apocrine adenosis of the breast: clonal evidence of neoplasia. Histopathology. 2001;38:221–224. doi: 10.1046/j.1365-2559.2001.01095.x. [DOI] [PubMed] [Google Scholar]
- 27.Kazakov DV, Bisceglia M, Sima R, et al. Adenosis tumor of anogenital mammary-like glands: a case report and demonstration of clonality by HUMARA assay. J Cutan Pathol. 2006;33:43–46. doi: 10.1111/j.0303-6987.2006.00391.x. [DOI] [PubMed] [Google Scholar]